Abstract
The performance of a commercially produced 62Zn/62Cu microgenerator system, and an associated kit-based radiopharmaceutical synthesis method, was evaluated for clinical site production of [62Cu]Cu-ETS (ethylglyoxal bis(thiosemicarbazonato)copper(II)), an investigational agent for PET perfusion imaging. Using 37 generators, containing 1.84 ± 0.23 GBq 62Zn at 9:00 AM on the day of clinical use, a total of 45 patient doses of [62Cu]Cu-ETS (672 ± 172 MBq) were delivered without difficulty. 62Cu elution yields were high (approximately 90%), accompanied by extremely low 62Zn breakthrough (<0.001%). Radiopharmaceutical preparation, from the start-of-elution to time-of-injection, consumed less than five minutes. The 62Zn/62Cu microgenerator was a dependable source of short-lived positron-emitting 62Cu, and the kit-based synthesis proved to be rapid, robust, and highly reliable for “on-demand” delivery of [62Cu]Cu-ETS for PET perfusion imaging.
Keywords: 62Zn/62Cu generator, PET radiopharmaceuticals, [62Cu]Cu-ETS (copper(II) ethylglyoxal bis(thiosemicarbazone)), tumor perfusion
1. Introduction
Copper-62 (β+ 98%, t1/2: 9.7 min.) is formed by the decay of cyclotron-produced 62Zn (t1/2: 9.2 hours) and offers attractive nuclear and chemical properties for many PET applications. Its nearly 10-minute half-life is compatible with relatively long image acquisition periods for good counting statistics, and yet remains short enough to permit repeat scanning within a single imaging session (e.g., performance of stress/rest perfusion studies, or combination studies with other radiopharmaceuticals such as 18F-FDG).
A number of designs have been reported for 62Zn/62Cu generator systems that deliver 62Cu in forms suitable for radiopharmaceutical synthesis (Robinson, Zielinski, and Lee 1980; Fujibayashi, et al., 1989; Green, et al., 1990; Zweit, et al., 1992; Haynes, et al., 2000). One widely used approach to 62Zn/62Cu generator construction has been reliance on a design reported by Robinson, et al., (1980) in which selective 62Zn(II) retention is provided by an anion exchange resin. For maximum recovery of 62Cu from the generator column, an acidic elution solvent containing 2.0 molar chloride ion is normally used. Under these conditions, the anion exchange resin avidly adsorbs the 62Zn(II) as [ZnCl3(H2O)]1−, while the 62Cu2+ ions are more loosely bound as CuCl2(H2O)2 and can be selectively eluted from the column on-demand (Kraus and Moore, 1952).
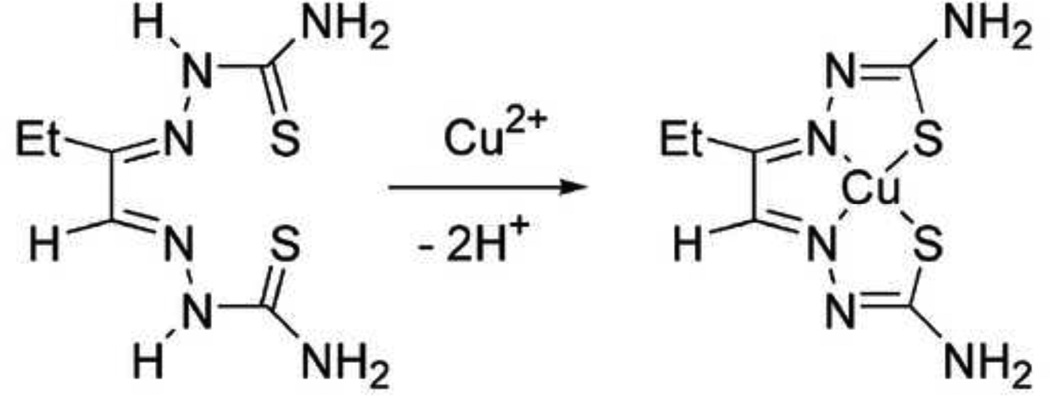
62Cu-labeled ethylglyoxal bis(thiosemicarbazone) ([62Cu]Cu-ETS) (Figure 1), a lipophilic agent, has been identified as having favorable characteristics for in vivo organ perfusion imaging with PET (John and Green, 1989; Green, et al., 2007, 2009; Lacy, et al., 2007, 2010; Basken and Green, 2009). This compound can afford high first-pass tissue extraction of radiotracer following intravenous administration (John and Green, 1989; Green, et al., 2007). Upon tissue uptake, the radiocopper is trapped by intracellular reductive decomposition of the copper(II) bis(thiosemicarbazone) chelate, for example by reaction with ubiquitous intracellular thiols like glutathione, liberating the 62Cu ion to the endogenous copper pool of the cell (Petering, 1980; John and Green, 1990; Shelton, et al., 1989, 1990; Mathias, et al., 1990; Baerga, Michael, and Green, 1992; Green, et al., 2007). This prolonged ‘microsphere-like’ tissue retention of 62Cu, together with its 10-minute physical half-life, allows use of extended image acquisition periods for improved counting statistics, as well as implementation of whole-body acquisition protocols similar to those employed with [18F]-FDG.
Figure 1.
Synthesis and chemical structure of the [62Cu]Cu-ETS radiopharmaceutical.
Practical clinical use of the 62Cu requires a rapid and robust radiopharmaceutical synthesis method for routine on-demand radiopharmaceutical delivery. The present study reports the performance of a commercially manufactured 62Zn/62Cu microgenerator system (Lacy, Stephens, and Yue, 2006; Stephens, et al., 2008), and a lyophilized H2ETS kit formulated for point-of-use [62Cu]Cu-ETS synthesis, in the delivery of [62Cu]Cu-ETS for whole-body PET perfusion imaging in patients with advanced renal carcinoma and head and neck cancer.
2 Materials and Methods
2.1 Preparation of the generator
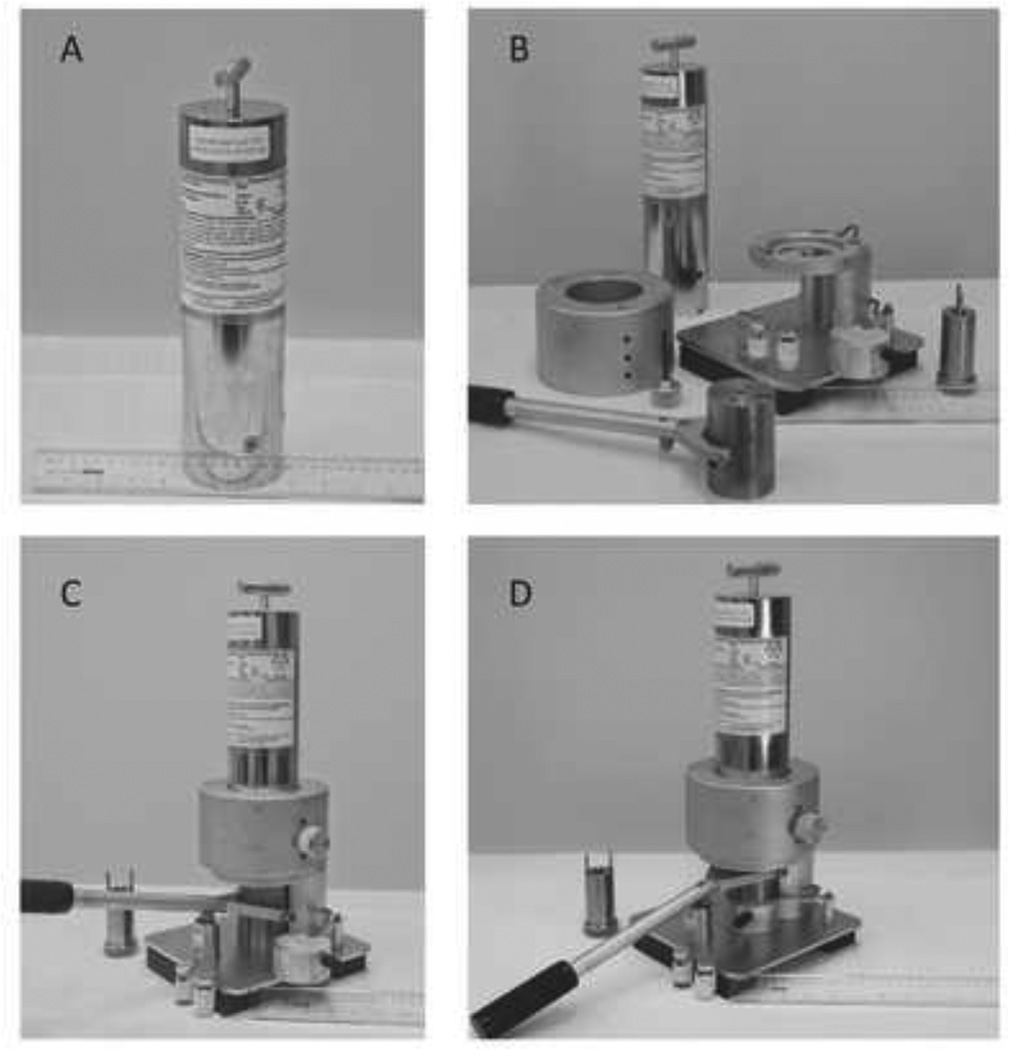
All 62Zn/62Cu microgenerators were manufactured by Proportional Technologies, Inc., (PTI, Houston, Texas) and shipped to the Melvin and Bren Simon Cancer Center PET/CT at Indiana University. The generators were calibrated for 9:00 AM on the day of clinical use. The microgenerator houses a 50-µL AGl×8 (200–400 mesh) anion exchange resin connected to a pressurized eluent vessel containing 0.2M HCL and 1.8M NaCl elution buffer. The tungsten-shielded generator-column is contained within a cylindrical stainless steel outer housing. Upon arrival, the generator unit was removed from the shipping packaging, and installed on the elution stand (Figure 2) as described in PTI’s IND documentation and Investigator Brochure. While PTI has an electronic control box that can automatically open the generator’s elution valve for the standard 30-second elution, for the studies described we instead elected to simply manually open and close that single-rotation valve using a stopwatch timer to track the elution period.
Figure 2.
The 62Zn/62Cu microgenerator produced by Proportional Technologies, Inc. (Houston). A: Generator unit as delivered to the clinical site via overnight shipment. B: Generator and disassembled stand and shielding. C: Assembled generator stand and shielded elution vial. The fulcrum on the base of the stand allows the shielded vial to be raised into the generator housing, whereupon the generator outlet needle punctures the septum of the vial. D: Generator with shielded vial positioned for elution. In the background one can also see the tungsten shield for the 5-mL syringe used to withdraw the patient dose from the shielded product vial. This syringe shield is designed to securely mate with guide holes in the vial shield, thereby controlling the position and depth of needle penetration into the vial septum, and allowing nearly quantitative dose withdrawal with limited radiation exposure to the hands of the operator.
2.2 Generator elution
An initial 90-second flushing elution was always performed before any patient dose was prepared. For this flushing elution, after venting to atmospheric pressure a 5.0 mL empty sterile elution vial was placed in the vial shield, positioned underneath the generator, and raised into position for elution (Figure 2D). A total volume of approximately 0.75 mL is eluted into the vial during this 90-second period. Eluted radioactivity was measured using a dose calibrator (Atomlab 100 Medical System, calibration setting of 9.5). After the assay, the vial was stored for later measurement of 62Zn breakthrough after complete decay of the initial 62Cu.
For subsequent radiopharmaceutical preparations, a standard 30-second elution was performed to deliver 62Cu2+ in a volume of 0.25 mL directly into the reconstituted reaction vials. If the elapsed time between two consecutive elution was more than one hour, a 30-second flushing elution was carried out 30–40 minutes before the planned dose delivery, as elution yields were observed to drop and become more variable when more than one hour elapsed between elutions.
The 62Cu elution efficiency was determined by comparison of the recovered 62Cu radioactivity (decay corrected to time-of-elution) and the calculated 62Cu available at time-of-elution (Finn, et al., 1983; IAEA, 2010). 62Zn breakthrough levels were determined after the eluted 62Cu radioactivity had decayed (>20 hours post-elution), measuring the residual 62Zn/62Cu radioactivity using a Nal(TI) well counter with a counting window centered at 511 keV. The measured count rates were converted to dpm based on the measured efficiency of the gamma counter for 511 keV photons in the counting window, and decay-corrected to 62Zn breakthrough at time-of-elution.
2.3 [62Cu]Cu-ETS radiopharmaceutical synthesis
The sterile and pyrogen-free supplies and reagents used in the production of [62Cu]Cu-ETS were supplied by PTI unless otherwise stated. For each synthesis, a lyophilized vial of H2ETS ligand (10-mL vial, 2 µg H2ETS, 20 mg sucrose as excipient) was aseptically reconstituted with 3.75 mL 25mM NaOAc sterile solution (pH 6.5). The vial was then mixed with gentle swirling until all of the lyophilized cake was dissolved, yielding within seconds a clear and colorless solution with no visible particles. Then, the reconstituted ligand vial was placed inside the vial shield, and positioned for generator elution.
A volume of approximately 0.25 mL 62Cu2+ in the acidic eluate was delivered directly into the ligand vial over a 30-second elution period. The reaction vial was removed from the generator stand, and mixed thoroughly (30 seconds) in order to ensure homogeneous mixing and completion of the chelation process between the 62Cu2+ and H2ETS ligand. Each preparation of the day was considered a sub-batch, with the first daily sub-batch employed solely for a required radiochemical purity assay.
For each patient administration, a new [62Cu]Cu-ETS synthesis was carried out. The synthesis was initiated with generator elution approximately 5-minutes before the intended injection. Immediately after the synthesis, all 4-mL of injectable solution was directly withdrawn from the shielded reaction vial into a shielded 5-mL sterile syringe through a BD TwinPak device. Finally, the product was filtered through a sterile 0.22-µm PVDF membrane filter (Whatman Puradisc™ 13 mm) into another sterile 5-mL BD syringe (BD, Franklin Lakes NJ, USA). Although the radiopharmaceutical synthesis uses sterile and pyrogen-free reagents, and the synthesis process was carried out with aseptic technique in a closed system, the final terminal filtration was performed as a redundant process for ensuring product sterility. (This terminal filtration was not the primary control for product sterility.)
Immediately after the filtration process was completed, the radioactivity in the syringe was measured using a dose calibrator, the time of assay recorded, and the dosage was delivered for patient administration. The time of the patient injection was recorded, followed by measurement of residual radioactivity in the injection syringe, and the injected radioactivity calculated.
2.4 Radiochemical purity assay
Standard assay: Oasis HLB cartridge method
An Oasis HLB (hydrophilic lipophilic balance) cartridge (Waters, USA) was connected to a 3.0 mL receiving syringe using a clip assembly, and the entire set-up was mounted onto a mini lab jack. Before use, the Oasis HLB column was pre-conditioned with 1.0 mL of methanol (Mallinckrodt Chemicals, USA), followed by 2.0 mL NaOAc buffer (25 mM, pH 4.7). The waste was collected in a 3.0-mL syringe at the outlet of the cartridge and was discarded, leaving the column bed wetted with buffer.
Immediately after the [62Cu]Cu-ETS synthesis, a 0.1 mL sample of the quality control sub-lot was withdrawn from the vial with a 1.0 mL syringe and diluted with 0.9 mL of NaOAc buffer. The sample was mixed thoroughly, and loaded onto the prepared Oasis HLB column. The column was eluted by drawing 1.0 mL NaOAc buffer through the column, collecting the eluate in a 3.0 mL receiving syringe. The radioactivity of the aqueous HLB column eluate, and remaining on the HLB column was measured, and the times of measurements were recorded. Total time for the entire procedure was also recorded. [62Cu]Cu-ETS radiochemical purity was calculated using the decay-corrected radioactivity measurements for the aqueous eluate and the HLB column as follows:
Alternative assay: C18 Sep-Pak® cartridge method
The C18 Sep-Pak® method was carried out in tandem with the standard Oasis method. In the Sep-Pak® method, a single-use solid phase extraction (SPE) C18 SepPak Light Cartridge (Waters, Milford, MA, USA) was pre-hydrated and pre-conditioned manually with 5.0 mL of absolute ethanol followed by 10.0 mL of deionized water. A volume of 0.05 mL [62Cu]Cu-ETS product solution was withdrawn from the quality control sub-lot reaction vial, and diluted with 0.95 mL deionized water. The sample was mixed thoroughly and then loaded into the SPE column. The loading eluate was collected into a test tube (“A”). Next, the C18 Sep-Pak® Light was eluted with 10.0 mL of water, followed by 10.0 mL of air flush. This aqueous eluate was also collected in tube “A”, and contains ionic impurities (e.g. 62Cu2+ and 62Zn2+). In order to recover the lipophilic [62Cu]Cu-ETS complex, the Sep-Pak® was then eluted with 1.0 mL ethanol, followed by 5.0 mL of air flush. The ethanol fraction was collected in tube “B”. The ‘dry’ cartridge was placed in test tube “C”. Finally, the radioactivity of samples A, B, and C was determined using a radionuclide dose calibrator, and the radiochemical purity of the [62Cu]Cu-ETS was calculated as:
3 Results
3.1 Performance of the 62Zn/62Cu microgenerator
All 42 generators arrived as scheduled, but five were not used to prepare the patient doses because of last-minute patient cancellations, or local cyclotron problems that prevented delivery of the [15O]-water required by the imaging protocol as a reference tracer for tumor perfusion assessment. The average 62Zn at 9:00 AM Eastern Time on the day of clinical use was 1.84 ± 0.23 GBq (49.8 ± 6.2 mCi, range: 1.67 to 2.04 GBq or 45 to 55 mCi). These results include two generators loaded with relatively low 62Zn activity (24 and 30 mCi), due to manufacturing problems. If these two generators are excluded from the calculations, the average activity of 62Zn at calibration time was 1.88 ± 0.13 GBq (50.9 ± 3.43 mCi). The entire generator set-up process, from receiving the generator shipment to delivery of the first patient dose, took approximately 1.5 hours.
3.2 Evaluation of 62Cu yield
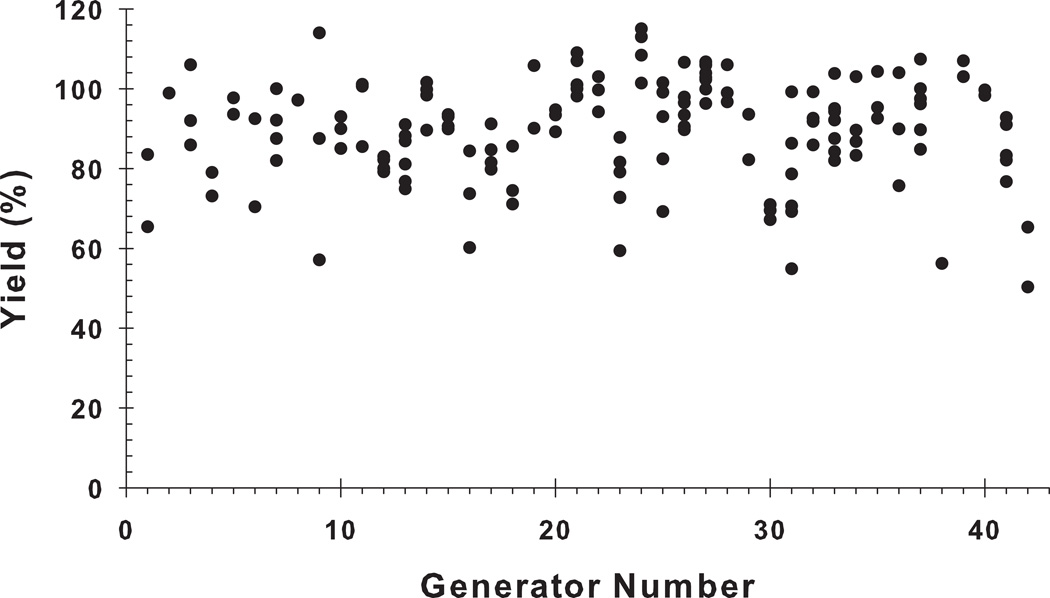
The generator elution yield was calculated based on the elutions made in the clinical study day (Day 1; 30-second elution period; elapsed time between two successive elutions of <1 hour). The generators were not used for patient doses on Day 2, due to the reduced level of available 62Cu, but remained useful on Day 2 for other radiochemistry studies. The 62Cu elution yield of all generators in Day-1 is shown in Figure 3. The results show that all of the generators exhibited a high elution yield that ranged from 60% to 100% with an overall average of 89.9 ± 12.9%.
Figure 3.
The elution yields of 62Cu from 42 generators on the day of receipt (overall mean: 89.9 ± 12.9%; 30-second elution period, elapsed time from prior elution < 1 hour).
We occasionally observed elution yields calculated to exceed 100%. This could reflect the calculated available level of 62Cu neglecting in-growth of 62Cu during the 30-second elution process itself, as well as 62Cu radioactivity partially remaining (and recovered) from the previous elution if the successive elution were carried out at short time intervals. Additionally, the loaded 62Zn radioactivity may slightly exceed the amount shown on the package label, reflecting within-limits variations in the accuracy of the dose calibrators employed at the manufacturing and clinical sites.
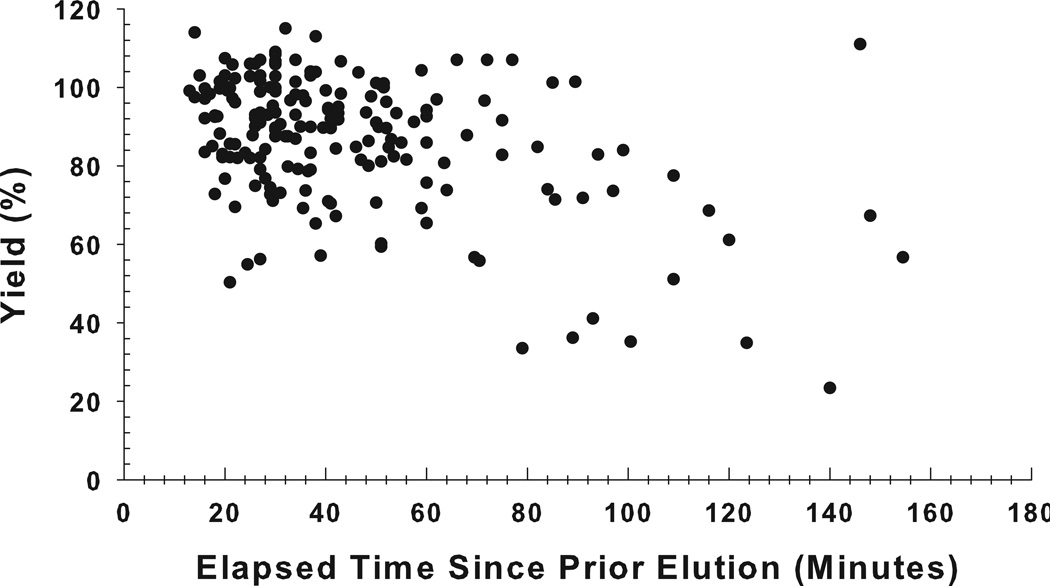
Figure 4 shows the generator elution yields plotted as a function of the time period between elutions, with intervals varying from 10 minutes to more than 1.5 hours. The results show that within the 10-to-60 minute intervals, the yield was high, consistent, and reproducible, averaging 90% (n = 152). However, when the elapsed time from the preceding elution exceeded 1 hour, the yield decreased (73.4 ± 24.3%; n = 34) and became more variable.
Figure 4.
The 62Cu elution yield on the day of generator receipt plotted as a function of the elapsed time since the preceding elution for 42 generators. The elution yield was observed to decline, and become more variable, when the elapsed time between elutions was greater than 60 minutes.
This variation in elution yield with the period between elutions was never observed on Day 2, when the radioactivity level on the column was over 4-fold lower than on Day 1. Because the observed declines in yield were clearly reversible in subsequent elutions (yield was readily recovered by a 30-second flushing elution 30–40 minutes prior to the next elution), there does not appear to be channeling damage to the column. Instead, we suspect that the altered yield reflects a reversible physical change, such as formation of gas bubbles in the column bed, due to the high Day-1 concentration of radioactivity on the column. The high elution efficiency observed when controlling the period between elutions assured that even at 5:00 pm on Day 1 the generator could deliver levels of 62Cu sufficient for patient studies.
3.3 Evaluation of 62Zn breakthrough
Table 1 summarizes the 62Zn breakthrough levels found in the first elution (90-second flushing elution, 0.75 mL), and the average value of breakthrough in the subsequent 30-second elutions (0.25 mL/elution; n ≥ 3) for a subset of 20 of the generators used. Overall, the eluted 62Zn constituted less than 0.001% of the total eluted 62Cu activity. All the generators had breakthrough levels that were less than 0.1 µCi of 62Zn, with values ranging as low as 0.0001 µCi.
Table 1.
Levels of 62Zn-breakthrough in the initial 90-second (0.75-mL) flushing elution, and subsequent 30-second (0.25-mL) elutions. Values shown are decay-corrected to time-of-elution.
| Generator I.D. |
62Zn Breakthrough in Initial 90-sec Elution (µCi) |
62Zn Breakthrough in Subseque 30-sec Elutions (µCi) (Mean ± SD; n ≥ 3) |
|---|---|---|
| Z260 | 0.01 | 0.0006 ± 0.0004 |
| Z261 | 0.002 | 0.003 ± 0.005 |
| Z262 | 0.01 | 0.009 ± 0.0007 |
| Z263 | 0.0002 | 0.0001 ± 0.00006 |
| Z264 | 0.0006 | 0.00025 ± 0.0002 |
| Z266 | 0.0075 | 0.006 ± 0.0005 |
| Z267 | 0.006 | 0.01 ± 0.006 |
| Z268 | 0.003 | 0.002 ± 0.0009 |
| Z269 | 0.0039 | 0.03 ± 0.02 |
| Z270 | 0.0002 | 0.0001 ± 0.00007 |
| Z271 | 0.01 | 0.04 ± 0.03 |
| Z273 | 0.09 | 0.05 ± 0.006 |
| Z274 | 0.0076 | 0.012 ± 0.008 |
| Z275 | 0.004 | 0.034 ± 0.03 |
| Z276 | 0.0002 | 0.0006 ± 0.0005 |
| Z280 | 0 | 0 |
| Z281 | 0.003 | 0.001 ± 0.001 |
| Z283 | 0.002 | 0.002 ± 0.001 |
| Z286 | 0 | 0 |
| Z393 | 0 | 0 |
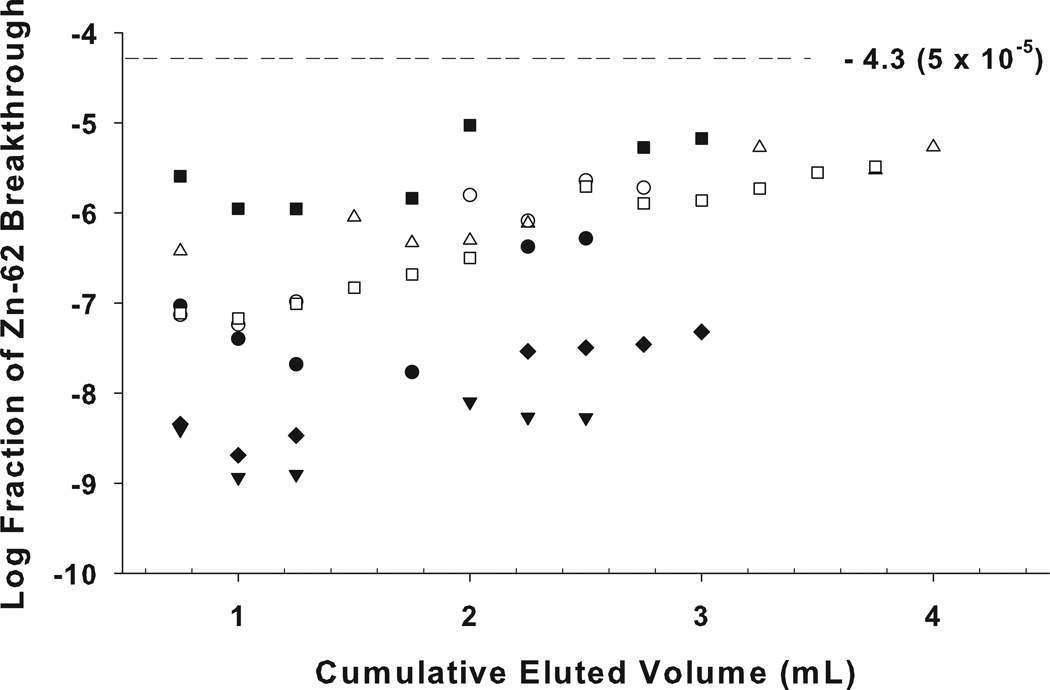
The fractional breakthrough of the 62Zn was determined as the eluted 62Zn relative to the total 62Zn activity present on the column at the time-of-elution. The chloride concentration, elution flow rate, elution volume, and column resin bed size are all fixed, but one factor that could contribute to variations in parent breakthrough is the number of prior elutions (reflecting the total cumulative eluent volume passed through the column). Since even for strongly absorbed solutes there is always a bound ⇆ free equilibrium, the bound 62Zn nuclide could migrate from the top of the generator column to the lower part of the column with high elution volumes, potentially increasing 62Zn leakage in later elutions. However, examination of the pattern of breakthrough in a subset of eight generators (Figure 5), provides no indication of any significant bulk migration of 62Zn through the column bed, even at the highest elution volumes one could encounter in clinical generator use.
Figure 5.
62Zn breakthrough (62Zn breakthrough/total 62Zn at time-of-elution) from a subset of eight 62Zn/62Cu generators studied, each with total cumulative eluted volume of ≥ 2.5 mL The fraction of leakage remained < 5× 10−5 for up to a 3.5 mL cumulative elution volume (10–12 elutions).
These studies confirm that the radionuclidic purity of the delivered 62Cu was more than 99.99% throughout the shelf-life of these generators. The extremely low levels of 62Zn contaminant in the 62Cu-radiopharmaceutical product will make a negligible contribution to total patient radiation exposure. For example, even a 1.0 µCi 62Zn contaminant in the 62Cu product will contribute only a 0.16 mGy (16 mRad) exposure to the liver (the critical organ for 62Zn), based on published dosimetry, with a corresponding effective dose equivalent of only 0.018 mSv (1.8 mRem) (ICRP, 1988).
3.4 Performance of the kit-based method for [62Cu]Cu-ETS synthesis
The [62Cu]Cu-ETS synthesis protocol is relatively simple, straightforward, and easy to perform. Over a period of three years, using 37 generators, a total of 45 doses of the Cu-ETS were successfully prepared for use in PET perfusion imaging at Indiana University Hospital. Every batch passed the radiochemical purity test standard (>95%), with an average radiochemical purity of 98.1 ± 1.1% (n=40) using standard Oasis-based assay method. Equivalent radiochemical purity values were obtained using the alternative C18 Sep-Pak® method, where values averaged 97.8 ± 0.9% (n=28). The synthesis process was rapid and robust, taking approximately 3.5 minutes to complete, resulting in an average elapsed time of 4.5 ± 0.9 minutes (n = 45) from start-of-generator-elution to patient-injection. The mean injected dose was 18.2 ± 4.6 mCi (672 ± 172 MBq; n=45).
4 Discussion
Generator-produced positron-emitting radioisotopes provide a practical route to performance of PET with short-lived radionuclides, even in the absence of a nearby cyclotron. The results of the present study confirm the commercially produced 62Zn/62Cu microgenerator to be a reliable source for on-site production of 62Cu-radiopharmaceuticals. The generator set-up is easy and rapid, even in a space-limited PET hot lab. The generator module does not require a hot cell, as the integrated shielding is sufficient to protect the operator during generator elution and radiopharmaceutical synthesis. The elution process efficiently recovers nearly all of the available 62Cu from the column in a very small volume (0.25 mL) of dilute HCI solution. This small elution volume facilitates in situ adjustment of the final product’s pH and isotonicity.
The kit-based [62Cu]Cu-ETS synthesis procedure, from the beginning of the generator elution to the patient injection, involves only few simple steps: (1) a 30-second ligand reconstitution in the kit vial; (2) a 30-second generator elution of 62Cu; (3) 30 seconds of mixing; (4) 0.5 to 1 minutes for the 0.2-µm membrane filtration process; and finally, (5) radiopharmaceutical injection. The 62Cu-generator eluate is delivered directly into the kit vial, where it is buffered by sodium acetate and allowed to react with the bis(thiosemicarbazone) chelating ligand (Figure 1). The resulting 4.0-mL isotonic product solution (pH 4.5–5) is suitable for direct intravenous administration. This simple procedure minimizes the time needed for radiopharmaceutical synthesis, and thus minimizes pre-injection radionuclide loss due to decay. We did not encounter a single failure in [62Cu]Cu-ETS dose delivery over the three-year study period. This simple “elute and mix” synthesis protocol can be conveniently implemented in any clinical facility, with the lyophilized kit formulation enabling the robust and reliable on-demand production of Cu-labeled radiopharmaceuticals.
The sole disadvantage of the 62Zn/62Cu generator is the somewhat short half-life of the cyclotron-produced 62Zn parent (9.2 hours), as it limits the generator to a one to two day shelf-life. This limitation is somewhat offset by the capability of the generator to produce radiopharmaceutical doses at 30–40 minute intervals throughout the day of use. In addition to the present example, where we focused only on delivery of [62Cu]Cu-ETS for perfusion imaging, the kit-based methodology can also be applied to [62Cu]Cu-PTSM or [62Cu]Cu-ETSM for cerebral perfusion imaging, [62Cu]Cu-ATSM for tumor hypoxia imaging, and 62Cu-labeling of peptide-chelate conjugates (John and Green, 1989; Mathias, et al., 1990; Fujibayashi, et al., 1997; Lewis, et al., 1999; Castle, et al., 2003; Vavere and Lewis, 2007; Mathias, et al., 2013).
The production process for the 62Zn/62Cu microgenerator is fully scalable in response to clinical demand. The 62Zn can be readily produced in very large quantities via the 63Cu(p,2n)62Zn nuclear reaction using a medium energy cyclotron. A GMP-compliant cyclotron facility could centrally produce 62Zn/62Cu generators for nationwide distribution relying on standard overnight delivery services.
The manufacturer’s Oasis-column method, and the alternative C18 Sep-Pak® method, were equally effective as approaches to verifying product radiochemical purity. However, we preferred the C18 Sep-Pak® method, due to its speed and simplicity (notably requiring less operator care than the Oasis method in conditioning the stationary phase, since one does not need to visually monitor the solvent being drawn down to the top of the stationary phase bed).
5 Conclusions
The 62Zn/62Cu microgenerator has served as a reliable source for “on-demand” delivery of short-lived positron-emitting 62Cu at a clinical site. The generator consistently provided high 62Cu elution yields, coupled with extremely low 62Zn breakthrough. The kit-based [62Cu]Cu-ETS synthesis method has proven to be convenient, rapid, reliable, and robust. Analysis and validation of the performance of [62Cu]Cu-ETS in whole-body tumor perfusion imaging is in progress (Fletcher, et al., 2014).
Highlights.
The microgenerator system is a dependable high-yield source of positron-emitting 62Cu.
Synthesis of the [62Cu]Cu-ETS radiopharmaceutical is rapid and reliable.
Kit-based production methods offer the convenience required for clinical PET with 62Cu-agents.
Acknowledgements
This work was supported by the National Cancer Institute of the National Institutes of Health under award number R01-CA140299. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. In addition, Yen Ng would like to thank The Public Service Department of Malaysia for her Ph.D fellowship funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baerga ID, Maickel RP, Green MA. Subcellular-distribution of tissue radiocopper following intravenous administration of Cu-67 labeled Cu-PTSM. Nucl Med Biol. 1992;19(6):697–701. doi: 10.1016/0883-2897(92)90104-7. [DOI] [PubMed] [Google Scholar]
- Basken NE, Green MA. Cu(II) bis(thiosemicarbazone) radiopharmaceutical binding to serum albumin: Further definition of species dependence and associated substituent effects. Nucl Med Biol. 2009;36(5):495–504. doi: 10.1016/j.nucmedbio.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle TC, Maurer RI, Sowrey FE, Went MJ, Reynolds CA, McInnes EJ, et al. Hypoxia-targeting copper bis(selenosemicarbazone) complexes: Comparison with their sulfur analogues. J Am Chem Soc. 2003;125(33):10040–10049. doi: 10.1021/ja035737d. [DOI] [PubMed] [Google Scholar]
- Finn RD, Molinski VJ, Hupf HB, Kramer H. Technical Information Center. Springfield, VA: U.S. Department of Energy; 1983. Radionuclide generators for biomedical applications (NAS-NS-3202) [Google Scholar]
- Fletcher JW, Eitel JA, Logan TF, et al. Whole-body PET/CT evaluation of tumor perfusion using generator-based [62Cu]Cu ETS: Validation by direct comparison to [15O]-water. J Nucl Med. 2014 doi: 10.2967/jnumed.114.148106. Meeting Abstracts, May 2014 (SNM annual meeting, St. Louis, June, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujibayashi Y, Matsumoto K, Yonekura Y, Konishi J, Yokoyama A. A new zinc-62/copper-62 generator as a copper-62 source for PET radiopharmaceuticals. J Nucl Med. 1989;30(11):1838–1842. [PubMed] [Google Scholar]
- Fujibayashi Y, Taniuchi H, Yonekura Y, Ohtani H, Konishi J, Yokoyama A. Copper-62-ATSM: A new hypoxia imaging agent with high membrane permeability and low redox potential. J Nucl Med. 1997;38(7):1155–1160. [PubMed] [Google Scholar]
- Green MA, Mathias CJ, Welch MJ, et al. [62Cu]Cu-labeled pyruvaldehyde bis(N4 - methylthiosemicarbazonato)copper(II): Synthesis and evaluation as a positron emission tomography tracer for cerebral and myocardial perfusion. J. Nucl. Med. 1990;31:1989–1996. [PubMed] [Google Scholar]
- Green MA, Mathias CJ, Willis LR, Handa RK, Lacy JL, Miller MA, et al. Assessment of Cu-ETS as a PET radiopharmaceutical for evaluation of regional renal perfusion. Nucl Med Biol . 2007;34(3):247–255. doi: 10.1016/j.nucmedbio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Haynes NG, Lacy JL, Nayak N, Martin CS, Dai D, Mathias CJ, Green MA. Performance of a 62Zn/62Cu generator in clinical trials of PET perfusion agent 62Cu-PTSM. J Nucl Med . 2000;41(2):309–314. [PubMed] [Google Scholar]
- IAEA. IAEA Radioisotopes and Radiopharmaceuticals, Series No. 2. Vienna: IAEA; 2010. Production of long-lived parent radionuclides for generators: Ga-68, Sr-82, Sr-90 and W-188. [Google Scholar]
- ICRP. Oxford: Pergamon Press; 1988. Radiation dose to patients from radiopharmaceuticals, a report of a task group of Committee 2 of the International Commission on Radiological Protection (ICRP Publication 53) [PubMed] [Google Scholar]
- John EK, Green MA. Structure-activity relationship for metal-labeled blood flow tracers: comparison of keto aldehyde bis(thiosemicarbazonato)copper(II) derivatives. J. Med. Chem. 1989;33:1765–1770. doi: 10.1021/jm00168a035. [DOI] [PubMed] [Google Scholar]
- Kraus KA, Moore GE. Anion exchange studies. VI: The divalent transition elements manganese to zinc in hydrochloric acid. J. Am. Chem. Soc. 1952;75:1460–1462. [Google Scholar]
- Lacy J, Stephen A, Yue Z. 62Zn/62Cu microgenerator for interchangeable kit-based synthesis of bis(thiosemicarbazone)62Cu radiopharmaceuticals. J Nucl Med. 2006;47(Supp 1):162p. [Google Scholar]
- Lacy JL, Guerrero L, Chiu R, Sun L, Hall L, Stone CK. PET imaging with 62Cu-ETS in a human clinical trial at the University of Wisconsin-Madison; IEEE Nuclear Science Symposium Conference 2007; 2007. pp. 4027–4030. [Google Scholar]
- Lacy JL, Guerrero L, Christian B, Stine C. Renal perfusion with 62Cu-ETS in comparison with 15O-water PET. J Nucl Med. 2010;51(Supp 2):587p. [Google Scholar]
- Lewis JS, McCarthy DW, McCarthy TJ, Fujibayashi Y, Welch MJ. Evaluation of Cu-64-ATSM in vitro and in vivo in a hypoxic tumor model. J Nucl Med. 1999;40(1):177–183. [PubMed] [Google Scholar]
- Mathias CJ, Ng Y, Lacy J, Green MA. Rapid synthesis of 62Cu-DOTA-NOC and 62Cu-NOTA-NOC as a potential short-lived generator-based PET tracers for somatostatin receptor imaging. J Nucl Med. 2013;54(Supp 1):1140p. [Google Scholar]
- Mathias CJ, Welch MJ, Raichle ME, Mintun MA, Lich LL, Mcguire AH, et al. Evaluation of a potential generator-produced PET tracer for cerebral perfusion imaging - single pass cerebral extraction measurements and imaging with radiolabeled Cu-PTSM. J Nucl Med . 1990;31(3):351–359. [PubMed] [Google Scholar]
- Petering DH. Carcinostic copper complexes. New York: Marcel Dekker; 1980. [Google Scholar]
- Robinson GD, Zielinski FW, Lee AW. The zinc-62/copper-62 generator: A convenient source of copper-62 for radiopharmaceuticals. Int J Appl Radiat Isot . 1980;31(2):111–116. doi: 10.1016/0020-708x(80)90053-8. [DOI] [PubMed] [Google Scholar]
- Shelton ME, Green MA, Mathias CJ, Welch MJ, Bergmann SR. Kinetics of copper-PTSM in isolated hearts - a novel tracer for measuring blood-flow with positron emission tomography. J Nucl Med. 1989;30(11):1843–1847. [PubMed] [Google Scholar]
- Shelton ME, Green MA, Mathias CJ, Welch MJ, Bergmann SR. Assessment of regional myocardial and renal blood-flow with copper-PTSM and positron emission tomography. Circulation. 1990;82(3):990–997. doi: 10.1161/01.cir.82.3.990. [DOI] [PubMed] [Google Scholar]
- Stephens A, Yue ZW, Bui H, Thrash T, Martin C, Lacy J. Automation and improved performance of a 62Zn/62Cu microgenerator system. J Nucl Med. 2008;49(Supp 1):95p. [Google Scholar]
- Vavere AL, Lewis JS. Cu-ATSM: A radiopharmaceutical for the PET imaging of hypoxia. Dalton Trans. 2007;43:4893–4902. doi: 10.1039/b705989b. [DOI] [PubMed] [Google Scholar]
- Zweit J, Goodall R, Cox M, Babich JW, Potter GA, Sharma HL, et al. Development of a high-performance zinc-62/copper-62 radionuclide generator for positron emission tomography. Eur J Nucl Med. 1992;19(6):418–425. doi: 10.1007/BF00177368. [DOI] [PubMed] [Google Scholar]