Significance
Highly mobile staphylococcal pathogenicity islands (SaPIs) are the only source of toxic shock toxin and certain other superantigens, especially enterotoxin B. To promote their survival and spread, the SaPIs parasitize and interfere with certain bacteriophages. Unlike the interference of the clustered regularly interspaced short palindromic repeats (CRISPRs), the interference of SaPIs is never complete, allowing horizontal gene transfer and adaptation. We report a novel SaPI-determined interference mechanism that targets a phage gene essential for both phage and SaPI. Because SaPI is not self-destructive, it must modulate this inhibition to ensure production of its own infectious particles, as well as those of the phage, and it does so by means of a novel SaPI protein that binds to the inhibitor.
Keywords: helper phage, transcription regulation, bacteriophage resistance
Abstract
Having gone to great evolutionary lengths to develop resistance to bacteriophages, bacteria have come up with resistance mechanisms directed at every aspect of the bacteriophage life cycle. Most genes involved in phage resistance are carried by plasmids and other mobile genetic elements, including bacteriophages and their relatives. A very special case of phage resistance is exhibited by the highly mobile phage satellites, staphylococcal pathogenicity islands (SaPIs), which carry and disseminate superantigen and other virulence genes. Unlike the usual phage-resistance mechanisms, the SaPI-encoded interference mechanisms are carefully crafted to ensure that a phage-infected, SaPI-containing cell will lyse, releasing the requisite crop of SaPI particles as well as a greatly diminished crop of phage particles. Previously described SaPI interference genes target phage functions that are not required for SaPI particle production and release. Here we describe a SaPI-mediated interference system that affects expression of late phage gene transcription and consequently is required for SaPI and phage. Although when cloned separately, a single SaPI gene totally blocks phage production, its activity in situ is modulated accurately by a second gene, achieving the required level of interference. The advantage for the host bacteria is that the SaPIs curb excessive phage growth while enhancing their gene transfer activity. This activity is in contrast to that of the clustered regularly interspaced short palindromic repeats (CRISPRs), which totally block phage growth at the cost of phage-mediated gene transfer. In staphylococci the SaPI strategy seems to have prevailed during evolution: The great majority of Staphylococcus aureus strains carry one or more SaPIs, whereas CRISPRs are extremely rare.
The staphylococcal pathogenicity islands (SaPIs) are prototypical members of a increasingly recognized family of highly mobile ∼15-kb phage-inducible chromosomal islands, which were discovered serendipitously because of their carriage of the gene for toxic shock syndrome toxin-1, tst, of which the SaPIs are the only source (1). The SaPIs are intimately related to certain helper phages whose life cycles they parasitize to enable their propagation and spread.
SaPIs exist quiescently at specific chromosomal sites under the control of a master repressor. A defining connection between the helper phage and the SaPI lifestyle is induction by helper phage-encoded antirepressor proteins (2). These proteins lift the repression, setting in motion the SaPI life cycle: excision, replication, and encapsidation of SaPI DNA into infectious phage-like particles. The resulting SaPI transfer frequencies approach the pfu titer of the helper phage (3). A key SaPI feature is that the SaPIs encode homologs of the phage terminase small subunit, referred to herein as “TerSS,” that recognize a specific SaPI packaging (pac) site to initiate packaging into proheads composed of helper phage virion proteins (4, 5). The phage and the SaPI TerS do not cross-react so that packaging is DNA-specific (6).
The SaPI life cycle is initiated only when the life cycle of a helper phage is in progress; SaPI particle production, however, is not quite able to keep up with helper phage maturation rate, and, perhaps to gain an advantage, SaPIs interfere with the reproduction of their helper phages (7). Thus far, two distinctly different SaPI-coded interference mechanisms have been identified, namely the capsid morphogenesis genes cpmA and B, which cause the formation of small capsids commensurate with the SaPI genome, and the phage packaging inhibition gene ppi, which blocks phage terminase small subunit (TerSP), favoring the packaging of SaPI DNA. ppi is present in all of the ∼50 SaPI genomes analyzed thus far; cpmAB is present in most, but not all. Both are encoded by and are functional in three prototypical SaPIs, SaPI1, SaPI2, and SaPIbov1.
In studies of SaPI interference with helper phage 80 (of the international typing set, a member of the famous 80/81 pair) (8), which is related to but is distinctly different from the more extensively studied 80α, we observed that SaPI2, the major cause of menstrual toxic shock, inhibits phage 80 reproduction more stringently than do other SaPIs, and preliminary results suggested that SaPI2 uses a third interference mechanism (7). In this report we characterize this third interference mechanism used by SaPI2 against phage 80 and other related phages but not against phage 80α.
Results
Identification of the SaPI2-Coded Inhibitor(s) of Phage 80.
To determine whether SaPI2 uses the same interference mechanisms against phage 80 as it does against phage 80α, we cloned the cpmAB and ppi loci of SaPI2 separately to the vector pCN51 (9), behind the exogenous cadmium-inducible promoter (Pcad) (10) and tested these clones for inhibition of phage 80. Although either of these clones completely blocked plaque formation by phage 80α (7), we were surprised to find that neither, even when induced, had any effect on plaque formation by phage 80 (Fig. 1A). To confirm this effect in situ, we deleted both ppi and cpmAB from SaPI2 and found that the interference was unaffected. This result showed that there must be a third interference mechanism; to identify it, we cloned each of the SaPI2 ORFs separately, using the same vector, and found that one of the ORFs, 17, caused interference. ORF17, when cloned, blocked plaque formation by phage 80 (Fig. 1A) (7). One other ORF, ORF13, was toxic to the host cells and could not be expressed adequately from a plasmid. It was found to cause interference on the basis of a deletion (see below).
Fig. 1.
(A) Phage interference mediated by cloned SaPI2 genes. The indicated genes were cloned to plasmid pCN51 under a cadmium-inducible promoter (Pcad). Strain RN4220 containing the indicated plasmids was infected with phages 80α (∼150 pfu per plate) or 80 (∼250 pfu per plate) plated on phage bottom agar containing 1.0 μM CdCl2 and incubated for 48 h at 32 °C. Plates were stained with 0.1% TTC in TSB and photographed. (B) Map of SaPI2 operon 1. Genes previously shown to cause phage interference are shown in red; hypothetical genes of unknown function are shown in blue; numbered ORFs newly determined to be involved in interference are shown in orange; and the SaPI2 small terminase subunit is shown in green.
ORF13 and -17 are located in operon 1, which includes cpmA and B, ORF16, and terSS. ppi is upstream of the operon and is equivalent to ORF10 (Fig. 1B). No other SaPI2 gene had any detectable inhibitory effect on phage 80.
ORF17-Mediated Interference.
We began with ORF17 because it could be overexpressed. To identify the phage gene(s) targeted by ORF17, we isolated ORF17-resistant mutants as rare plaques on a strain containing the cloned and overexpressed ORF17. Thirteen of the mutant phages were sequenced. All 13 had point mutations in a single gene, 32, and all had amino acid replacements at a single site in gp32: tyrosine 67 was replaced by histidine in 11 mutants and by cysteine in two mutants (Fig. S1).
The sequence of gene 32 showed it to be late transcriptional regulator C, ltrC (11, 12), which corresponds to 80α rinA (13). LtrC and RinA are regulatory proteins that activate the transcription of the late phage operon (morphogenetic and lysis genes) (Fig. 2A). Five families of these proteins have been described, constituting a superfamily of late transcriptional regulators (Ltr) (11, 12). These are essential phage genes (14, 15) corresponding to coliphage lambda gene Q. To test phage 80 LtrC for its role in transcriptional activation, we needed to delete the gene. Because the gene is presumed to be essential for phage reproduction, this deletion would have to be done with the prophage. This deletion could not be done with phage 80, which cannot form a lysogen (13), so we turned to a close relative, phage 52A, which can form lysogens, can induce SaPI2, and whose LtrC and late gene operon are identical to those of phage 80. Using a phage 52A lysogen, we introduced an in-frame deletion in ltrC and found that the phage, as predicted, was unable to lyse the cell or to produce any infectious phage particles. We next cloned the β-lactamase reporter downstream of the late phage promoter in plasmid pCN41 in RN450 with either the WT or ltrC mutant 52A prophage. The ltrC mutant did not detectably activate the late gene promoter (Fig. 2B); following mitomycin C treatment, the culture did not undergo lysis, and no detectable phage were produced, confirming the essentiality of the gene for phage 52A and, by inference, for phage 80. The presence of SaPI2 had no effect on the β-lactamase activity in the strain with the ltrC mutant but, as predicted, had a profound effect on the activity in the strain with the WT phage (Fig. 2B). Using the WT phage, we next demonstrated inhibition of late phage transcription by the cloned SaPI2 ORF17. These results are presented in Fig. 2C and have suggested the designation “phage transcription inhibitor A” (ptiA) for the gene (ORF17). Because PtiA blocked expression of the entire late phage gene module, we predicted it would block lysis as well as plaque formation. Note that the lysis of a culture and the formation of plaques on an indicator strain are rather different tests of phage function: Plaque formation can be blocked by a modest reduction in burst size, whereas lysis can be blocked only by preventing the expression of the lysis module. Unlike the other two interference mechanisms, which blocked plaque formation but not lysis (7), PtiA, as predicted, blocked both (Fig. 3A), making it the most severe of the interference mechanisms yet identified. Also, predictably, the cloned ptiA blocked plaque formation by other phages encoding LtrC, as shown in Fig. S2.
Fig. 2.
(A) Comparison of the rinA and ltrC genomic region from 80α and 80. Note that the position of ltrC (ORF32) in phage 80 corresponds to that of rinA in phage 80α. “P” represents the late gene promoter. (B) Activation of phage 52A late transcript promoter by LtrC. The late transcript promoter was cloned to plasmid pCN41 as a transcriptional fusion to β-lactamase. The resulting reporter plasmid was introduced into strains lysogenic for either the WT prophage or a derivative prophage containing a deletion of ltrC in the absence or presence of SaPI2. β-Lactamase activity was measured 2 h after induction with mitomycin C (MC) and in a parallel culture without induction. (C) Effects of cloned ptiA and ptiM on the phage late transcript promoter. ptiA and ptiM were cloned singly or together to pCN51, and their effects on LtrC activation of the late transcript promoter–β-lactamase fusion were determined. For these tests, the pti genes were induced with 1.0 µm CdCl2, the β-lactamase fusion construct was integrated in the chromosome at the SaPI4 att site (lab plasmids collection), and LtrC was provided by superinfection with phage 80. Cultures were assayed at 2 h postinfection, and assays were performed in triplicate.
Fig. 3.
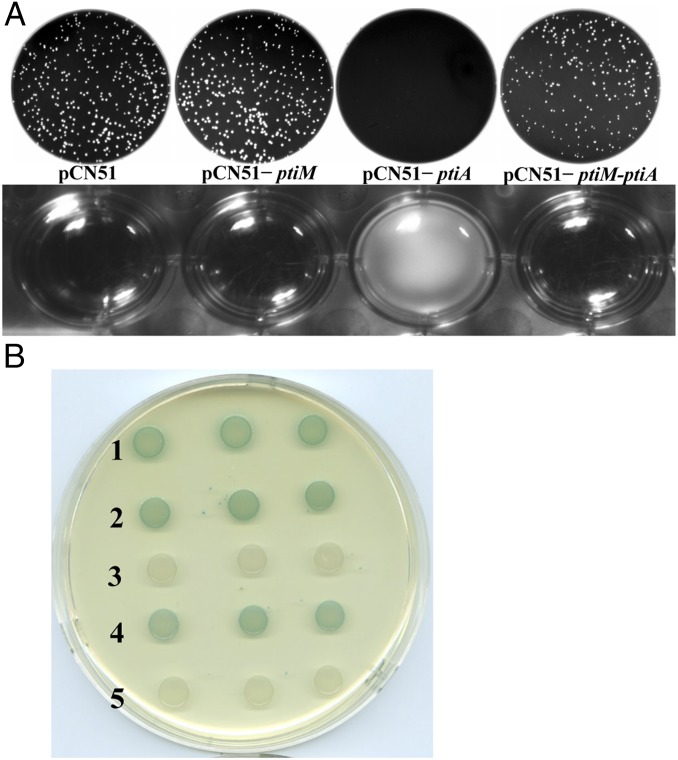
(A) Modulation of PitA activity by PtiM. (Upper) Plaque assay. Phage platings were as in Fig. 1A. (Lower) Lysis assay. RN4220strains carrying pCN51 (vector) or carrying the pti clones used were grown with the inducer (1.0 μM CdCl2), infected with phage 80 at a multiplicity of infection (MOI) of 0.2, and incubated at 32 °C at 60 rpm for 3 h. (B) Bacterial adenylate cyclase-based two-hybrid (BACTH) analysis. Spots in each row represent three independent colonies. Plasmid combinations are numbered as follows: 1, pKT25-zip + pUT18C-zip (positive control); 2, pKT25-LtrC (WT80) + pUT18-PtiAS2; 3, pKT25-LtrC (PtiAS2-resistant 80) + pUT18-PtiAS2; 4, pKT25-PtiMS2 + pUT18-PtiAS2; 5, pKT25 + pUT18 (negative control). Blue color indicates cAMP-dependent lacZ expression following reconstitution of adenylate cyclase activity by the interaction of fusion proteins.
Mechanism of Action of PtiA.
Because all the PtiA-resistant mutations mapped in the LtrC coding sequence, it was likely that PtiA acted by direct protein binding. Accordingly, we performed a two-hybrid assay, as shown in Fig. 3B. As can be seen (Fig. 3B, row 2), there was a strong positive reaction between PtiA and the LtrC WT protein, but no reaction was detected between PtiA and a PtiA-resistant mutant of LtrC (Fig. 3B, row 3). Therefore we conclude that PtiA interferes with phage 80 growth by binding to LtrC and directly inhibiting its ability to activate late gene transcription.
It is interesting that the phage 80 proteins corresponding to the 80α targets of Ppi (TerSP) and of CpmAB (presumably the phage head scaffolding protein) are considerably different from the corresponding 80α proteins and are unaffected by these inhibitors. In fact, phage 80 virion proteins are not formed into small capsids by any of the three prototypical SaPIs (13); moreover, 80α is indifferent to PtiA, presumably because of the insensitivity of RinA to the inhibitor (Fig. 1A). PtiA, in turn, also occurs in variant forms, and we have cloned those of SaPI1 and SaPI1bov1. The SaPI1 variant PtiAS1 differs from PtiAS2 by a single amino acid substitution and is equally effective against phage 80; however, the SaPI1bov1 variant PtiASB1 differs at 10 sites and does not inhibit phage 80 (Fig. S3). PtiA homologs are encoded by all intact SaPIs, and their sequences suggest that they fall into different groups. Perhaps PtiA variants encoded by different SaPIs inhibit LtrC variants encoded by different phages.
Modulation of PtiA Activity.
As noted above, when overexpressed (cloned to a plasmid and driven by an inducible promoter), PtiAS2 not only blocks phage 80 plaque formation but totally prevents lysis (Fig. 3A). The logic of SaPI-phage parasitism would indicate that lysis prevention cannot be the normal effect of PtiAS2 because it would be suicidal for the SaPI as well as for the phage. However, SaPI2 is not a suicide bomber: Although WT SaPI2 eliminates plaque formation by phage 80, the infected cultures undergo lysis. So we next attempted to find the basis for this difference. Because, when overproduced, PtiAS2 totally blocks phage 80 reproduction, and because overproduction is expected during SaPI replication, it seemed unlikely that the difference is based simply on relative gene dosage. In fact, even in single copy, ptiAS2 has a profound effect on phage 80 reproduction (Fig. S4). Therefore, we considered the possibility that PtiAS2 action is modulated by a second SaPI2 gene. As seen in Fig. 1B, ORF16 is immediately upstream of ptiA, and sequence analysis suggested that the two genes probably are translationally coupled. Therefore, ORF16 was a likely candidate for the putative modulator of PtiAS2. To test this possibility, we compared the effects of the cloned ptiAS2 with those of a clone containing both ORFs on the activity of phage 80. As seen in Fig. 3A, the cloned ptiAS2 totally inhibited phage 80, whereas the ORF16-ptiAS2 clone caused only modest inhibition of the phage and did not prevent lysis. Furthermore, ORF16 also reduced the PtiAS2-mediated inhibition of the phage late gene transcription (Fig. 2C). Because the clone of ORF16 alone had little, if any, effect on plaque formation, it is suggested that ORF16 is, in fact, the modulator of PtiAS2. If so, ORF16 could act at the level of ptiAS2 transcription or at the level of translation of the product, or it could form an inhibitory complex with the putative protein. Because the two genes almost certainly are cotranscribed, it seemed highly unlikely that ORF16 controlled the transcription of ptiAS2. As noted above, modulation of gene dosage is unlikely to be the mechanism of control by ORF16. Therefore, it also seemed unlikely that ORF16 controlled the translation of ptiAS2. Accordingly, we considered it more likely that there would be direct binding between the two proteins. We tested for this direct binding by performing a second bacterial two-hybrid assay to test for possible binding of the ORF16 product, gp16, to PtiAS2. As shown in Fig. 3B row 4, a positive binding reaction was readily demonstrated, suggesting that gp16 modulates the activity of PtiAS2 by a direct binding interaction. Because PtiAS2 has two binding partners, which could be mutually exclusive, the equilibrium between these partners could account for a subtle and precise modulation system. On the basis of these results we propose that gp16 be designated “modulator of PtiAS2,” PtiMS2.
To confirm the effects of PtiAS2 and PtiMS2 in the intact SaPI, we constructed in-frame deletions of the two genes and tested them individually and together for their effects on phage 80 plaque formation. As seen in Fig. 4, deletion of ptiAS2 resulted in small plaques; deletion of ptiMS2 eliminated plaque formation, as expected, because of the absence of its modulatory effect on ptiAS2. Also as expected, deletion of both caused the formation of small plaques indistinguishable from those seen with deletion of ptiAS2 alone; however, the failure of these deletions to restore the formation of full-sized plaques is consistent with interference by ORF13, because, as noted above, none of the other SaPI2 ORFs showed interference. Accordingly, we then constructed an in-frame deletion of ORF13 and, indeed, this deletion resulted in partial restoration of plaque formation (Fig. 4); deletion of both ptiAS2 and ORF13 restored plaques to full size (Fig. 4). More definitive results were obtained by one-step growth analysis of phage 80 in the presence of SaPI2 with and without deletions of the interference genes studied here. As shown in Fig. 5A, deletions of the various interference genes restored the growth of phage 80 to different extents, roughly paralleling the plaque formation tests.
Fig. 4.
Effects of SaPI2 ptiA, -B, and -M deletions on SaPI2 interference with phage 80. Strain RN4220 containing WT SaPI2 or various SaPI2 deletion mutants was infected with phage 80 (∼250 pfu per plate), plated on phage bottom agar, and incubated for 48 h at 32 °C. Plates were stained with 0.1% TTC in TSB and photographed.
Fig. 5.
(A) One-step phage growth analysis of ptiA- and ptiB-mediated interference of phage 80. Bacteria were infected with phage 80 at an MOI of 10, washed to remove unadsorbed phage, diluted to contain ∼104 infective centers/mL, and then incubated for 90 min. Samples were removed at the indicated times and plated for plaque formation using RN4220 as the indicator. Three replicates were used for each strain. (B) Effect of deletion of ptiA, ptiB, ptiM, and their combinations on phage 80 LtrC activity. The late phage transcript promoter–β-lactamase reporter used for the experiments in Fig. 2 B and C was tested in RN4220 with SaPI2 and several of its deletion mutants. Strains were infected with phage 80 at an MOI of 0.2. The infected cultures were incubated at 32 °C, 60 rpm and assayed for β-lactamase at 2.5 h postinfection. Three replicates were used for each strain.
ORF13-Mediated Interference.
Because of its bacterial toxicity, ORF13 could not be cloned in Escherichia coli and could be cloned in Staphylococcus aureus only behind a promoter (Ptet) with an extremely low basal level of expression. Bacteria could grow on plates only at concentrations of the inducer anhydrotetracycline (ATc) ≤2 ng/mL; growth on an ATc gradient plate (0–20 ng/mL) is shown Fig. S5A. Plaque size was slightly larger at the borderline of inhibition, suggesting that the host cells are more sensitive to ORF13 than the phage. Thus, our usual strategy for identifying interference targets, namely the formation of rare mutant plaques by the inhibited phage, could not be used in this case. Because we already had a very useful β-lactamase fusion to the late phage promoter, it seemed worthwhile to perform the extremely simple test of measuring the effects of the ORF13 deletion on that promoter before attempting to identify the unknown phage function that was inhibited. In fact, the ORF13 deletion had a profound effect on the late phage promoter, as shown in Fig. 5B (light green bar). It relieved the inhibition by SaPI2 to about the same extent as the ptiAS2 deletion (aqua bar) and, more strikingly, in conjunction with the ptiAS2 deletion, it fully restored promoter activity (orange bar). On the basis of these results, we have designated ORF13 ptiBS2. It is likely, but not certain without mutational evidence, that this is the only interference effect of PtiBS2.
The question that then arises is whether PtiBS2 is toxic to host cells in its native context. In the absence of SaPI or SOS induction, it would not be expressed. Because the host cells lyse after the induction of a helper phage, PtiBS2 could not be so toxic to the host cells that it would interfere with the phage lytic cycle. Indeed, after Ptet induction in broth, the ptiBS2-containing strain grows for six generations and then stops sharply (Fig. S5B), suggesting that the synthesis of an essential protein is inhibited and that there is an ∼64-fold excess of this protein in exponential cells, an amount that surely is more than sufficient to support the lytic cycle. Therefore, its host cell toxicity is irrelevant to phage propagation. In principle, one could test for host inhibition in the absence of any phage, because SaPI operon 1, in which the gene is located, is repressed by LexA (16). Although SOS induction relieves LexA repression, the effect is transient, and transient expression of ptiBS2 would not provide a clear answer because cells would have six generations to recover from any toxicity. We suggest that, in any case, the possible toxicity of PtiBS2 is of little or no importance to the phage or SaPI life cycle.
Another question that arises is whether PtiBS2, like PtiAS2, is modulated by a separate SaPI gene product. The simplest route to answering this question would be via host mutants resistant to PtiBS2. Although colonies appear on inducer-containing plates at a frequency of ∼10−6, analysis of 25 such colonies revealed that all have mutations in Ptet. Screening of additional colonies is in progress. Alternatively, an approach involving further cloning and deletion studies has been initiated; however, that work is beyond the scope of this report.
Discussion
Among the remarkable abilities of bacteria to adapt to unfavorable environmental contingencies is their resistance to bacteriophages. Especially as exemplified by the lactic acid bacteria, resistance has been developed to every imaginable phase of the bacteriophage life cycle (17). Also remarkable is that most of the bacteriophage resistance mechanisms are encoded by plasmids and other mobile elements. Plasmids and other mobile elements seem particularly suited to the carriage and transfer of resistance genes because such resistance would not involve mutational alterations of core functions. We suggest that phage resistance may well exemplify this principle, and we point to the special case of the SaPIs; ultimately derived from ancestral phages or protophages, the SaPIs have undergone an evolutionary divergence that has included the acquisition of a special category of phage-resistance mechanisms not used by other genetic elements. These mechanisms differ from the usual phage-resistance mechanisms in that they are carefully crafted to ensure the production of mature phage particles, most of which contain SaPI rather than phage DNA. Like other phage-resistance mechanisms, the SaPIs target essential phage functions, and all known thus far affect particle maturation and DNA packaging. The two SaPI resistance mechanisms first identified target helper phage capsid morphogenesis and DNA packaging, respectively, functions that do not compromise SaPI particle production. The third mechanism, described here, is unique in that it involves two proteins, PtiA and PtiB, that target the promoter of late phage gene transcription, which is responsible for the production of virion and lysis proteins. This interference mechanism must be modulated precisely so as not to interfere with SaPI particle formation, and we show here that a modulatory protein, PtiM, binds to one of the interference-mediating proteins, PtiA. The other protein, PtiB probably is modulated also, but, because its extreme toxicity for the host cell, it has not yet been analyzed. Thus, as shown in the diagram in Fig. 6, PtiA has two binding partners, one that it inhibits and one that inhibits it. This interference mechanism, like the others, favors the SaPI over its helper phage, and one would assume that phage and SaPI are in competition for particle maturation so that deletion of the interference genes would result in an increase in phage titer concomitantly with diminished production of SaPI particles. In reality, the situation is rather more complex. In a previous study (7), we observed that deletion of an interference gene increased phage titer but did not decrease SaPI titer. The biological function of SaPI-mediated interference was, as expected, to diminish phage particle production relative to SaPI particle production. The increase in phage titer upon deletion of an interference gene is consistent with the well-known excess of virion proteins in the infected cell. To explain the failure of SaPI particle production to respond to the presence or absence of the interference genes, we suggest that a SaPI product must be rate-limiting for SaPI particle production and that this limitation is not overcome by increasing the availability of virion proteins. This limiting factor could only be TerSS or the SaPI-specific replication initiator protein. Because there always is excess SaPI DNA at the time of cellular lysis, the latter possibility could be ruled out.
Fig. 6.
Summary of SaPI2-mediated interference with phage 80. SaPI2 operon 1 encodes three proteins, PtiA, PtiM, and PtiB, involved in phage 80 interference. PtiA binds to the phage 80 late gene transcription activator protein, LtrC, and blocks LtrC-mediated phage late gene activation, which is essential for the expression of packaging and lysis genes. A modulator protein, PtiM, binds to and precisely modulates the activity of PtiA, attaining the required level of interference. Preliminary data show that another SaPI2 protein, PtiB, also inhibits the phage late gene transcription, but its mechanism remains to be elucidated.
However, once it was determined that both ptiA and ptiB inhibit transcription of the bacteriophage late gene promoter (Fig. 5B), which is responsible for production of virion proteins, the expectation would be that phage and SaPI titers would be affected equally, because the reduction in the overall level of virion proteins should take precedence over the putative rate limitation of TerSS. This expectation was not realized: As shown in Table S1, deletion of either ptiAS2 alone or of ptiAS2 and ptiBS2 had no significant effect on SaPI titer, although phage titer was increased significantly. Given that the morphogenetic (late) phage proteins generally are present in considerable excess, the effect of this rather modest inhibition of the Ltr promoter (Fig. 5B) suggests that production of one or more late phage proteins, such as TerSP, is rate-limiting for the phage and that this rate limitation would be overcome by relief of LtrC inhibition. The biological basis for interference by SaPI with late phage transcription would be precisely parallel to that suggested for the previously described ppi gene (7), despite the nature of the target of inhibition—namely, to give the SaPI an advantage over its helper phage. Because the inhibited protein is required for both SaPI and phage particle production, it must be modulated precisely to ensure adequate production of both types of particles. Note, however, that, as shown in Table S1, deletion of ptiBS2, unlike the deletion of ptiAS2, did cause a modest reduction in SaPI titer. To explain this effect, we hypothesize that, in addition to its inhibitory role, PtiB may have a regulatory role, such as up-regulation of operon 1 transcription, which would increase TerSS production. Why deletion of both genes reverses this effect is unexplained. The location of terSS in the same operon as two of the three interference genes (Fig. 1B) raises an interesting regulatory question: terSS surely would be up-regulated during SaPI induction, certainly would be up-regulated by relief of LexA repression of operon 1, and perhaps also would be up-regulated by the up-regulation of one or more promoters upstream of operon, which would be needed for the transcription of ppi (ORF10), which is immediately upstream of operon 1 (18). The other interference genes would be up-regulated also, leading to diminution of phage particle production, as observed. However, because TerSS, like other TerS's, is presumed to be a multisubunit enzyme, an increase in the TerS holoenzyme would necessarily be accompanied by a much greater increase in the interference protein copy numbers. The effects of this increase would necessarily be mitigated by PtiM for PtiA and by a putative, as yet unknown, modulator for PtiB. Experiments to sort out this interesting regulatory circuitry are in progress.
We and others (19) predict that since the SaPIs package their DNA the same way that phages do, the SaPIs should be able to mediate generalized transduction, using pac site homologs in host DNA that would be recognized by TerSS. By interfering with helper phage reproduction, the SaPIs would thus promote horizontal transfer of a wide variety of host genes in addition to their own transfer. It is interesting to compare this mechanism with an entirely different phage-interference mechanism, that of the clustered regularly interspaced short palindromic repeats (CRISPRs) (20, 21), which block phage propagation completely by degrading incoming phage DNA and thus block all phage-mediated horizontal gene transfer (22). Because the SaPIs are extremely common, with most S. aureus strains harboring at least one, whereas the CRISPRs are extremely rare in S. aureus, it could be argued that the SaPIs have won that particular evolutionary contest. All-in-all, the SaPI-phage interactions represent a remarkable microcosm within the bacterial intracellular universe.
Materials and Methods
Bacterial Strains and Growth Conditions.
Bacterial strains and plasmids used in these studies are listed in Tables S2 and S3, respectively. Growth media and conditions are described in SI Materials and Methods. Staphylococcal temperate phages 80, 80α, 52A, 71, ETA, and ϕNM4 were used in this study.
DNA Methods.
All general DNA manipulations (e.g., digestion and ligation) were carried out by standard methods (23). Oligonucleotides used for this work are listed in Table S4. All restriction enzymes and T4 DNA ligase were purchased from New England Biolabs. Primers were obtained from Macrogen. Integrated DNA Technologies Inc. performed DNA sequencing.
Mutant Construction.
Mutants (in-frame deletion) were generated using the allelic exchange vector pMAD (24) as previously described (18). Details are given in SI Materials and Methods.
Phage Infection and Induction of Lysogen.
S. aureus strains were inoculated in casamino acids yeast extract glycerophosphate (CYGP) (25) broth at an initial OD600 = 0.1 (∼1 × 108 cfu/mL) and were grown at 37 °C and 225 rpm. For phage infection, cultures were adjusted to OD600 = 1.0 (∼1 × 109 cfu/mL) with CYGP broth and diluted 1:1 with phage buffer (25) infected with phage 80. To induce expression during phage growth of genes cloned in plasmid pCN51, 1.0 μM of CdCl2 was added at the time of infection. For lysogen induction, cultures were adjusted to OD600 = 1.0 (∼1 × 109 cfu/mL) with CYGP broth and diluted 1:1 with CYGP broth containing mitomycin C (final concentration, 2 μg/mL).
Plaque Assay.
Approximately 100–200 phage 80 particles were mixed with ∼108 cells (100 μL culture adjusted to OD600 = 1.0; ∼1 × 109 cfu/mL) of RN4220 and its derivative strains. This mixture was incubated at room temperature for 15 min, subsequently mixed with 3 mL of phage top agar (25), and immediately poured on a phage bottom agar (25) plate without or with 1.0 μM CdCl2. Plates were incubated at 32 °C for 48 h and stained with 0.1% triphenyl tetrazolium chloride (TTC) (Difco) (26) in tryptic soy broth (TSB).
Enzyme Assays.
Phage late gene transcription was measured using the β-lactamase enzyme assay using nitrocefin as substrate. The assays were performed as mentioned (27) using a Thermomax (Molecular Devices) microtiter plate reader. β-Lactamase units are defined as Vmax/OD650. Details of preparation of samples for this assay are described in SI Materials and Methods.
Bacterial two-hybrid assays, the one-step growth curve, and PtiB toxicity assays are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R01AI022159 (to R.P.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.M.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1406749111/-/DCSupplemental.
References
- 1.Novick RP, Christie GE, Penadés JR. The phage-related chromosomal islands of Gram-positive bacteria. Nat Rev Microbiol. 2010;8(8):541–551. doi: 10.1038/nrmicro2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tormo-Más MA, et al. Moonlighting bacteriophage proteins derepress staphylococcal pathogenicity islands. Nature. 2010;465(7299):779–782. doi: 10.1038/nature09065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruzin A, Lindsay J, Novick RP. Molecular genetics of SaPI1—a mobile pathogenicity island in Staphylococcus aureus. Mol Microbiol. 2001;41(2):365–377. doi: 10.1046/j.1365-2958.2001.02488.x. [DOI] [PubMed] [Google Scholar]
- 4.Tallent SM, Langston TB, Moran RG, Christie GE. Transducing particles of Staphylococcus aureus pathogenicity island SaPI1 are comprised of helper phage-encoded proteins. J Bacteriol. 2007;189(20):7520–7524. doi: 10.1128/JB.00738-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tormo MA, et al. Staphylococcus aureus pathogenicity island DNA is packaged in particles composed of phage proteins. J Bacteriol. 2008;190(7):2434–2440. doi: 10.1128/JB.01349-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ubeda C, et al. Specificity of staphylococcal phage and SaPI DNA packaging as revealed by integrase and terminase mutations. Mol Microbiol. 2009;72(1):98–108. doi: 10.1111/j.1365-2958.2009.06634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ram G, et al. Staphylococcal pathogenicity island interference with helper phage reproduction is a paradigm of molecular parasitism. Proc Natl Acad Sci USA. 2012;109(40):16300–16305. doi: 10.1073/pnas.1204615109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLeo FR, et al. Molecular differentiation of historic phage-type 80/81 and contemporary epidemic Staphylococcus aureus. Proc Natl Acad Sci USA. 2011;108(44):18091–18096. doi: 10.1073/pnas.1111084108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charpentier E, et al. Novel cassette-based shuttle vector system for gram-positive bacteria. Appl Environ Microbiol. 2004;70(10):6076–6085. doi: 10.1128/AEM.70.10.6076-6085.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corbisier P, Ji G, Nuyts G, Mergeay M, Silver S. luxAB gene fusions with the arsenic and cadmium resistance operons of Staphylococcus aureus plasmid pI258. FEMS Microbiol Lett. 1993;110(2):231–238. doi: 10.1111/j.1574-6968.1993.tb06325.x. [DOI] [PubMed] [Google Scholar]
- 11.Ferrer MD, et al. RinA controls phage-mediated packaging and transfer of virulence genes in Gram-positive bacteria. Nucleic Acids Res. 2011;39(14):5866–5878. doi: 10.1093/nar/gkr158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quiles-Puchalt N, et al. A super-family of transcriptional activators regulates bacteriophage packaging and lysis in Gram-positive bacteria. Nucleic Acids Res. 2013;41(15):7260–7275. doi: 10.1093/nar/gkt508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christie GE, et al. The complete genomes of Staphylococcus aureus bacteriophages 80 and 80α—implications for the specificity of SaPI mobilization. Virology. 2010;407(2):381–390. doi: 10.1016/j.virol.2010.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grayhack EJ, Roberts JW. The phage lambda Q gene product: Activity of a transcription antiterminator in vitro. Cell. 1982;30(2):637–648. doi: 10.1016/0092-8674(82)90260-4. [DOI] [PubMed] [Google Scholar]
- 15.Deighan P, Hochschild A. The bacteriophage lambdaQ anti-terminator protein regulates late gene expression as a stable component of the transcription elongation complex. Mol Microbiol. 2007;63(3):911–920. doi: 10.1111/j.1365-2958.2006.05563.x. [DOI] [PubMed] [Google Scholar]
- 16.Ubeda C, et al. SaPI operon I is required for SaPI packaging and is controlled by LexA. Mol Microbiol. 2007;65(1):41–50. doi: 10.1111/j.1365-2958.2007.05758.x. [DOI] [PubMed] [Google Scholar]
- 17.S. L 2012. Lactic Acid Bacteria: Microbiological and Functional Aspects, Chapter 9, (CRC, Boca Raton, FL), 4th Ed.
- 18.Ubeda C, et al. SaPI mutations affecting replication and transfer and enabling autonomous replication in the absence of helper phage. Mol Microbiol. 2008;67(3):493–503. doi: 10.1111/j.1365-2958.2007.06027.x. [DOI] [PubMed] [Google Scholar]
- 19.Bento JC, et al. Sequence determinants for DNA packaging specificity in the S. aureus pathogenicity island SaPI1. Plasmid. 2014;71:8–15. doi: 10.1016/j.plasmid.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrangou R, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315(5819):1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- 21.Deveau H, Garneau JE, Moineau S. CRISPR/Cas system and its role in phage-bacteria interactions. Annu Rev Microbiol. 2010;64:475–493. doi: 10.1146/annurev.micro.112408.134123. [DOI] [PubMed] [Google Scholar]
- 22.Marraffini LA, Sontheimer EJ. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science. 2008;322(5909):1843–1845. doi: 10.1126/science.1165771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook JRD. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2001. [Google Scholar]
- 24.Arnaud M, Chastanet A, Débarbouillé M. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Appl Environ Microbiol. 2004;70(11):6887–6891. doi: 10.1128/AEM.70.11.6887-6891.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novick RP. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- 26.Pattee PA. Use of tetrazolium for improved resolution of bacteriophage plaques. J Bacteriol. 1966;92(3):787–788. doi: 10.1128/jb.92.3.787-788.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji G, Beavis R, Novick RP. Bacterial interference caused by autoinducing peptide variants. Science. 1997;276(5321):2027–2030. doi: 10.1126/science.276.5321.2027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.