Significance
We tested the hypothesis that gelsolin (GSN) plays an important role in gynecological chemoresistance through the following: We provided strong evidence in support of GSN as an important etiologic factor in chemoresistance in vitro. We also determined the mechanism by which GSN exerts its prosurvival action. Our findings also suggest that the application of C-terminal GSN may represent a new therapeutic strategy for chemoresistant gynecologic cancer. We have also validated our in vitro findings with a clinical investigation that determines the relationship between GSN expression and cis-Diammine dichloroplatinium (II) sensitivity in human ovarian tumor. These findings agree with the notion that GSN plays a key role in the regulation of gynecological cell fate as reflected in chemoresistance.
Keywords: ovarian cancer, cervical cancer
Abstract
Chemoresistance is a major hurdle in cancer treatment. Down-regulation of apoptosis pathways is one of the key determinants for chemoresistance. Here, we report higher gelsolin (GSN) levels in chemoresistant gynecological cancer cells compared with their sensitive counterparts. cis-Diammine dichloroplatinium (II) (CDDP)-induced GSN down-regulation is associated with its cleavage and apoptosis. Although the C-terminal GSN fragment (C-GSN) sensitized chemoresistant cells to CDDP, intact GSN and its N-terminal fragment (N-GSN) attenuated this response. GSN silencing also facilitated CDDP-induced apoptosis in chemoresistant cells. In contrast, intact GSN (I-GSN) was prosurvival in the presence of CDDP through a FLICE-like inhibitory protein (FLIP)-Itch interaction. This interaction was colocalized in the perinuclear region that could be dissociated by CDDP in sensitive cells, thereby inducing FLIP ubiquitination and degradation, followed by apoptosis. In resistant cells, GSN was highly expressed and CDDP failed to abolish the I-GSN-FLIP-Itch interaction, resulting in the dysregulation of the downstream responses. In addition, we investigated the association between GSN expression in ovarian serous adenocarcinoma and progression free survival and overall survival, as well as clinical prognosis. GSN overexpression was significantly associated with more aggressive behavior and more cancer deaths and supported our hypothesis that high GSN expression confers chemoresistance in cancer cells by altering the GSN-FLIP-Itch interaction. These findings are in agreement with the notion that GSN plays an important role in the regulation of gynecological cell fate as reflected in dysregulation in chemosensitivity.
Cell-fate decision is the underpinning of cancer-therapy effectiveness, which is dependent on chemosensitivity. cis-Diammine dichloroplatinum (II) (cisplatin or CDDP) is a widely used chemotherapeutic agent (1) for gynecological, testicular, lung, and head-and-neck cancer. Clinical evidence supports that the platinum-taxane combination for ovarian cancer (OVCA) remains the standard regimen of choice (2). CDDP-based cancer chemotherapy, however, is often limited by acquired or intrinsic chemoresistance. OVCA patients are clinically divided into “resistant,” “partially sensitive,” and “sensitive” to platinum according to the progression-free interval (PFI) of <6 mo, 6–12 mo, and >12 mo (3, 4). This concept is supported by evidence of lower response rates to subsequent platinum retreatment in patients with PFI < 12 mo compared with those with PFI > 12 mo.
Cellular mechanisms potentially contributing to CDDP resistance include changes in cellular drug uptake and accumulation, drug detoxification, apoptosis inhibition, and repair of the DNA adducts. Inability of the cells to undergo apoptosis is critical in CDDP resistance (5), and dysregulation of proapoptotic (6) and antiapoptotic (7–9) pathways plays an important role in chemoresistance.
Gelsolin (GSN) controls actin dynamics and plays a role as a multifunctional regulator for cell metabolism and survival (10, 11). GSN participates in multiple important cellular signaling for motility, apoptosis, proliferation, differentiation, epithelial mesenchymal transition (12), and carcinogenesis phenotypes (13, 14). GSN plays roles as both effecter and inhibitor of apoptosis, which underlines its association in a wide variety of cancer types.
We screened potential proteins interacting with GSN using a yeast-two-hybrid system combined with site-directed mutagenesis. A panel of proteins involving drug resistance and antiapoptosis pathways was found to potentially interact with GSN. These interactions could render a mobile docking hypothesis to affect critical functional pathways in chemoresistance. Elucidation of GSN cross-talks with intracellular intermediates is important to better understand the molecular and cellular mechanisms of chemoresistance in gynecological cancer.
We have demonstrated the role of Fas-associated death domain-like interleukin-1β–converting enzyme (FLICE)-like inhibitory protein (FLIP) in CDDP resistance in cervical cancer cells (8). FLIP, a Fas-associated death domain (FADD)-binding inhibitor of apoptosis, exists as 55-kDa (FLIPL) and 28-kDa (FLIPS) splice variants. CDDP-induced FLIP degradation in chemosensitive cells involves FLIP-p53-Itch interaction and its translocation to the cell membrane for Itch-mediated FLIP ubiquitination and proteasomal degradation (15, 16).
To date, there has been no clinical investigation to examine the relationship between GSN and cancer progression in OVCA. In the present study, we investigated GSN expression in human ovarian cancerous tumors, its influence on patient survivals, and its association with PFI. Moreover, we compared the GSN expression between chemosensitive and chemoresistant cell lines of ovarian and cervical cancer. The molecular mechanisms governing the role of GSN and FLIP in modulating cancer chemosensitivity were also examined. Our clinical results are complemented with in vitro findings illustrating the important role GSN plays in the regulation of gynecological cell fate as reflected in dysregulation in chemosensitivity. They support the hypothesis that GSN forms a complex with FLIP and Itch and stabilizes FLIP in a nonstress state whereas CDDP dissociates GSN from the complex in chemosensitive cells, thereby facilitating FLIP ubiquitination and degradation, caspase-3 activation, and GSN cleavage.
Results
GSN Expression and Cancer Progression in Vivo.
A total of 102 patients diagnosed with ovarian serous adenocarcinoma were recruited into the study. Nine patients were each at stages I and II (4.4%), 72 at stage III (70.6%), and 12 at stage IV (11.8%). During follow-up (median = 33.4 mo; range from 1.6 mo to 225.9 mo), 71 patients (69.6%) developed progressive disease, and 51 patients (50.0%) died. The relationship between patient demographic variables and ovarian cancer progression was analyzed (Table S1).
The association between GSN expression at diagnosis and the treatment outcome was examined (Table 1). Notably, GSN overexpression was associated with tumor progression (P = 0.008), risk of cancer death (P = 0.001), and extrapelvic peritoneal nodules when diseases progressed (P = 0.012). However, there was no correlation between GSN expression and age, stage, tumor differentiation, or PFI of 6 mo. High GSN expression was significantly correlated with tumor progression (P = 0.008) and PFI ≤ 6 mo (P = 0.042) in 84 late-stage but not in 18 early-stage patients.
Table 1.
Correlation between GSN expression and tumor progression in all patients (n = 102), early-stage and late-stage subgroups
| GSN expression | ||||
| Characteristics: All patients | Total | No. (%) | Yes (%) | P value |
| Age (years) | 0.362 | |||
| ≤55 | 56 | 15 (26.8) | 41 (73.2) | |
| >55 | 46 | 10 (21.7) | 36 (78.2) | |
| FIGO stage | 0.465 | |||
| Early stage (I plus II) | 18 | 5 (27.8) | 13 (72.2) | |
| Late stage (III plus IV) | 84 | 20 (23.8) | 64 (76.2) | |
| Differentiation | 0.367 | |||
| Poor | 54 | 12 (22.2) | 42 (77.8) | |
| Well and moderate | 48 | 13 (27.1) | 35 (72.9) | |
| Tumor progression | 0.008** | |||
| No | 31 | 13 (41.9) | 18 (58.1) | |
| Yes | 71 | 12 (16.9) | 59 (83.1) | |
| Progression-free interval | 0.138 | |||
| >6 mo | 62 | 18 (29.0) | 44 (71.0) | |
| ≤6 mo | 40 | 7 (17.5) | 33 (82.5) | |
| Tumor-progression site | 0.012* | |||
| Extrapelvis | 46 | 11 (23.9) | 35 (76.1) | |
| Pelvis | 18 | 1 (7.6) | 17 (94.4) | |
| Others | 7 | 0 (0.0) | 7 (100.0) | |
| Vital status | 0.001** | |||
| Alive | 51 | 18 (35.3) | 33 (64.7) | |
| Death | 51 | 7 (13.7) | 44 (86.3) | |
| Early-stage tumor progression | 0.5 | |||
| No | 9 | 3 (33.3) | 6 (66.7) | |
| Yes | 9 | 2 (22.2) | 7 (77.8) | |
| Early-stage progression-free interval | 0.172 | |||
| >6 mo | 15 | 3 (20.0) | 12 (80.0) | |
| ≤6 mo | 3 | 2 (66.7) | 1 (33.3) | |
| Late-stage tumor progression | 0.008** | |||
| No | 22 | 10 (45.5) | 12 (54.5) | |
| Yes | 62 | 10 (16.1) | 52 (83.9) | |
| Late-stage progression-free interval | 0.042* | |||
| >6 mo | 47 | 15 (31.9) | 32 (68.1) | |
| ≤6 mo | 37 | 5 (13.5) | 32 (86.5) | |
Data are given as n (%). Data were analyzed by χ2 test and Fisher’s exact method. *P < 0.05, **P < 0.01.
GSN Expression and Patient Survivals.
The long-term survival curves are illustrated in Fig. 1 A and B. GSN-positive patients had significantly poorer overall survival (OS) and progression-free survival (PFS) than GSN-negative patients (P = 0.032 and P = 0.035, respectively). The median times to progression and death in the GSN-positive group were 1.4 y and 3.8 y, respectively. The hazard ratio for the progression risk was 1.79 [95% confidence interval (CI), 1.07–3.01; P = 0.03] and for the death risk was 1.97 (95% CI, 1.06–3.66; P = 0.03) compared with the GSN-negative group.
Fig. 1.

Overall survival (OS) and progression-free survival (PFS) curves of all-stage patients and subgroups with serous ovarian cancer, stratified according to GSN expression. High GSN expression significantly correlated with the long-term OS and PFS in all patients (A and B), subgroup patients with PFI ≤ 12 mo (n = 50) (C and D), and late-stage subgroup patients (n = 84) (E and F). Among late-stage subgroup patients with PFI > 12 mo, the OS (G) was significantly poorer in those with GSN-positive tumors than those with GSN-negative tumors. Although the negative impact of GSN overexpression on PFS (H) was found in this subgroup, it was not statistically significant.
Among patients with PFI ≤ 12 mo (n = 50), the GSN-positive subgroup had significantly shorter OS (Fig. 1C) and PFS (Fig. 1D) than the GSN-negative subgroup (P = 0.041 and P = 0.028, respectively). We also observed a significantly negative association of GSN overexpression with OS (Fig. 1E) and PFS (Fig. 1F) among late-stage subgroup patients (P = 0.010 and P = 0.015, respectively). Among the late-stage subgroup with PFI > 12 mo, significantly shorter OS was found in those with GSN-positive (Fig. 1G) than GSN-negative tumors (P = 0.049). Although the negative impact of GSN overexpression on PFS was observed in the late-stage subgroup with PFI > 12 mo (Fig. 1H) or > 6 mo (Fig. S1), it did not achieve statistical significance (P = 0.076 and P = 0.080, respectively).
CDDP-Induced Apoptosis in Cancer Cells Is Associated with Decreased Intact GSN Protein Content.
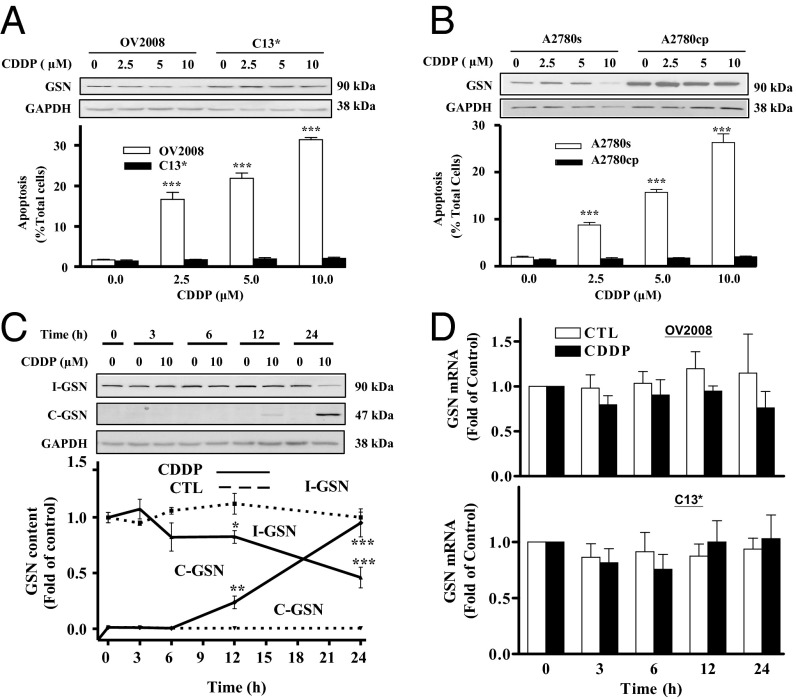
To examine the influence of CDDP on GSN level, chemosensitive OVCA (A2780s) and cervical carcinoma (CECA; OV2008) cells and their resistant variants (A2780cp and C13*, respectively) were cultured with CDDP (0-10 μM; 24 h). The chemoresistant cells expressed higher intact GSN (I-GSN) protein than their chemosensitive counterpart (Fig. 2 A and B). CDDP decreased I-GSN content in CDDP-sensitive cells but not in resistant cells. In addition, CDDP induced concentration-dependent apoptosis in sensitive cells but not in resistant cells (P < 0.001 vs. P > 0.05) (Fig. 2 A and B). The GSN expression profile induced by CDDP in chemosensitive cells is time-dependent (Fig. 2C). GSN mRNA levels were not affected by CDDP treatment in both cell lines (Fig. 2D), suggesting that changes in the GSN in chemosensitive cells did not result from transcriptional regulation.
Fig. 2.
CDDP-induced apoptosis in cancer cells is associated with decreased intact GSN (I-GSN) protein content. (A and B) CDDP induced GSN down-regulation and apoptosis. CECA cells (A) (OV2008 and C13*) and OVCA cells (B) (A2780s and A2780cp) were cultured with CDDP (0-10 μM; 24 h) and analyzed for I-GSN and GAPDH contents by Western blotting (Upper). Apoptosis was determined morphologically by Hoechst 33258 staining (Lower; ***P < 0.001 vs. control). (C) CDDP induced I-GSN down-regulation and enhanced GSN protein cleavage into C-GSN in a time-dependent manner. OV2008 was cultured with CDDP (0-10 µM; 0–24 h) and analyzed for I-GSN, C-GSN, and GAPDH contents by Western blotting. (D) CDDP failed to alter GSN mRNA abundance in sensitive CECA cells. OV2008 was cultured with CDDP (0-10 µM; 0–24 h) and analyzed for GSN mRNA contents by RT-PCR. CTL, control.
Chemoresistant Cancer Cells Exhibit Higher I-GSN and Lower C-Terminal GSN Content.
To further explore the association of GSN isoforms to chemoresistance, the above studies were extended to examine the changes of endogenous I-GSN and C-terminal GSN (C-GSN) contents in chemosensitive (OV2008 and A2780s) and resistant (C13* and A2780cp, respectively) cells following CDDP treatment (0-10 μM, 24 h). CDDP promoted the conversion of I-GSN into cleaved GSN (C-GSN) in the sensitive but not resistant cells (Fig. S2A).
We previously demonstrated that CDDP activates caspases in chemosensitive cells (8). Although caspase-3 inhibitor DEVD pretreatment (0-20 μM, 3 h) alone had no effect on I-GSN levels, it significantly attenuated the CDDP-induced cleavage of I-GSN (Fig. S2B) and apoptosis (P < 0.01) (Fig. S2C). Together, it suggests that caspase-3 activation following CDDP challenge may be responsible for the decrease in I-GSN content and in part for the CDDP sensitivity.
GSN Is Involved in the Regulation of CDDP Responsiveness in Cancer Cells.
To further investigate whether GSN plays a role in CDDP resistance, C13* and A2780cp were transfected with either I-GSN or control small interfering RNA (siRNA; 50–200 nM) and treated with CDDP (0–10 µM). I-GSN siRNA significantly down-regulated I-GSN content and sensitized the cells to CDDP-induced apoptosis (Fig. 3 A and B) (P < 0.001). These findings suggest that I-GSN plays essential roles in CDDP chemoresistance and imply that down-regulation of GSN may be an important mechanism to sensitize chemoresistant cancer cells to CDDP. Because caspase-3 cleaves GSN between residues Asp352 and Gly353, resulting in the generation of N-terminal (N-GSN) and C-GSN fragments, we constructed the N- and C-GSN and the cleavage site mutant GSN (M-GSN; DQTN352S in place of DQTD352G GSN sequence) plasmids in the pCMVtaq5C vector. OV2008 cells and their resistant variants (C13*) were transiently transfected with different GNS fragments or the empty vector plasmids and treated with CDDP (0-10 µM; 24 h) to test whether these GSN fragments differentially regulate CDDP sensitivity. Although expression of different GSN constructs (Fig. 3 C and D) alone had no effects on the baseline apoptotic cell death, CDDP-induced apoptosis in chemosensitive OV2008 cells was enhanced in the presence of C-GSN (P < 0.001) (Fig. 3 C and E) but was not significantly affected by N-GSN, I-GSN, and M-GSN (P > 0.05) (Fig. 3C). CDDP was ineffective in inducing apoptosis in the C13* cells transfected with PCMV, N-GSN, I-GSN, or M-GSN whereas C-GSN overexpression sensitized them to CDDP (P < 0.001) (Fig. 3 D and F).
Fig. 3.
GSN regulates CDDP-induced apoptosis in cancer cells. GSN silencing facilitated CDDP-induced apoptosis in C13* cells (A) and A2780cp cells (B). Cells were transfected with GSN or control siRNA (50-200 nM; 24 h) and then cultured with CDDP (0-10 μM; 24 h). I-GSN, C-GSN, and GAPDH contents were assessed by Western blotting (Upper), and apoptosis was determined morphologically by Hoechst 33258 staining (Lower; ***P < 0.001 vs. control; n = 3). Expression of C-terminal GSN (C-GSN; 0–2 µg, 24 h) facilitated CDDP-induced apoptosis [0 and 10 µM; 24 h; Lower; ***P < 0.001 (vs. control); n = 3] in both OV2008 (C and E) and C13* (D and F) whereas full-length (I-GSN; 0–2 µg, 24 h), N-terminal GSN (N-GSN; 0–2 µg, 24 h) and caspase cleavage mutant (M-GSN, 0–2 µg, 24 h) attenuated this response in OV2008 cells (C).
CDDP Attenuates FLIP-GSN Colocalization at OV2008 but Not C13* Chemoresistant Lines.
We further analyzed the possibility that the antiapoptotic action of GSN may be mediated by FLIP. Moreover, CDDP down-regulates FLIP and induces apoptosis in chemosensitive but not resistant gynecologic cancer cells (7) by facilitating the interaction between FLIP, p53 and Itch, FLIP ubiquitination, and proteasomal degradation (15). Considering the role of FLIP in CDDP resistance in OVCA (7, 15) and to investigate whether GSN interacts with FLIP and Itch and whether such a complex plays a role in chemoresistance, OV2008 and C13* cells were transfected with I-GSN (2 μg; 24 h), infected [multiplicity of infection (MOI) = 25; 24 h] with adenoviral V5- FLIPL (Fig. 4A) and V5- FLIPS (Fig. 4B), and treated with CDDP (0-10 µM; 0–24 h). Using coprecipitation, GSN-FLIP and GSN-Itch interactions were evident in both sensitive and resistant cells in the absence of CDDP. CDDP decreased these interactions in a time-dependent manner only in chemosensitive cells (Fig. 4 A and B, Upper). FLIP and GSN binding analysis demonstrated the same trend in the absence and presence of CDDP (Fig. 4 A and B, Lower).
Fig. 4.
CDDP attenuates the GSN-FLIP-Itch interaction in chemosensitive but not resistant counterparts. The FLIP-GSN-Itch interaction was attenuated by CDDP in chemosensitive (OV2008) but not resistant (C13*) cells as detected by immunocoprecipitation. Cells were transfected with I-GSN (2 μg; 24 h), infected (MOI = 25; 24 h) with either adenoviral V5-FLIPL, (A) or V5-FLIPS (B) and cultured with CDDP (0-10 μM; 0–24 h). Cell lysates were immunoprecipitated with IgG (control; lanes 1 and 5) or corresponding antibody, as indicated. Protein–protein interaction was determined by immunoprecipitation–Western blots (IP-Western). GSN and V5-FLIP immunoprecipitates were immunoblotted [IP: GSN, WB: V5 and Itch; IP: V5-tagged FLIPL or FLIPS, WB: GSN and Itch (A and B, Upper)].
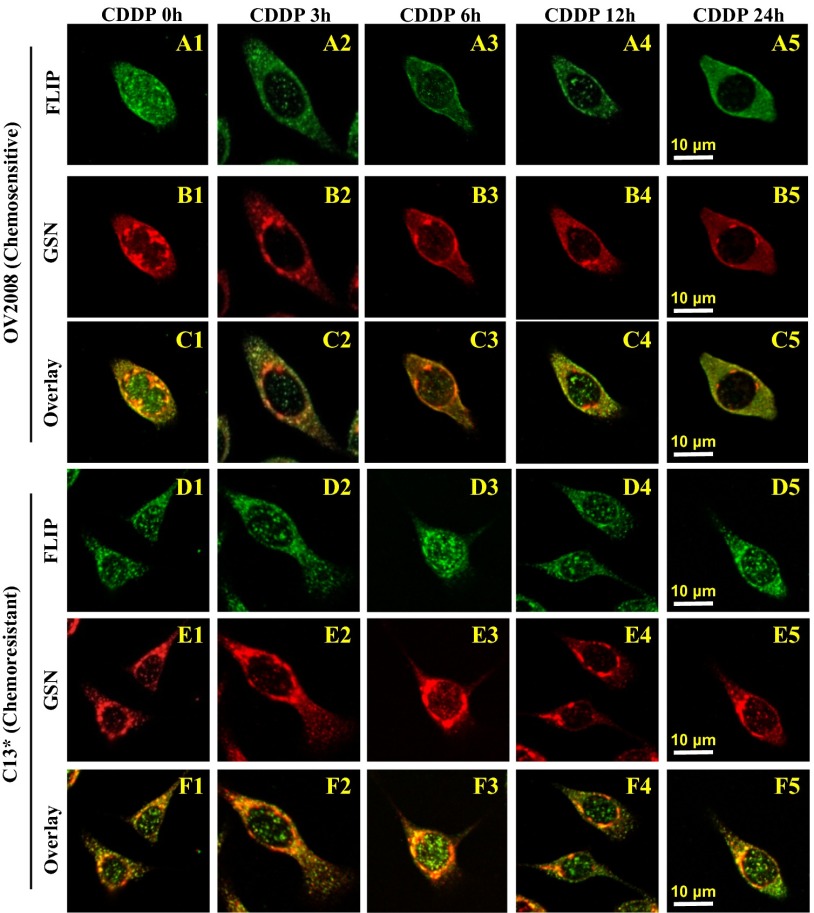
The relationship between GSN and FLIP was further examined by immunofluorescence when OV2008 and C13* cells were treated with CDDP (2.5 µM, 0–24 h) (Fig. 5). In OV2008 cells treated with DMSO (control), both cytoplasm and nucleus showed clusters of FLIP immunoreactivity (Fig. 5A1). CDDP resulted in nucleus FLIP depletion with its cytoplasmic redistribution by 12 h (Fig. 5 A1–A5). In contrast, C13* cells exhibited persistent nuclear FLIP localization (Fig. 5D1) and were unaffected by the CDDP treatment (Fig. 5 D1–D5). OV2008 cells showed nuclear GSN staining clustering near the nuclear membrane, and the intensity decreased with time when exposed to CDDP (Fig. 5 B1–B5). In contrast, GSN was localized in the perinuclear region of the cytoplasm of C13* cells but independent of CDDP treatment (Fig. 5 E1–E5). The FLIP-GSN colocalization was primarily detected in the nucleus near the nuclear membrane in OV2008 cells (Fig. 5C1) and was markedly decreased with CDDP in a time-dependent manner (Fig. 5 C1–C5) whereas it was prominent in the perinuclear region of the cytoplasm in C13* cells, with signal intensity remaining high while peaking at 3–6 h of CDDP treatment (Fig. 5 F1–F5). These results suggest that GSN-FLIP interaction in the perinuclear region of the cytoplasm may be an important factor in conferring resistance in cancer cells to CDDP.
Fig. 5.
CDDP attenuates FLIP-GSN colocalization at OV2008 but not C13* cells. Confocal imaging of double-stained FLIP and GSN in OV2008 and C13* cells. FLIP (A1) distributed throughout the cytoplasm and nucleus in OV2008 treated with DMSO (control). CDDP treatment (0–2.5 μM) induced relocation of FLIP to the cytoplasm from 3 h to 24 h (A2–A5). GSN was detected in the perinuclear region (B1) and decreased over time in the presence of CDDP (B2–B5). FLIP and GSN nuclear colocalization was not detected in OV2008 cells in the absence (C1) or presence (C2–C5) of CDDP, but they were colocalized in the perinuclear area and decreased over time with CDDP (C2–C5). In resistant C13* cells, although perinuclear and nuclear FLIP (D1) and perinuclear of GSN (E1) were extensively detected, they were not affected by CDDP (D2–D5 and E2–E5, respectively). FLIP-GSN was found to be colocalized in the perinuclear area (F1) independent of the CDDP treatment (F2–F5). Scale bars in A5 and D5 apply to A1–C5 and D1–F5, respectively (representative of total of 65 cells for each treatment group, n = 3).
Discussion
In this paper, we observed that the OVCA patients with GSN overexpression had cancer progression and shorter survivals after first-line platinum-based regimens, indicating that elevated GSN expression is an important tumor progression marker of poor long-term survival. This finding was also demonstrated in chemoresistant cells derived from gynecologic cancers (OVCA and CECA). The resistant cells exhibited higher endogenous GSN expression than their chemosensitive counterparts. In the nonstress state, GSN formed a complex with FLIP and Itch, which could be attenuated by CDDP in chemosensitive cells, resulting in FLIP degradation, caspase-3–mediated GSN cleavage, and apoptosis. In chemoresistant cells, CDDP failed to alter the GSN-FLIP-Itch interaction, resulting in the attenuation of the downstream responses to CDDP and protection of the cells from cytotoxic insults. Based on these discoveries, we propose that GSN regulates cancer chemosensitivity through apoptosis modulation and thus could serve as a therapeutic target for human gynecologic cancers.
GSN plays important roles in apoptosis regulation, which is highly associated with chemotherapy-induced cell death (17–19). To our knowledge, the present clinical study is the first report to investigate GSN expression by immunohistochemistry (IHC) analysis in OVCA specimens and their potential applications for predicting long-term clinical outcomes. Our results indicated that GSN overexpression was significantly associated with more aggressive behavior and increased cancer deaths in serous OVCA. Specifically in the late-stage subgroup, GSN overexpression was significantly correlated with the clinically defined platinum resistance based on PFI at 6 mo. This observation is consistent with a prognostic significance of GSN expression in non-small cell lung cancers (20) and oral cancer (21). Cellular GSN levels were found to be an important factor for tumor recurrence in high-grade tumors (22). Furthermore, GSN expression could be predictive of the patient subgroup with more cancer-specific deaths in the late-stage group whose PFI was >12 mo. These clinical findings are compatible with the observation that chemoresistant gynecological cancer cells exhibited higher endogenous GSN expression than their chemosensitive counterparts in vitro.
Evidence indicates that longer PFI corresponds to better responses to recurrence therapy in OVCA (23). The development of platinum resistance is complex and dynamic (3). Therefore, development of new early predictors for platinum resistance in conjunction with personalized interventions is required to improve patient outcome. Our observation that GSN was highly expressed in platinum-resistant cell lines is consistent with the positive associations between GSN overexpression and aggressive or potentially progressive OVCA tumors. High GSN expression at diagnosis may provide predicting value of unfavorable outcome. This marker could prompt early individualized therapies, such as combination chemotherapy with an antiangiogenic agent as first-line or maintenance therapy (24), dose-dense first-line chemotherapy (25), or paclitaxel maintenance chemotherapy (26) to prolong PFI and to improve long-term prognosis. Its clinical applications may be important not only for patient groups with PFI ≤ 12 mo, but also for late-stage patients with PFI > 12 mo.
We also found that CDDP-induced apoptosis in chemosensitive cells could be rescued by overexpression of GSN and that CDDP resistance could be overcome by GSN down-regulation. However, full-length GSN has also been reported to enhance apoptotic activity in MCF-7 cells (27). The mechanism underlying the differential responses among different cancer types in apoptosis responses remains to be further investigated.
GSN is a caspase-3 substrate (17). We demonstrated that GSN could be cleaved in chemosensitive CECA cells but not in chemoresistant cells where apoptosis was absent or minimal, suggesting that the cleavage of GSN may be a determinant of chemosensitivity. More importantly, this study showed the proapoptotic role of C-GSN where overexpression of C-GSN not only enhanced apoptosis in chemosensitive cells, but also sensitized chemoresistant cells to CDDP-induced apoptosis. These observations raise the interesting possibility that C-GSN expression may be a novel therapeutic strategy for chemoresistant cancers. However, the mechanism(s) by which C-GSN facilitates CDDP-induced apoptosis in chemoresistant cells remains unclear. Although the notion that C-GSN interferes with GSN-FLIP-Itch interaction is intriguing, it is not supported by the present findings: (i) CDDP-induced FLIP degradation in sensitive cells precedes the increased C-GSN, and (ii) overexpression of C-GSN had no effect on FLIP-GSN and FLIPs-Itch interactions irrespective of the presence of CDDP (Fig. S3), suggesting that C-GSN does not act by interfering with GSN-FLIP-Itch binding.
FLIP, a key antiapoptotic regulator, is down-regulated in chemosensitive but not in chemoresistant cells (7) and interacts with the E-3 ligase Itch in response to CDDP, resulting in its ubiquitination and proteasomal degradation and apoptosis (15, 16). Our immunofluorescence and immunoprecipitation results showed that overexpression of GSN in chemosensitive cells leads to chemoresistance, possibly through formation of a FLIP-GSN complex, thus preventing FLIP or GSN from being degraded and initiation of apoptosis. The interaction may prevent CDDP-induced FLIP ubiquitination and degradation in these cells. This study presents the first report, to our knowledge, on the interaction of GSN and FLIP in chemoresistant but not chemosensitive cells under CDDP challenge. Thus, this study suggests that GSN may be an important determinant of chemoresistance by sequestering FLIP and preventing it from ubiquitination and proteasomal degradation (15).
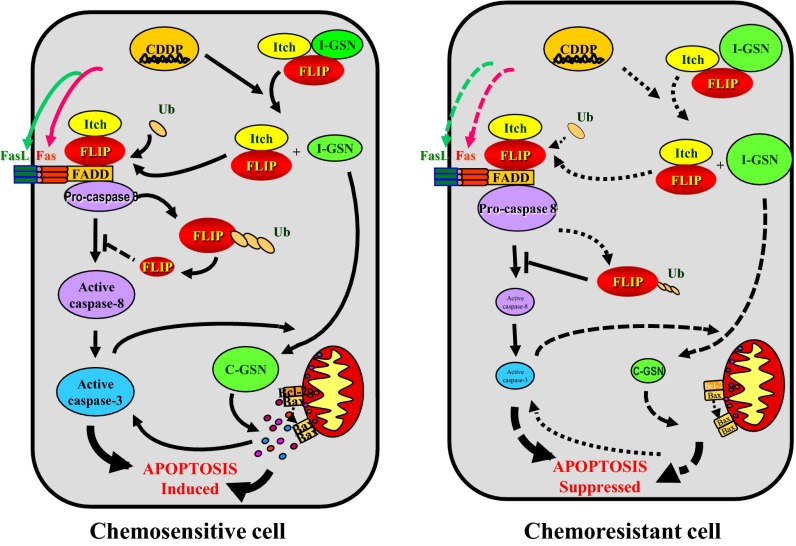
In conclusion, we examined GSN expression in OVCA patients and its association with chemoresistance. This association was further confirmed by analyzing the endogenous GSN level in paired chemosensitive and resistance lines derived from gynecologic cancer cells. We found that GSN overexpression conferred chemoresistance in human OVCA and CECA cells by suppressing apoptosis induced by chemotherapeutic agents. These findings support our hypothesis that GSN forms a complex with FLIP and Itch and stabilizes FLIP in the nonstress state whereas CDDP would dissociate GSN from the complex in chemosensitive cells, thereby facilitating FLIP ubiquitination and degradation, and caspase-3 activation and GSN cleavage. In chemoresistant cells, CDDP fails to alter the GSN-FLIP-Itch interaction, resulting in the dysregulation of the downstream responses to CDDP (Fig. 6). Further, we found that GSN fragments could modulate CDDP sensitivity in cancer cells, which may lead to novel therapeutic strategies for chemoresistant cancers.
Fig. 6.
A hypothetical model illustrating the role and regulation of GSN in the control of chemosensitivity in cancer cells. In nonstress state, GSN forms a complex with FLIP and Itch. CDDP leads to the dissociation of GSN from the GSN-FLIP-Itch complex in sensitive cells, thereby inducing FLIP ubiquitination and degradation, caspase-8 and -3 activation, caspase-3-mediated GSN cleavage, and apoptosis. In resistant cells, CDDP fails to alter the GSN-FLIP-Itch interaction, resulting in the attenuation of the downstream responses to CDDP.
Materials and Methods
Patient Population.
The research protocol and consent form were approved by the Medical Ethics Committee of National Cheng Kung University Hospital (approval A-ER-I02-051). We included consecutive patients who were diagnosed with serous type OVCA between November 1993 and May 2011 at National Cheng Kung University Hospital, Taiwan. These patients underwent comprehensive staging or cytoreductive surgery with adjuvant chemotherapy, which consisted of platinum-based chemotherapeutic agents. Staging was performed according to the criteria of the International Federation of Gynecology and Obstetrics (FIGO).
Cancer progression was defined according to the objective Response Evaluation Criteria in Solid Tumors 1.1 or the Gynecologic Cancer Intergroup definition for CA125 progression. Both the progression-free survival (PFS) and overall survival (OS) were calculated from diagnosis. The OS time was measured to the date of death from any cause; data on survivors were censored on the date at which they were last known to be alive. The PFS duration was measured to the date of first clinical progression or death from any cause, unless the patient was progression-free at the time of last contact, in which case PFI was measured to the date of last contact. The date of the latest record retrieved was May 31, 2013. We reviewed the medical records and pathological slides, which provided information on the patient demographics, clinical characteristics, and treatment outcome. After the primary surgical intervention, the cancerous tissues from the ovarian site were fixed in formaldehyde, embedded in paraffin, and sectioned (4 μm thick) for pathological confirmation of the diagnosis.
Immunohistochemical Grading of GSN Expression Levels in Ovarian Tumor Samples.
GSN protein expression levels in tissue samples were examined by immunohistochemistry (21), using the monoclonal anti-GSN antibody (clone 2C4; Sigma-Aldrich) specific for C-terminal GSN. The slides were then developed in 3,3′-diaminobenzidine (Zymed Laboratories) and counterstained with hematoxylin. Primary antibody was replaced with PBS in negative control. GSN protein expression levels were scored (Fig. S4) as 0–4. Low expression was assigned a score ≤ 1, and positive expression was assigned a score > 1.
Cell Culture and Adenovirus Infection.
Chemosensitive cancer cells (OV2008 and A2780s) were cultured infected with appropriate adenoviral constructs or LacZ as previously reported (15). All experiments were carried out in serum-free medium.
RNA Interference.
Cells transfected with GSN or control siRNA (Ambion and Dharmacon; 50–200 nM, 24 h) (8, 16) were treated with CDDP and harvested for subsequent analysis. GSN down-regulation was confirmed by Western blotting.
Protein Extraction and Western Blot Analysis.
Western blotting (WB) was carried out as described (6). Membranes were incubated with anti-GSN (Sigma-Aldrich), anti–caspase-3 (Cell Signaling Technology), anti-GAPDH (Abcam), anti-Itch (BD Bioscience), or anti-V5 (Bethyl Laboratories) and subsequently with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody in 5% (wt/vol) Blotto. Peroxidase activity was visualized with the enhanced chemiluminescent kit (Amersham Biosciences) and analyzed (Scion Image software). WBs shown in figures are representative of at least three independent experiments.
RNA Isolation and Quantitative PCR.
Relative differences in GSN mRNA levels in experimental groups were determined by quantitative PCR (16).
Assessment of Apoptosis.
Apoptosis was determined morphologically, using Hoechst 33258 nuclear stain (16). The counter was “blinded” to avoid experimental bias.
Immunoprecipitation–Western Blots.
Immunoprecipitation (IP) was performed on whole-cell lysates using anti-V5 and anti-GSN antibodies (16) and lysates were immunoblotted for GSN, Itch, and V5.
Immunocytochemistry and Confocal Microscopy.
OV2008 cells were fixed and then incubated with anti-GSN (Sigma-Aldrich) and anti-FLIP (Cell Signaling) and subsequently with secondary donkey-conjugated antibodies: anti-goat (Cy5), anti-mouse (Cy3), and anti-rabbit (FITC), as previously reported (16).
Statistical Analyses.
Frequency distributions between categorical variables were compared using the χ2 test and Fisher’s exact method. OS and PFS were estimated according to the Kaplan–Meier method and compared using log-rank tests. Cox proportional hazards models were used to estimate hazard ratios and confidence intervals for death and progression. Results from in vitro studies are expressed as mean ± SEM of three or more experiments. Data were analyzed by two-way ANOVA and Bonferroni tests. Statistical significance was inferred at P < 0.05.
Supplementary Material
Acknowledgments
We thank Mr. Bao Kong for his technical support (Fig. 3) and staff of the Human Biobank, Research Center of Clinical Medicine and Cancer Data Bank of the Cancer Center, National Cheng Kung University Hospital for their assistance in the collection of clinical samples and information analyzed in the present study. This work was supported by the Canadian Institutes of Health Research (MOP-15691), the Aim for the Top University Project to the National Cheng Kung University, the National Research Program for Biopharmaceuticals (MOHW103-TDU-PB-211-113016), and the Ministry of Science and Technology, Taiwan (NSC 102-2120-M-006-003 and SC 101-2314-B-006-048-MY3).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1401166111/-/DCSupplemental.
References
- 1.Kartalou M, Essigmann JM. Mechanisms of resistance to cisplatin. Mutat Res. 2001;478(1-2):23–43. doi: 10.1016/s0027-5107(01)00141-5. [DOI] [PubMed] [Google Scholar]
- 2.Markman M, Bookman MA. Second-line treatment of ovarian cancer. Oncologist. 2000;5(1):26–35. doi: 10.1634/theoncologist.5-1-26. [DOI] [PubMed] [Google Scholar]
- 3.Ledermann JA, Kristeleit RS. Optimal treatment for relapsing ovarian cancer. Ann Oncol. 2010;21(Suppl 7):vii218–vii222. doi: 10.1093/annonc/mdq377. [DOI] [PubMed] [Google Scholar]
- 4.Sehouli J, Alfaro V, Gonzalez-Martin A. Trabectedin plus pegylated liposomal doxorubicin in the treatment of patients with partially platinum-sensitive ovarian cancer: Current evidence and future perspectives. Ann Oncol. 2012;23(3):556–562. doi: 10.1093/annonc/mdr321. [DOI] [PubMed] [Google Scholar]
- 5.Cheng JQ, et al. Role of X-linked inhibitor of apoptosis protein in chemoresistance in ovarian cancer: Possible involvement of the phosphoinositide-3 kinase/Akt pathway. Drug Resist Updat. 2002;5(3-4):131–146. doi: 10.1016/s1368-7646(02)00003-1. [DOI] [PubMed] [Google Scholar]
- 6.Fraser M, Bai T, Tsang BK. Akt promotes cisplatin resistance in human ovarian cancer cells through inhibition of p53 phosphorylation and nuclear function. Int J Cancer. 2008;122(3):534–546. doi: 10.1002/ijc.23086. [DOI] [PubMed] [Google Scholar]
- 7.Abedini MR, Qiu Q, Yan X, Tsang BK. Possible role of FLICE-like inhibitory protein (FLIP) in chemoresistant ovarian cancer cells in vitro. Oncogene. 2004;23(42):6997–7004. doi: 10.1038/sj.onc.1207925. [DOI] [PubMed] [Google Scholar]
- 8.Fraser M, et al. p53 is a determinant of X-linked inhibitor of apoptosis protein/Akt-mediated chemoresistance in human ovarian cancer cells. Cancer Res. 2003;63(21):7081–7088. [PubMed] [Google Scholar]
- 9.Sasaki H, Sheng Y, Kotsuji F, Tsang BK. Down-regulation of X-linked inhibitor of apoptosis protein induces apoptosis in chemoresistant human ovarian cancer cells. Cancer Res. 2000;60(20):5659–5666. [PubMed] [Google Scholar]
- 10.Sun HQ, Yamamoto M, Mejillano M, Yin HL. Gelsolin, a multifunctional actin regulatory protein. J Biol Chem. 1999;274(47):33179–33182. doi: 10.1074/jbc.274.47.33179. [DOI] [PubMed] [Google Scholar]
- 11.Silacci P, et al. Gelsolin superfamily proteins: Key regulators of cellular functions. Cell Mol Life Sci. 2004;61(19-20):2614–2623. doi: 10.1007/s00018-004-4225-6. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka H, et al. siRNA gelsolin knockdown induces epithelial-mesenchymal transition with a cadherin switch in human mammary epithelial cells. Int J Cancer. 2006;118(7):1680–1691. doi: 10.1002/ijc.21559. [DOI] [PubMed] [Google Scholar]
- 13.Rahman M, Miyamoto H, Chang C. Androgen receptor coregulators in prostate cancer: mechanisms and clinical implications. Clin Cancer Res. 2004;10(7):2208–2219. doi: 10.1158/1078-0432.ccr-0746-3. [DOI] [PubMed] [Google Scholar]
- 14.Mielnicki LM, Ying AM, Head KL, Asch HL, Asch BB. Epigenetic regulation of gelsolin expression in human breast cancer cells. Exp Cell Res. 1999;249(1):161–176. doi: 10.1006/excr.1999.4461. [DOI] [PubMed] [Google Scholar]
- 15.Abedini MR, Muller EJ, Bergeron R, Gray DA, Tsang BK. Akt promotes chemoresistance in human ovarian cancer cells by modulating cisplatin-induced, p53-dependent ubiquitination of FLICE-like inhibitory protein. Oncogene. 2010;29(1):11–25. doi: 10.1038/onc.2009.300. [DOI] [PubMed] [Google Scholar]
- 16.Abedini MR, et al. Cisplatin induces p53-dependent FLICE-like inhibitory protein ubiquitination in ovarian cancer cells. Cancer Res. 2008;68(12):4511–4517. doi: 10.1158/0008-5472.CAN-08-0673. [DOI] [PubMed] [Google Scholar]
- 17.Kothakota S, et al. Caspase-3-generated fragment of gelsolin: Effector of morphological change in apoptosis. Science. 1997;278(5336):294–298. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- 18.Koya RC, et al. Gelsolin inhibits apoptosis by blocking mitochondrial membrane potential loss and cytochrome c release. J Biol Chem. 2000;275(20):15343–15349. doi: 10.1074/jbc.275.20.15343. [DOI] [PubMed] [Google Scholar]
- 19.Kusano H, et al. Human gelsolin prevents apoptosis by inhibiting apoptotic mitochondrial changes via closing VDAC. Oncogene. 2000;19(42):4807–4814. doi: 10.1038/sj.onc.1203868. [DOI] [PubMed] [Google Scholar]
- 20.Shieh DB, et al. Cell motility as a prognostic factor in Stage I nonsmall cell lung carcinoma: The role of gelsolin expression. Cancer. 1999;85(1):47–57. doi: 10.1002/(sici)1097-0142(19990101)85:1<47::aid-cncr7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.Shieh DB, et al. Tissue expression of gelsolin in oral carcinogenesis progression and its clinicopathological implications. Oral Oncol. 2006;42(6):599–606. doi: 10.1016/j.oraloncology.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Rao J, et al. Tissue microarray analysis of cytoskeletal actin-associated biomarkers gelsolin and E-cadherin in urothelial carcinoma. Cancer. 2002;95(6):1247–1257. doi: 10.1002/cncr.10823. [DOI] [PubMed] [Google Scholar]
- 23.Bookman MA. Extending the platinum-free interval in recurrent ovarian cancer: The role of topotecan in second-line chemotherapy. Oncologist. 1999;4(2):87–94. [PubMed] [Google Scholar]
- 24.Monk BJ, Dalton H, Farley JH, Chase DM, Benjamin I. Antiangiogenic agents as a maintenance strategy for advanced epithelial ovarian cancer. Crit Rev Oncol Hematol. 2013;86(2):161–175. doi: 10.1016/j.critrevonc.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Katsumata N, et al. Japanese Gynecologic Oncology Group Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: A phase 3, open-label, randomised controlled trial. Lancet. 2009;374(9698):1331–1338. doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]
- 26.Markman M, et al. Impact on survival of 12 versus 3 monthly cycles of paclitaxel (175 mg/m2) administered to patients with advanced ovarian cancer who attained a complete response to primary platinum-paclitaxel: Follow-up of a Southwest Oncology Group and Gynecologic Oncology Group phase 3 trial. Gynecol Oncol. 2009;114(2):195–198. doi: 10.1016/j.ygyno.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, et al. Gelsolin, but not its cleavage, is required for TNF-induced ROS generation and apoptosis in MCF-7 cells. Biochem Biophys Res Commun. 2009;385(2):284–289. doi: 10.1016/j.bbrc.2009.05.078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.