Significance
Females of several mosquito species have evolved obligatory blood-feeding to supply amino acids and other nutrients necessary for egg development, providing a mechanism for the spread of many devastating diseases in humans. The mosquito-specific microRNA (miRNA), miR-1174, is significantly upregulated post blood meal and specific to the female mosquito midgut, suggesting a role in blood meal-associated events. We have found that miR-1174 targets serine hydroxymethyltransferase and is required for key functions in Aedes aegypti and Anopheles gambiae. Its inhibition disrupts sugar absorption, fluid excretion, blood intake in the gut, and, consequently, egg maturation and survival. Mosquito-specific miRNAs, such as miR-1174, have the potential for influencing the development of a future generation of mosquito control.
Keywords: insect disease vector, antagomir
Abstract
Lineage-specific microRNAs (miRNAs) may contribute to functions specific to hematophagous mosquitoes and, as such, have potential for contributing to the development of future mosquito control approaches. Here we report that the mosquito- and gut-specific miRNA, miR-1174, is required for proper sugar absorption, fluid excretion, blood intake, and, consequently, egg maturation and survival in female mosquitoes. miR-1174 is highly expressed and localized in the posterior midgut, the blood-digesting portion of the mosquito alimentary canal. Depletion of miR-1174 results in severe defects in sugar absorption and blood intake. We identified serine hydroxymethyltransferase (SHMT) is a direct miR-1174 target. The adverse phenotypes caused by miR-1174 silencing were rescued by SHMT RNA interference. Our results suggest that miR-1174 is essential for fine-tuning the SHMT transcript to levels necessary for normal mosquito gut functions.
Females of the vector mosquito species have evolved obligatory blood-feeding requirements to supply amino acids and other nutrients for egg development (1). Consequently, they serve as vectors of many devastating human diseases. Combined, vector-borne diseases result in about a million deaths and cause illness for more than half a billion people annually. Disease pathogens enter the mosquito gut with ingested blood. Hence, understanding the molecular basis of gut physiology is of great significance for the development of future approaches toward vector and pathogen control (2–4).
MicroRNAs (miRNAs) are noncoding 22- to 23-nt RNAs that usually repress translation and promote transcript decay (5–7). Most animal miRNAs imperfectly pair with complementary sites within the 3′ UTRs of mRNA (8, 9). Lineage-specific miRNAs may contribute to developmental novelties in blood-feeding mosquito species and represent attractive targets for vector control approaches. Although several mosquito miRNAs have been characterized functionally (10–14), little is known regarding the role of mosquito-specific miRNAs and their contribution to mosquito-specific events (5).
Many miRNAs are elevated in the female mosquito gut after a blood meal, suggesting that they regulate blood-digestive events (15–17). Previously, the mosquito-specific miR-1174/miR-1175 microRNA (miRNA) cluster was shown to be highly expressed in the Anopheles gambiae gut post blood meal (PBM) (15); however, its role remained undetermined. In this report, we examine the role of the mosquito- and gut-specific miR-1174/miR-1175 cluster and present evidence that miR-1174 and its target, serine hydroxymethyltransferase (SHMT), control key gut function in the yellow fever mosquito, Aedes aegypti, and malaria vector, A. gambiae.
Results
Expression Analysis of the miR-1174/miR-1175 Cluster in the Female Mosquito Midgut.
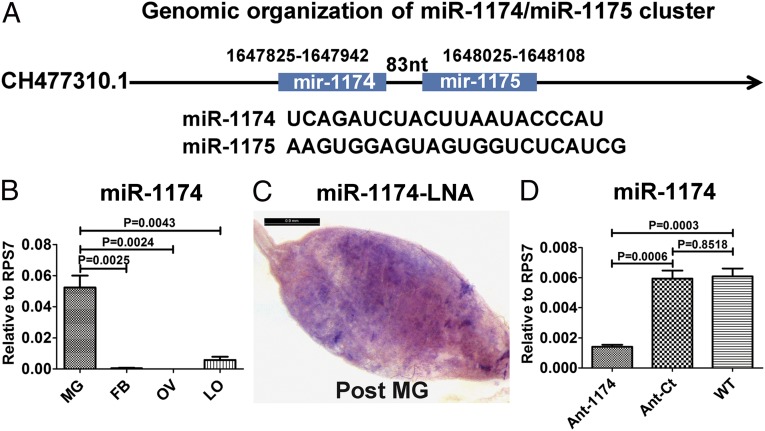
An ortholog of aga–miR-1174 is present in A. aegypti and differs from that in A. gambiae by 2 nucleotides. Similarly, genomic clustering of miR-1174 and miR-1175 is conserved—located at the same locus 83 nucleotides apart (Fig. 1A)—suggesting that these two miRNAs may be coregulated. We measured mature miR-1174 and miR-1175 relative expression levels in the midgut of the female mosquito by quantitative real-time (qRT) PCR analysis. A. aegypti miR-1174 and miR-1175 exhibit similar patterns of expression, reaching their peak levels at 36 h PBM and decreasing to background levels by 72 h PBM (Fig. S1A), further supporting coregulation. Expression of both miRNAs was gut-specific in A. aegypti (Fig. 1B and Fig. S1B). Moreover, in situ hybridization indicated that miR-1174 was localized in the posterior midgut, the part of the alimentary canal where the ingested blood is digested (Fig. 1C and Fig. S1D). High levels of expression of miR-1174 and miR-1175 in the female mosquito midgut PBM suggest that these miRNAs may play an important role in regulating midgut functions related to blood digestion.
Fig. 1.
Characterization of miR-1174 and miR-1175 in A. aegypti. (A) Genomic organization of miR-1174/miR-1175. (B) Tissue-specific expression of miR-1174. mg, midgut; FB, fat body; OV, ovary; LO, leftover. (C) In situ hybridizations using a 5′ and 3′ digoxigenin (DIG)-labeled locked nucleic acid-modified DNA oligonucleotide complementary to miR-1174 (1174LNA). (D) miR-1174 level in the whole body after antagomir silencing (Ant-1174) compared with the effect of scrambled control antagomir (Ant-Ct) in female A. aegypti mosquitoes. WT shows miR-1174 level in untreated mosquitoes. qRT-PCR, error bars depict ±SEM.
miR-1174 Depletion Results in Severe Defects in Sugar Absorption.
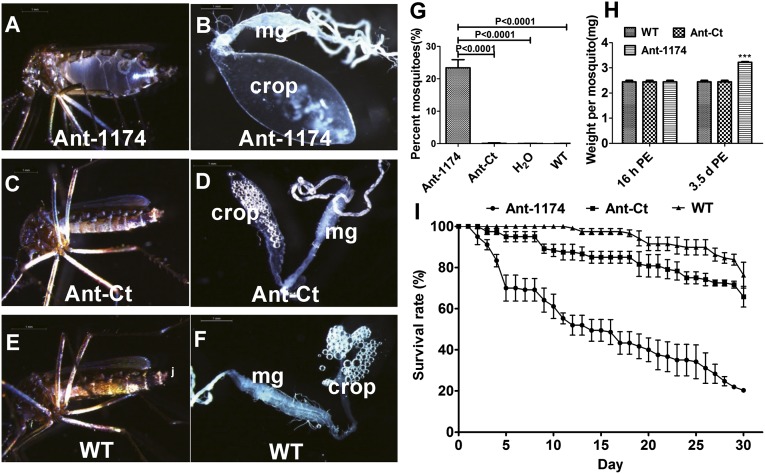
To determine the function of miR-1174 in the mosquito, we inhibited miR-1174 by antisense oligonucleotides (antagomirs, Ant-1174) (18). We injected Aedes female mosquitoes with 100 pmol of Ant-1174 at 12 h after their eclosion from pupae (posteclosion, PE). The level of miR-1174 decreased significantly after its specific inhibition, whereas no change was observed after the injection of a scrambled oligonucleotide sequence antagomir (Ant-Ct) (Fig. 1D). There was no change in the miR-1175 level in Ant-1174–treated mosquitoes (Fig. S1C). When maintained on a 10% (wt/vol) sugar solution for 3 d postinjection (PIJ), over 20% of the Ant-1174–treated mosquitoes had bloated abdomens (bloated-abdomen phenotype, BAP) (Fig. 2A). The BAP was mostly due to the extended fluid-filled crop, a part of the mosquito digestive system used for storing nectar under normal conditions (Fig. 2B). Ant-Ct–treated female mosquitoes maintained under the same conditions had a normal appearance, similar to that of WT and H2O-treated mosquitoes, and their crops were not extended (Fig. 2 C–G). The weight of each Ant-1174–treated female mosquito increased from ∼2.5 mg before injection to 3.3 mg after being maintained on a 10% sugar solution for 3 d PIJ, whereas the weight of Ant-Ct–treated mosquitoes and WT did not change (Fig. 2H). Female mosquitoes with the BAP were unable to fly or feed on blood. However, when Ant-1174–treated mosquitoes were maintained only on water for 3 d PIJ, they had normal appearance and behavior similar to those of WT untreated, H2O-treated, and Ant-Ct–treated mosquitoes. Inhibition of miR-1174 affected the life span of female mosquitoes with 80% mortality within 30 d compared with 30% in Ant-Ct–treated mosquitoes (Fig. 2I). We injected 40 pmol of antagomir into male Aedes mosquitoes due to their smaller size and found that over 20% Ant-1174–treated males displayed BAP. Similarly, A. gambiae female mosquitoes were injected with 40 pmol of the Ant-1174, specific to A. gambiae miR-1174, at 12 h PE. Twenty-five percent of female mosquitoes developed the BAP after maintenance on a 10% sugar solution for 3 d PIJ (Figs. S2 and S3 A and B). Thus, miR-1174 plays a role in regulation of sugar absorption and fluid excretion that is conserved in both Aedes and Anopheles mosquitoes.
Fig. 2.
Effect of miR-1174 silencing in A. aegypti female mosquitoes maintained on 10% sugar solution. (A) A female mosquito with the bloated-abdomen phenotype after miR-1174 antagomir (Ant-1174) injection. (B) Digestive system isolated from a female mosquito with bloated-abdomen phenotype. Note an extremely extended crop. mg, midgut. (C) A female mosquito injected with control antagomir (Ant-Ct). (D) Digestive system from isolated Ant-Ct–treated mosquito. (E) Untreated WT mosquito. (F) Digestive system isolated from a WT mosquito. (G) Percentage of female mosquitoes injected with Ant-1174, Ant-Ct, H2O, or untreated WT displaying the bloated-abdomen phenotype. (H) Weight changes per mosquito 3 d PIJ. (I) Survival of female mosquitoes injected with Ant-1174, Ant-Ct, or untreated WT. Error bars depict ±SEM.
miR-1174 Depletion Results in Severe Defects in Blood Digestion.
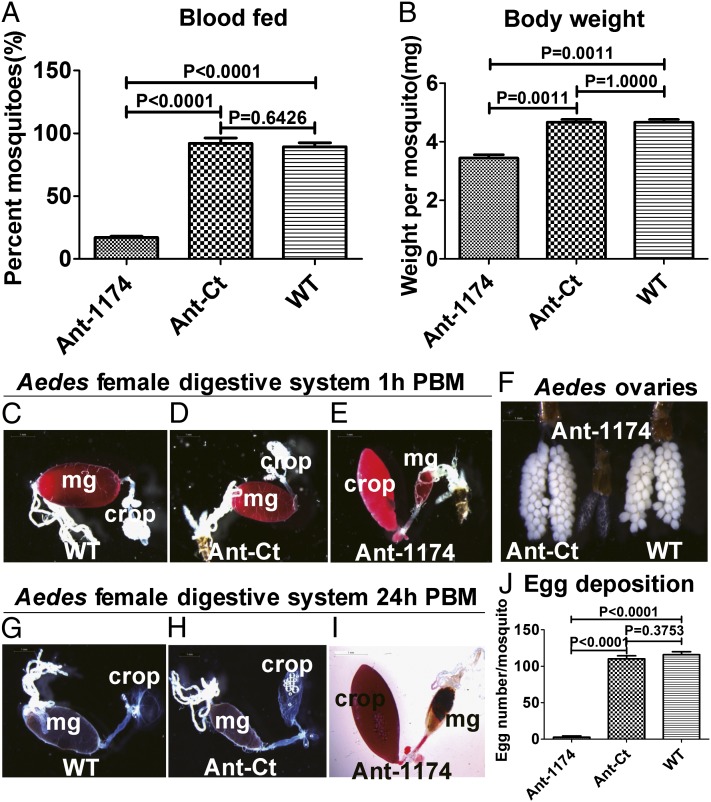
Next, we tested whether Ant-1174–treated female A. aegypti mosquitoes that did not exhibit the BAP could feed on blood. Only about 20% of these mosquitoes took blood, compared with over 80–90% of Ant-Ct–treated or WT-untreated females (Fig. 3A), and the weight per Ant-1174–treated mosquito was significantly lower than that of WT or Ant-Ct–treated mosquitoes at 0.5 h PBM (Fig. 3B). Examination of WT and Ant-Ct–treated female mosquitoes at 1.0 h PBM showed that the ingested blood bypassed the crop and was directed into the posterior midgut (Fig. 3 C and D). In contrast, blood was mostly present in the crop of the Ant-1174–treated female mosquitoes, indicating that blood was directed to the crop of these mosquitoes during feeding (Fig. 3E). Unlike WT and Ant-Ct–treated female mosquitoes at 24 h PBM (Fig. 3 G and H), most of the blood was retained in the crop of Ant-1174–treated mosquitoes (Fig. 3I). A similar phenotype was observed in Ant-1174–treated female A. gambiae mosquitoes (Fig. S3 C–E). Inhibition of miR-1174 in Aedes and Anopheles female mosquitoes by their respective Ant-1174s arrested egg development (Fig. 3F and Fig. S3F). These mosquitoes laid significantly fewer eggs than the WT and Ant-Ct–treated mosquitoes (Fig. 3J and Fig. S3G). We observed no abnormalities in sugar-feeding or absorption in female Aedes mosquitoes injected with the miR-1175 antagomir (Ant-1175) (Fig. S4 A–F). They fed normally on blood, had no blood in the crop, and laid the same number of eggs as WT and H2O-treated mosquitoes (Fig. S4 G–I). The lack of any adverse phenotypes in Ant-1175–treated mosquitoes serves as an additional confirmation of the specificity of Ant-1174 action. Taken together, these results provide evidence that miR-1174 is essential for proper blood intake and, consequentially, egg development in the female mosquito.
Fig. 3.
Effect of miR-1174 silencing in blood-fed A. aegypti female mosquitoes. (A and B) Mosquitoes that blood-fed post miR-1174 silencing. (A) Percent of mosquitoes for sucking blood. (B) Body weight post blood-feeding. (C–E) Isolated digestive systems from mosquitoes 1 h after blood-feeding. (C) Untreated WT. (D) Ant-Ct–injected. (E) Ant-1174–injected. mg, midgut. (F) Ovaries isolated from female mosquitoes injected with Ant-Ct, Ant-1174, or untreated WT. (G–I) Isolated digestive systems from mosquitoes 24 h after blood-feeding. (G) Untreated WT. (H) Ant-Ct–injected. (I) Ant-1174–injected. (J) Number of eggs per female mosquito injected with Ant-1174, Ant-Ct, or untreated WT. Error bars depict ±SEM.
Computational and in Vitro Analysis Indicates miR-1174 Targets Serine Hydroxymethyltransferase.
To identify the putative gene targets of miR-1174, we used five miRNA target prediction tools—Probability of Interaction by Target Accessibility (PITA) (19), miRanda (20), RNAhybrid (21), TargetScan (22), and an in-house program developed on the basis of the features proposed by Grimson et al. (23). To weigh for putative binding site conservation, we predicted miR-1174 targets using both A. aegypti and A. gambiae 3′UTRs. All programs were run locally, and the targets predicted by each of these programs were mapped against each other to determine the overlaps. A list of top candidate targets was produced on the basis of predictions by multiple algorithms and their conservation (Table S1). Targets that were either identified by all five programs in A. aegypti (AAEL002510 and AAEL012079) or in A. gambiae (AAEL001779 ortholog of AGAP005775), or by four different programs in both A. aegypti (AAEL005411 and AAEL010558) and their A. gambiae orthologs (AGAP003892 and AGAP000964), were shortlisted for further analysis. Following computational prediction, we assessed the 3′UTRs of the top five potential targets for their responses to miR-1174 using the Dual Luciferase Reporter Assay. The 3′UTR for each putative miR-1174 target was cloned downstream of the Renilla translational stop codon within the psiCheck-2 vector to produce 3′UTR-fused luciferase reporters. When transfected into Drosophila Schneider 2 (S2) cells along with miR-1174 mimic, the luciferase reporter containing the full-length 3′UTR of SHMT yielded less than 50% luciferase activity compared with the negative control mimic and “no mimic” control samples (Fig. 4A). Four other tested genes elicited no response (Fig. S5 B–E). Hence, of the top five candidate targets, only SHMT was validated as the miR-1174 target in vitro. Luciferase reporter assay was optimized using a synthetic miR-1174 sensor containing three repeat miR-1174 binding sites cloned downstream of the Renilla translational stop codon (Fig. S5A).
Fig. 4.
SHMT is a direct target of miR-1174. (A) miR-1174 directly targets AAEL002510 (SHMT) 3′UTR in in vitro Dual Luciferase Reporter Assay. Data represent the average percentage activity (∆ Fold Activity × 100) of triplicate samples. (B) miR-1174 silencing (Ant-1174) results in an increase of the SHMT transcript level. Injection of Ant-Ct has no effect on SHMT levels. (C) Injection of miR-1174 mimic (Mimic-1174) results in a decrease of the SHMT transcript level, similar to that of SHMT RNAi (dsSHMT). (D) Coinjection of Ant-1174 and dsSHMT results in a lower level of the SHMT transcript (Ant-1174/dsSHMT). Coinjection of Ant-1174 and control dsRNA (dsLuc) does not affect an elevated level of the SHMT transcript caused by miR-1174 silencing (Ant-1174). (B–D) qRT-PCR. Error bars in A–D depict ±SEM.
miR-1174 Regulates Expression of Serine Hydroxymethyltransferase in Vivo.
To determine the effects of miR-1174 on SHMT expression in vivo, we first assayed for the levels of the SHMT transcript and miR-1174 expression in different tissues. The SHMT transcript level in the gut was low relative to other tissues at every sampled time point (Fig. S6 A and C). This is in contrast to the elevated level of miR-1174 in the gut at the same time points of the female mosquito life cycle (Fig. S6 B and D). However, the SHMT transcript level significantly increased in this tissue of Ant-1174–injected mosquitoes (Fig. 4B), suggesting that miR-1174 controls the SHMT transcript level in vivo. To verify this hypothesis, we injected 100 pmol of miR-1174 mimic and observed a significant increase in the level of miR-1174 in the guts of these mosquitoes (Fig. S6E), whereas the level of miR-1175 did not change (Fig. S6F). Indeed, injection of the miR-1174 mimic caused a significant decrease in gut SHMT transcript level (Fig. 4C), further validating SHMT as an authentic target of miR-1174 in vivo.
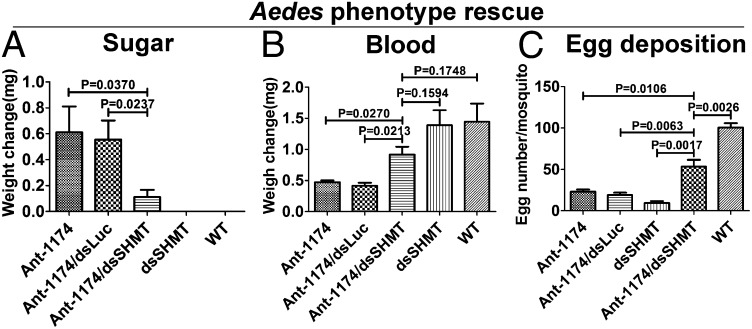
To further evaluate SHMT as the authentic miR-1174 target gene, we conducted phenotypic rescue experiments through the SHMT RNAi depletion in Ant-1174–treated mosquitoes. The RNAi of a physiologically relevant gene was expected to alleviate the adverse phenotypes caused by miR-1174 silencing. This approach has been successfully used in rescuing miRNA mutant phenotypes in Drosophila (24, 25). RNAi silencing of SHMT resulted in severe phenotypes manifested in the inability of female mosquitoes to normally digest blood, develop, and lay eggs. We then coinjected 100 pmol of Ant-1174 and 100 ng of dsSHMT into female A. aegypti mosquitoes at 12 h PE and maintained them for 3 d PIJ on a 10% sugar solution. The gut SHMT transcript level in these Ant-1174/dsSHMT mosquitoes was as low as that in untreated WT mosquitoes (Fig. 4D). The BAP was not observed in sugar-fed Ant-1174/dsSHMT mosquitoes. There was no weight gain in these mosquitoes, unlike those treated with Ant-1174 only (Fig. 5A); they exhibited normal behavior and were able to fly. SHMT RNAi also rescued the adverse post blood-feeding phenotypes caused by miR-1174 silencing. The Ant-1174/dsSHMT mosquitoes fed normally on blood, and their weight increased significantly post blood-feeding (Fig. 5B). No blood was observed in the crop of these mosquitoes, and they laid significantly more eggs than Ant-1174–treated mosquitoes (Fig. 5C). Thus, our data demonstrate that SHMT is the authentic miR-1174 target contributing to the adverse phenotypes observed in Ant-1174–treated female mosquitoes.
Fig. 5.
Rescue of phenotypes caused by miR-1174 silencing in female Aedes mosquitoes. (A) Mosquitoes were treated 12 h posteclosion, maintained on 10% sugar solution for 3 d, and then examined. Increase in weight over that of WT was plotted. (B) Mosquitoes treated as in A were blood-fed, and their weight was measured. Increase in weight was plotted. (C) Number of eggs per female mosquito from experimental treatments in B. Ant-1174, miR-1174 Antagomir; Ant-1174/dsLuc, coinjection of Ant-1174 with control Luciferase dsRNA; Ant-1174/dsSHMT, coinjection of Ant-1174 and dsSHMT; dsSHMT, injection of dsSHMT; and WT is untreated. Error bars in A–C depict ±SEM.
Discussion
Our results show that depletion of the mosquito- and gut-specific miRNA, miR-1174, results in severe defects linked to proper sugar absorption, fluid excretion, blood intake, and, consequently, egg maturation and survival in two important vectors of human disease, A. aegypti and A. gambiae. Using antagomir-based inhibition in A. aegypti and A. gambiae, this study represents a significant step toward defining regulatory roles of mosquito-specific miRNAs. Our work implicates that miR-1174 functions as a regulator of sugar absorption and blood intake in female mosquitoes by regulating the expression of SHMT. Depletion of miR-1174 in A. aegypti and A. gambiae female mosquitoes resulted in a dramatic bloated-abdomen phenotype, due to an extended fluid-filled crop, when maintained on a 10% sugar solution for 3 d postinjection. Females exhibiting this bloated-abdomen phenotype were unable to fly or feed on blood. Additionally, blood-fed miR-1174–depleted female mosquitoes displayed visually striking PBM phenotypes: blood was directed to the crop instead of the gut, and, as a result, primary follicle growth was drastically inhibited.
Using multiple miRNA target prediction algorithms, we identified SHMT as the physiological relevant miR-1174 target contributing to the miR-1174 depletion phenotypes. Dual Luciferase Reporter Assay using a luciferase reporter vector containing A. aegypti SHMT 3′UTR cotransfected along with miR-1174 mimic resulted in a decrease in Renilla luciferase activity in vitro, whereas SHMT RNAi rescue experiments recovered the miR-1174 depletion phenotype in vivo. SHMT catalyzes the interconversion of serine and glycine, with tetrahydrofolate (THF) serving as the one-carbon carrier in the de novo synthesis of deoxythymidine monophosphate (dTMP) (26, 27). dTMP serves as a precursor of deoxythymidine triphosphate (dTTP), the optimal dTTP cellular level of which is essential for normal replication of nuclear and mitochondrial DNA (28). Folate metabolism is regulated by iron availability, and, in humans, iron deficiency leads to many abnormalities, including intestinal malfunction. The iron-storage protein ferritin enhances SHMT mRNA translation rate and, thereby, de novo thymidine biosynthesis (29). Mosquitoes face the opposite task of removing massive amounts of iron from a blood meal to avoid its toxic effect. This is accomplished by converting iron to heme, which is rapidly excreted from the gut. Maintaining a low SHMT level in the mosquito gut is likely of critical importance to counterbalance the iron-stimulating effect. This may be achieved by the direct action of miR-1174 on the SHMT transcript, maintaining it at the level required for normal gut functions.
These experiments provide evidence that the lineage-specific miRNA, miR-1174, functions in anautogenous mosquito species to control gut functions essential for sugar absorption, blood intake, and digestion, and, as a consequence, ovary development in two important vectors of human disease. miR-1174 is present in the blood-feeding mosquito species, A. gambiae, A. stephensi, and A. aegypti and not found in the non-blood-feeding mosquito, T. amboinensis (17), further supporting that miR-1174 functions in a conserved mechanism essential for blood-feeding in anautogenous mosquito species. Moreover, miR-1175 has been identified in all four mosquito species, A. gambiae, A. stephensi, A. aegypti, and T. amboinensis (17). Due to the close proximity of miR-1174 and miR-1175 (Fig. 1A), it is possible that miR-1174 arose through a duplication event in the miR-1174/miR-1175 cluster. It remains unknown if miR-1174 was lost, evolved beyond recognition, or if a duplication event never occurred in T. amboinensis. However, our findings suggest that miR-1174’s essential role in blood-feeding and other gut functions placed a strict evolutionary pressure in blood-feeding mosquito species leading to miR-1174 conservation.
Materials and Methods
Mosquito Rearing.
Mosquito larvae were reared at 27 °C in water supplemented with a mixture of yeast and rat chow (1/1 ratio). Adult mosquitoes of the WT A. aegypti and A. gambiae were maintained at 27 °C and 80% humidity in cages with unlimited access to 10% sugar solution and water until blood-feeding. Three- to four-day-old female A. aegypti mosquitoes were blood-fed on white rats, and A. gambiae were blood-fed on White Leghorn chickens. Laboratory vertebrate animals were kept according to National Institutes of Health (NIH)-approved conditions at a certified facility at the University of California, Riverside. Mosquito tissues were dissected in the Aedes physiological solution (30).
RNA Isolation and Quantitative Real-Time PCR.
Total RNA were extracted using TRIzol Reagent (Invitrogen), and cDNA were synthesized using SuperScript II Reverse Transcriptase (Invitrogen). DNA were synthesized using Platinum PCR SuperMix High Fidelity (Invitrogen). Mature miRNA and mRNA cDNA were produced using the miScript II RT Kit (Qiagen). mRNA expression was analyzed quantitatively using the QuantiTect SYBR Green PCR kit (Qiagen), and mature miRNA expression was measured using the miScript SYBR Green PCR Kit (Qiagen).
Antagomir and dsRNA Treatments.
The dsRNA for serine hydroxymethyltransferase were synthesized using MEGAScript T7 (Ambion). The dsRNA for luciferase (dsLuc) were used as a negative control. Mosquitoes 12 h PE were injected with 2 µg of dsRNA in 0.3–0.5 μL distilled water. Transcript abundance was analyzed by means of qRT-PCR analysis, as described above. RNAi-treated mosquitoes were maintained on 10% (wt/vol) sucrose solution for 4 d and then given a blood meal. Transcript abundance was examined in the gut tissue isolated from RNAi-treated mosquitoes at 24 h PBM by means of qRT-PCR. RNAi depletion of luciferase (dsLuc) served as a control.
Antagomirs were purchased from Dharmacon and designed using the RNA module for custom single-stranded RNA synthesis available at www.dharmacon.com/rna/rna.aspx. Antagomirs were designed as previously described (10). Antagomir to miR-1174 was 5′ mA(*)mU(*)mGmGmGmUmAmUmUmAmAmGmUmAmGmAmU(*)mC(*)mU(*)mG(*)mA-Chl3′. Mosquitoes were anesthetized with CO2 12 h PE and microinjected into the thorax at a dose of 50 pmol per mosquito (200 μM in a volume of 0.25 μL). Mosquitoes were given a 4-d recovery period before phenotype analysis or blood-feeding.
Computational Prediction of microRNA Targets.
For the prediction of miR-1174 targets in A. aegypti and A. gambiae, we used five different target prediction programs. The features related to the miRNA/mRNA binding site interactions that these algorithms use were taken into account while selecting the programs. The readily available miRNA target prediction programs used for this analysis are PITA (19), miRanda (20), RNAhybrid (21), and TargetScan (22). In addition, we used a program that has been developed in-house. The algorithm for the “in-house” program is a relaxed implementation of the five features proposed by Grimson et al. (23): (i) perfect seed-matching; (ii) additional Watson–Crick base pairing in nucleotides 12–17 (complementary seed site); (iii) location in locally Adenine-Uracil (AU)-rich regions; (iv) sites in the first 15 nucleotides of the 3′ UTR are seemingly less effective; and (v) when long 3′ UTR’s are divided into quartiles, sites that reside within the first and last quartiles of the 3′UTR are more efficient. The program identifies all seed match sites and assigns scores on the basis of the features mentioned above. For example, one point is scored if the starting nucleotide of the seed region of a miRNA is beyond nucleotide 15 in the selected 3′UTR. For each nucleotide within the 3′ complementary motif (nucleotides 13–16) of a miRNA that matches the sequence on the targeted transcript, 0.25 points are scored (this includes Guanine-Uracil wobbles). If the seed region target resides inside the first or last quartile of the 3′UTR, another point is scored. Iteratively, 30 nucleotides upstream and downstream from the seed-matching site are scanned on the 3′UTR, and As or Us are registered and scored according to the following formula: 0.25/(distance from seed region edge). Thus, if the first nucleotide upstream or downstream is A or U, the score increases 0.25 points (0.25/1), whereas the score increases by 0.125 points (0.25/2) if the second one is an A or U, and so on, until all 30 nucleotides are analyzed. All points based on the different criteria are summed up, and the sites are ranked according to the scores—the higher the score, the more likely it is that the seed match is a putative miRNA target site. To account for the cross-species conservation, we identified A. aegypti genes that have orthologs in A. gambiae by reciprocal best BLAST. The 3′ UTRs of A. aegypti and A. gambiae genes were extracted using the information provided in their respective General Feature Format files. Either the mature miRNA sequence or the seed sequence was used, depending on the program requirements.
Cell Culture and Luciferase Assay.
Drosophila S2 cells (Invitrogen) were kept at 28 °C in Schneider Drosophila medium supplemented with 10% (vol/vol) heat-inactivated FBS (Gibco, Life Technologies) and 1× Antibiotic-Antimycotic (Gibco, Life Technologies). Luciferase constructs were made by inserting the A. aegypti putative target 3′UTR into the multiple cloning region located downstream of the Renilla translational stop codon within the psiCheck-2 vector (Promega). A “miR-1174 synthetic sensor” positive control was also produced by cloning a construct containing three sites reverse complementary to miR-1174 with four nucleotide linkers between each miR-1174 binding site into the multiple cloning region located behind the Renilla luciferase stop codon in the psiCheck-2 vector. 100 ng of psiCheck-2 reporters and synthetic Aedes miR-1174 miScript miRNA Mimic (Qiagen) or AllStars Negative Control siRNA (Qiagen) at a final concentration of 100 nm were cotransfected into Drosophila S2 cells using Attractene Transfection Reagent (Qiagen). A “no siRNA” treatment control was also used. Dual Luciferase Reporter Assay was performed 48 h posttransfection using the Dual Luciferase Reporter Assay System (Promega). Firefly luciferase in the psiCheck-2 Vector was used for normalization of Renilla luciferase expression. Treatments were made in triplicate, and transfections were repeated three times.
Statistical Analysis.
Data were analyzed with GraphPad using Student t tests. Probability values of less than 0.05 are considered significant. Data are presented as mean and the SEM.
Phenotypic Rescue Experiments.
Double-stranded RNA were synthesized following a method described previously (26). In brief, dsRNA to SHMT were synthesized using the MEGAscript kit (Ambion). Mosquitoes were anesthetized with CO2 at 12 h PE, and a mixture containing 2 μg/μL dsRNA and 200 μM antagomir was microinjected into the thorax at a volume of 0.5 μL. Double-stranded RNA for Luciferase (Luc) was used as a negative control. Mosquitoes were allowed to recover for 3–4 d before blood-feeding.
miRNA in Situ Hybridization.
Whole-mount in situ hybridizations were performed using a 5′ and 3′ digoxigenin (DIG)-labeled locked nucleic acid-modified DNA oligonucleotide (LNAs) complementary to miR-1174 (miRCURY LNA Detection probe, Exiqon). The miR-1174 LNA had the following sequence: 5′-ATGGGTATTAAGTAGATCTGA-3′. The Scramble-miR negative control LNA was provided by Exiqon. Female mosquito digestive systems were harvested at 72 h PE and fixed in 4% (vol/vol) paraformaldehyde at room temperature for 1 h with shaking. After 30 min, fixing solution was removed, and samples were thoroughly washed in PBST (1×PBS/0.1%Tween20). Prehybridizations were performed at 55°C for 2h with shaking in prewarmed hybridization buffer [50% (vol/vol) formamide, 5×SSC, 10% (vol/vol) Tween-20, 30μg/mL heparin]. Hybridization was performed at 55 °C with shaking overnight with 25 pmol LNA probe diluted 1:100 in prewarmed hybridization buffer. After hybridization, samples were thoroughly washed in prewarmed hybridization buffer multiple times over a 12-h period and then washed in PBST. Samples were incubated overnight at 4 °C with shaking in Anti-Digoxigenin–AP (Roche) diluted 1:1,500 in PBST. After washing, samples were incubated in alkaline phosphatase staining buffer (100 mM Tris, 150 mM NaCl, 1 mM MgCl2, pH 9.5) and developed using 1-Step nitro-blue tetrazolium chloride/5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt solution (Pierce) according to the manufacturer’s protocol.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R01 AI113729 (to A.S.R.), a scholarship (2010850541) from China Scholarship Council of Chinese Ministry of Education, and National Basic Research Program of China Grant 2012CB114602 (to S.L.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1416278111/-/DCSupplemental.
References
- 1.Attardo GM, Hansen IA, Raikhel AS. Nutritional regulation of vitellogenesis in mosquitoes: Implications for anautogeny. Insect Biochem Mol Biol. 2005;35(7):661–675. doi: 10.1016/j.ibmb.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Zhu J, Miura K, Chen L, Raikhel AS. Cyclicity of mosquito vitellogenic ecdysteroid-mediated signaling is modulated by alternative dimerization of the RXR homologue Ultraspiracle. Proc Natl Acad Sci USA. 2003;100(2):544–549. doi: 10.1073/pnas.0235695100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raikhel AS, et al. Molecular biology of mosquito vitellogenesis: From basic studies to genetic engineering of antipathogen immunity. Insect Biochem Mol Biol. 2002;32(10):1275–1286. doi: 10.1016/s0965-1748(02)00090-5. [DOI] [PubMed] [Google Scholar]
- 4.Hill CA, Kafatos FC, Stansfield SK, Collins FH. Arthropod-borne diseases: Vector control in the genomic era. Nat Rev Microbiol. 2005;3(3):262–268. doi: 10.1038/nrmicro1101. [DOI] [PubMed] [Google Scholar]
- 5.Lucas K, Raikhel AS. Insect microRNAs: Biogenesis, expression profiling and biological functions. Insect Biochem Mol Biol. 2013;43(1):24–38. doi: 10.1016/j.ibmb.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Djuranovic S, Nahvi A, Green R. miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science. 2012;336(6078):237–240. doi: 10.1126/science.1215691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466(7308):835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farh KK, et al. The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science. 2005;310(5755):1817–1821. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- 10.Bryant B, Macdonald W, Raikhel AS. microRNA miR-275 is indispensable for blood digestion and egg development in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2010;107(52):22391–22398. doi: 10.1073/pnas.1016230107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain M, Frentiu FD, Moreira LA, O’Neill SL, Asgari S. Wolbachia uses host microRNAs to manipulate host gene expression and facilitate colonization of the dengue vector Aedes aegypti. Proc Natl Acad Sci USA. 2011;108(22):9250–9255. doi: 10.1073/pnas.1105469108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osei-Amo S, Hussain M, O’Neill SL, Asgari S. Wolbachia-induced aae-miR-12 miRNA negatively regulates the expression of MCT1 and MCM6 genes in Wolbachia-infected mosquito cell line. PLoS ONE. 2012;7(11):e50049. doi: 10.1371/journal.pone.0050049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain M, Walker T, O’Neill SL, Asgari S. Blood meal induced microRNA regulates development and immune associated genes in the dengue mosquito vector, Aedes aegypti. Insect Biochem Mol Biol. 2013;43(2):146–152. doi: 10.1016/j.ibmb.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Zhang G, Hussain M, O’Neill SL, Asgari S. Wolbachia uses a host microRNA to regulate transcripts of a methyltransferase, contributing to dengue virus inhibition in Aedes aegypti. Proc Natl Acad Sci USA. 2013;110(25):10276–10281. doi: 10.1073/pnas.1303603110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winter F, Edaye S, Hüttenhofer A, Brunel C. Anopheles gambiae miRNAs as actors of defence reaction against Plasmodium invasion. Nucleic Acids Res. 2007;35(20):6953–6962. doi: 10.1093/nar/gkm686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mead EA, Tu Z. Cloning, characterization, and expression of microRNAs from the Asian malaria mosquito, Anopheles stephensi. BMC Genomics. 2008;9:244. doi: 10.1186/1471-2164-9-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S, Mead EA, Liang S, Tu Z. Direct sequencing and expression analysis of a large number of miRNAs in Aedes aegypti and a multi-species survey of novel mosquito miRNAs. BMC Genomics. 2009;10:581. doi: 10.1186/1471-2164-10-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krützfeldt J, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438(7068):685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 19.Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E. The role of site accessibility in microRNA target recognition. Nat Genet. 2007;39(10):1278–1284. doi: 10.1038/ng2135. [DOI] [PubMed] [Google Scholar]
- 20.Enright AJ, et al. MicroRNA targets in Drosophila. Genome Biol. 2003;5(1):R1. doi: 10.1186/gb-2003-5-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kruger J, Rehmsmeier M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res. 2006;34:W451–W454. doi: 10.1093/nar/gkl243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115(7):787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 23.Grimson A, et al. MicroRNA targeting specificity in mammals: Determinants beyond seed pairing. Mol Cell. 2007;27(1):91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varghese J, Cohen SM. microRNA miR-14 acts to modulate a positive autoregulatory loop controlling steroid hormone signaling in Drosophila. Genes Dev. 2007;21(18):2277–2282. doi: 10.1101/gad.439807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyun S, et al. Conserved MicroRNA miR-8/miR-200 and its target USH/FOG2 control growth by regulating PI3K. Cell. 2009;139(6):1096–1108. doi: 10.1016/j.cell.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 26.Szebenyi DM, Musayev FN, di Salvo ML, Safo MK, Schirch V. Serine hydroxymethyltransferase: Role of glu75 and evidence that serine is cleaved by a retroaldol mechanism. Biochemistry. 2004;43(22):6865–6876. doi: 10.1021/bi049791y. [DOI] [PubMed] [Google Scholar]
- 27.Schirch V, Szebenyi DM. Serine hydroxymethyltransferase revisited. Curr Opin Chem Biol. 2005;9(5):482–487. doi: 10.1016/j.cbpa.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Anderson DD, Quintero CM, Stover PJ. Identification of a de novo thymidylate biosynthesis pathway in mammalian mitochondria. Proc Natl Acad Sci USA. 2011;108(37):15163–15168. doi: 10.1073/pnas.1103623108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oppenheim EW, Adelman C, Liu X, Stover PJ. Heavy chain ferritin enhances serine hydroxymethyltransferase expression and de novo thymidine biosynthesis. J Biol Chem. 2001;276(23):19855–19861. doi: 10.1074/jbc.M100039200. [DOI] [PubMed] [Google Scholar]
- 30.Roy SG, Hansen IA, Raikhel AS. Effect of insulin and 20-hydroxyecdysone in the fat body of the yellow fever mosquito, Aedes aegypti. Insect Biochem Mol Biol. 2007;37(12):1317–1326. doi: 10.1016/j.ibmb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.