Mature adipocytes are postmitotic (1), and therefore development of adipocytes requires the proliferation and differentiation of progenitor cells into mature adipocytes. The identity of adipose precursor cells and the molecular mechanisms that control their proliferation and differentiation are only partially understood. Brown adipose tissue (BAT) is specialized for energy expenditure through a process mediated by uncoupling protein 1 (UCP1) that uncouples respiration from ATP synthesis and generates heat (2). Current knowledge defines two types of thermogenic adipocytes based on their distinct origins, anatomical locations, and differential abilities to respond to stimuli. The classical brown adipocytes mainly develop during embryonic stage, form a discrete depot, and exert a high level of basal thermogenic capacity. The other type of brown fat-like cells, called beige or brite adipocytes, are dispersed in white adipose tissue (WAT) and are recruited and induced in response to certain stimuli. Although beige adipocytes also generate heat by UCP1-mediated proton leak, they arise from a myogenic factor 5-negative (Myf5−) developmental lineage distinct from brown adipocytes, which develop from Myf5+ progenitors (Fig. 1) (3, 4). Aside from the adrenergic pathways that activate thermogenesis in both brown and beige adipocytes, whether there exists a common factor that marks and specifies the fate of these thermo-competent cells remains unknown. In PNAS, Wang et al. (5) identify early B-cell factor 2 (Ebf2) as a marker that specifies the brown and beige adipocyte lineage from muscle, dermal, and white adipocyte lineages and provide new insight into transcriptional targets of Ebf2 that may play a role in activating the thermogenic gene program.
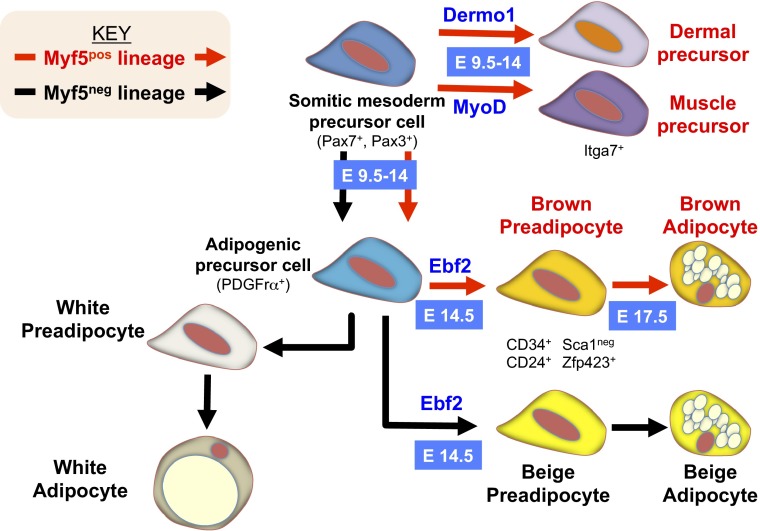
Fig. 1.
Ebf2 specifies thermogenic preadipocytes. During development, brown adipogenic precursors arise from a Myf5+ lineage (red arrows). Furthermore, adipogenic precursor cells that arise from a Myf5− lineage (black arrows) can be isolated from WAT. PDGFrα marks cells from both the Myf5+ and Myf5− lineages with adipogenic potential that can differentiate into white, brown, and beige adipocytes. Beginning at embryonic day (E)12.5, the transcription factor Ebf2 distinguishes thermogenically competent brown and beige preadipocytes from white adipocytes and activates a gene-expression profile that primes these cells for thermogenic gene expression. Ebf2 expression is common to thermogenically primed cells from both the Myf5+ and Myf5− lineages and may act to activate the thermogenic gene program that allows these cells to uncouple respiration from ATP production to generate heat.
Ebf proteins are members of the helix–loop–helix transcription factors originally identified in mouse B-lymphocytes (6). Although it belongs to the Ebf family of proteins, Ebf2 is not expressed in mature B cells; rather, it is abundantly expressed in adipocytes, neurons, and immature osteoblasts (7–9). Ebf2 has been shown to play a role in establishing the osteoblast niche for hematopoiesis (10), regulating somite development (11), and initiating white adipocyte differentiation (12). Recently, the same group of authors discovered that Ebf2 cooperates with peroxisome proliferator-activated recep=tor-γ (PPARγ) to regulate the expression of brown adipocyte-selective genes in classical brown adipocytes (13). In the current study, Wang et al. (5) use an unbiased approach to search for genes that mark committed brown fat precursors and the results lead them back to Ebf2. Interestingly, Ebf2 not only determines the identity of the classical brown fat cells, but also marks the beige fat progenitors present in WAT. Moreover, Ebf2 serves as a functional determinant for both brown and beige adipocytes and poises the precursor cells to become thermogenically competent (Fig. 1). Taken together, these results allow for the construction of a lineage roadmap that highlights platelet-derived growth factor receptor-α (PDGFrα) expression as key intersection in a common adipogenic differentiation. Furthermore, Ebf2 expression is common to different lineages that share thermogenic capacity.
A key technical advancement in the study of Wang et al. (5) is the ability to sort cells with white, brown, and beige adipogenic potential from one another using Myf5-lineage tracing coupled with the Ebf2 GFP reporter. Although brown and beige adipocytes are qualitatively similar in that they both expend energy in the form of heat for thermoregulation, the quantitative functional difference between these cells types can now be assessed. Wang et al. (5) investigated changes in gene transcription in these different cell types to identify a thermogenic gene-expression profile. It will be important to also assess functional differences in these cell types, such as differences in metabolism.
Several different pathways regulate Ebf family gene expression and transcriptional activity, including Notch (14), Hedgehog (15), and bone morphogenetic protein (BMP) signaling (11). Hedgehog signaling in adipose stromal vascular cells primes these precursors for osteogenic differentiation and suppresses adipogenesis (16), suggesting Hedgehog is unlikely to be upstream of Ebf2. That BMP signaling is capable of regulating Ebf2 expression makes it tempting to link this signaling pathway—specifically BMP7 signaling, which is known to play an essential role in embryonic brown fat development (17, 18) and regulate beige adipocyte formation (4)—directly to Ebf2-mediated transcriptional activity. Still, the impact of other upstream signals on Ebf2 expression and activity in brown preadipocytes cannot be discounted.
Previously, Ebf2 was identified as a transcription factor that cooperates with PPARγ to turn on BAT-specific genes, such as PRD1-BF1-RIZ1 homologous domain-containing 16 (PRDM16), UCP1, and PPARα, and reduce PPARγ binding to WAT-specific loci, including Agt2 and Rarres2 (13). Interestingly, Wang et al. (5) demonstrate that PPARγ is dispensable for establishing brown fat precursor identity, as expression of the selected Ebf2-regulated brown preadipocyte signature genes was not altered in PPARγ-null cells. These findings highlight differential roles of Ebf2 during the course of brown/beige adipocyte differentiation. Ebf2 alone appears to be sufficient and necessary for establishing brown/beige fat precursor cell identity; priming these cells to become thermogenically competent. As differentiation proceeds, PPARγ is recruited to the enhancer/promoter regions of PRDM16, UCP1, and PPARα and fully executes the brown adipogenic program. It would be critical to determine whether the Ebf2-regulated genes identified in the study of Wang et al. (5) merely serve as the “signatures” of brown/beige fat precursors or if they could contribute to the thermogenic program turned on during late-stage differentiation. Along this line of investigation, determining the direct targets of Ebf2 binding using a chromatin immunoprecipitation strategy would be useful to define the specific transcriptional program activated by Ebf2, as opposed to the transcriptional changes present in Ebf2 knockout cells described by Wang et al. This strategy might also uncover nodes of the Ebf2-driven transcriptional program that intersect with the PPARγ network and allow Ebf2 to compensate for PPARγ deletion. Indeed, the overlap of transcriptional targets seems to include UCP1 itself, as Ebf2 can bind to the UCP1 promoter and activate transcription (13). Interestingly, a long noncoding RNA called Blnc1 has recently been identified as a binding partner of Ebf2, and the ribonucleoprotein formed by Blnc1 binding to Ebf2 increases transcriptional activity more than Ebf2 alone (19).
Wang et al. use an unbiased approach to search for genes that mark committed brown fat precursors and the results lead them back to Ebf2.
Unfortunately, the sorting strategy developed in the study of Wang et al. (5) relies on transgenes to mark the Myf5+ lineage, as well as cells that express Ebf2, making it difficult to apply to human cells. It is possible, however, that genes regulated by Ebf2 are expressed on the cell surface, raising the possibility that these downstream markers could be used to label human cells for isolation. This prospect is exciting for several reasons. First, although it is clear that adult humans do indeed have thermogenically competent adipose tissue (20), it is unclear whether this is brown, beige, or a mixture of both types of adipocytes (21). Second, isolating these progenitors and expanding them in culture could potentially lead to autologous brown/beige fat tissue transplants as an obesity therapy.
In summary, Wang et al. (5) tease out the distinct thermogenic gene program active in preadipocytes primed to differentiate into energy-dissipating cells. Perhaps most excitingly in terms of translational aspects, this thermogenic gene program regulated by Ebf2 appears to be co-opted by brown and beige preadipocytes arising from distinct lineages. Is the activation of the thermogenic gene program by promoting Ebf2 target gene transcription possible in any cell type? The identification of the Ebf2-regulated gene network in brown and beige preadipocytes provides a road map showing how cells activate thermogenesis, and enriches our understanding of the development of adipose tissue.
Acknowledgments
This work was supported in part by National Institutes of Health (NIH) Grants R01DK077097 (to Y.-H.T.), and Joslin Diabetes Center's Diabetes Research Center (P30DK036836), a research grant from the American Diabetes Foundation (ADA 7-12-BS-191), and by funding from the Harvard Stem Cell Institute (to Y.-H.T.). M.D.L. was supported by NIH fellowships (T32DK007260 and F32DK102320).
Footnotes
The authors declare no conflict of interest.
See companion article on page 14466.
References
- 1.Simon G. Histogenesis. In: Renold AE, Cahill GF, editors. Handbook of Physiology. Section 5: Adipose Tissue. American Physiological Society; Washington, DC: 1965. [Google Scholar]
- 2.Cannon B, Nedergaard J. Metabolic consequences of the presence or absence of the thermogenic capacity of brown adipose tissue in mice (and probably in humans) Int J Obes (Lond) 2010;34(Suppl 1):S7–S16. doi: 10.1038/ijo.2010.177. [DOI] [PubMed] [Google Scholar]
- 3.Seale P, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454(7207):961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulz TJ, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci USA. 2011;108(1):143–148. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W, et al. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc Natl Acad Sci USA. 2014;111:14466–14471. doi: 10.1073/pnas.1412685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Travis A, Hagman J, Hwang L, Grosschedl R. Purification of early-B-cell factor and characterization of its DNA-binding specificity. Mol Cell Biol. 1993;13(6):3392–3400. doi: 10.1128/mcb.13.6.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garel S, et al. Family of Ebf/Olf-1-related genes potentially involved in neuronal differentiation and regional specification in the central nervous system. Dev Dyn. 1997;210(3):191–205. doi: 10.1002/(SICI)1097-0177(199711)210:3<191::AID-AJA1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 8.Kieslinger M, et al. EBF2 regulates osteoblast-dependent differentiation of osteoclasts. Dev Cell. 2005;9(6):757–767. doi: 10.1016/j.devcel.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Malgaretti N, et al. Mmot1, a new helix-loop-helix transcription factor gene displaying a sharp expression boundary in the embryonic mouse brain. J Biol Chem. 1997;272(28):17632–17639. doi: 10.1074/jbc.272.28.17632. [DOI] [PubMed] [Google Scholar]
- 10.Kieslinger M, Hiechinger S, Dobreva G, Consalez GG, Grosschedl R. Early B cell factor 2 regulates hematopoietic stem cell homeostasis in a cell-nonautonomous manner. Cell Stem Cell. 2010;7(4):496–507. doi: 10.1016/j.stem.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 11.El-Magd MA, Allen S, McGonnell I, Otto A, Patel K. Bmp4 regulates chick Ebf2 and Ebf3 gene expression in somite development. Dev Growth Differ. 2013;55(8):710–722. doi: 10.1111/dgd.12077. [DOI] [PubMed] [Google Scholar]
- 12.Akerblad P, Lind U, Liberg D, Bamberg K, Sigvardsson M. Early B-cell factor (O/E-1) is a promoter of adipogenesis and involved in control of genes important for terminal adipocyte differentiation. Mol Cell Biol. 2002;22(22):8015–8025. doi: 10.1128/MCB.22.22.8015-8025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajakumari S, et al. EBF2 determines and maintains brown adipocyte identity. Cell Metab. 2013;17(4):562–574. doi: 10.1016/j.cmet.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crozatier M, Valle D, Dubois L, Ibnsouda S, Vincent A. Collier, a novel regulator of Drosophila head development, is expressed in a single mitotic domain. Curr Biol. 1996;6(6):707–718. doi: 10.1016/s0960-9822(09)00452-7. [DOI] [PubMed] [Google Scholar]
- 15.Vervoort M, Crozatier M, Valle D, Vincent A. The COE transcription factor Collier is a mediator of short-range Hedgehog-induced patterning of the Drosophila wing. Curr Biol. 1999;9(12):632–639. doi: 10.1016/s0960-9822(99)80285-1. [DOI] [PubMed] [Google Scholar]
- 16.James AW, et al. Sonic Hedgehog influences the balance of osteogenesis and adipogenesis in mouse adipose-derived stromal cells. Tissue Eng Part A. 2010;16(8):2605–2616. doi: 10.1089/ten.tea.2010.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tseng Y-H, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454(7207):1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz TJ, et al. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature. 2013;495(7441):379–383. doi: 10.1038/nature11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao X-Y, Li S, Wang GX, Yu Q, Lin JD. A long noncoding RNA transcriptional regulatory circuit drives thermogenic adipocyte differentiation. Mol Cell. 2014;55(3):372–382. doi: 10.1016/j.molcel.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cypess AM, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013;19(5):635–639. doi: 10.1038/nm.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu J, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]