Significance
Chronic hepatitis B virus (HBV) infection puts >250 million humans at risk for developing liver cirrhosis and liver cancer. Current therapies are not curative because they do not target HBV´s persistence reservoir, the plasmid-like covalently closed circular DNA (cccDNA). RNA production from cccDNA initiates the generation of progeny virus via protein-primed reverse transcription, yielding viral polymerase-linked relaxed-circular DNA (RC-DNA). Its conversion, upon infection, into cccDNA requires multiple poorly understood steps, including polymerase removal. We found that the host enzyme tyrosyl-DNA-phosphodiesterase 2 (TDP2), important for repair of cellular protein–DNA adducts, performed this step in vitro and that TDP2 depletion impaired the conversion of RC-DNA to cccDNA in cells. These data establish a functional link between HBV and cellular DNA repair and pave the way for targeting HBV persistence.
Keywords: hepatitis B virus persistence, TDP substrate specificity, virus–DNA repair interface
Abstract
Hepatitis B virus (HBV), the causative agent of chronic hepatitis B and prototypic hepadnavirus, is a small DNA virus that replicates by protein-primed reverse transcription. The product is a 3-kb relaxed circular DNA (RC-DNA) in which one strand is linked to the viral polymerase (P protein) through a tyrosyl–DNA phosphodiester bond. Upon infection, the incoming RC-DNA is converted into covalently closed circular (ccc) DNA, which serves as a viral persistence reservoir that is refractory to current anti-HBV treatments. The mechanism of cccDNA formation is unknown, but the release of P protein is one mandatory step. Structural similarities between RC-DNA and cellular topoisomerase–DNA adducts and their known repair by tyrosyl-DNA-phosphodiesterase (TDP) 1 or TDP2 suggested that HBV may usurp these enzymes for its own purpose. Here we demonstrate that human and chicken TDP2, but only the yeast ortholog of TDP1, can specifically cleave the Tyr–DNA bond in virus-adapted model substrates and release P protein from authentic HBV and duck HBV (DHBV) RC-DNA in vitro, without prior proteolysis of the large P proteins. Consistent with TPD2’s having a physiological role in cccDNA formation, RNAi-mediated TDP2 depletion in human cells significantly slowed the conversion of RC-DNA to cccDNA. Ectopic TDP2 expression in the same cells restored faster conversion kinetics. These data strongly suggest that TDP2 is a first, although likely not the only, host DNA-repair factor involved in HBV cccDNA biogenesis. In addition to establishing a functional link between hepadnaviruses and DNA repair, our results open new prospects for directly targeting HBV persistence.
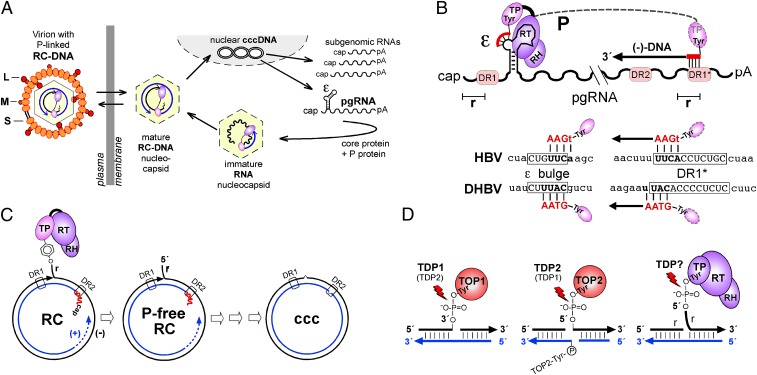
More than 250 million people worldwide are chronically infected with hepatitis B virus (HBV) (1) and are at a highly increased risk for developing end-stage liver disease (2). Current treatments with IFN-α or nucleos(t)ide analogs (NAs) are only partially effective (3, 4). Importantly, they do not directly target the viral persistence reservoir, an episomal covalently closed circular (ccc) DNA form of the viral genome that serves as template for all viral transcripts; hence a few cccDNA molecules present in the liver can reactivate full viral replication. Eliminating infection thus will require the elimination of cccDNA. cccDNA is generated, upon infection, from the viral polymerase (P protein)-linked relaxed circular (RC) DNA present in incoming virions (Fig. 1A). The mechanism by which RC-DNA is converted to cccDNA is poorly understood, but it must involve multiple steps (see below). Because the ∼3-kb genomes of HBV and the other hepadnaviruses [e.g., from ducks (DHBV)] are too small to encode all the activities required for this conversion, these activities must be provided by the host cell.
Fig. 1.
Study-relevant hepadnavirus features. (A) Replication cycle. Incoming P-protein–linked RC-DNA is converted into nuclear cccDNA from which viral RNAs are transcribed, exported, and translated. Via ε, pgRNA is copackaged with P into new nucleocapsids and reverse transcribed into RC-DNA. Mature nucleocapsids can be secreted as virions or reenter the intracellular cycle. (B) Establishment of the 5′-phosphotyrosyl linkage. (Upper) P protein uses a Tyr residue in its TP domain to initiate synthesis of an ε bulge-templated DNA oligo primer; its extension after translocation to 3′ DR1* yields minus-strand (−) DNA bearing a terminal redundancy (r). (Lower) HBV and DHBV primer and DR1* acceptor sequences are depicted. Subsequent RC-DNA formation is not depicted. DR, direct repeat; RH, RNase H domain of P protein; RT, reverse transcriptase domain of P protein. (C) P protein release is one of several obligatory steps in the conversion of RC-DNA to cccDNA. Before final ligation, all structural peculiarities of RC-DNA [i.e., linked P protein and r on minus-strand DNA, and RNA primer (red line) and the gap in plus-strand DNA] must be removed. (D) Similarity of abortive TOP1 and TOP2 cleavage complexes to P-protein–linked RC-DNA. TOP1 is bound to DNA via 3′- and TOP2 via 5′-phosphotyrosyl bonds. Major repair pathways include TDP1 for TOP1 and TDP2 for TOP2 adducts, but TDP2 may substitute for TDP1 and possibly vice versa. Alternative nucleolytic repair pathways are not indicated.
RC-DNA from all hepadnaviruses bears several molecular peculiarities that result from its generation by protein-primed reverse transcription (for reviews, see refs. 5 and 6). The pregenomic RNA (pgRNA) acts as mRNA for the capsid (core) protein and P protein and then is copackaged with P into nucleocapsids via P protein's interaction with the 5′-proximal ε RNA stem-loop (Fig. 1A). A Tyr residue in the terminal protein (TP) domain of P protein acts as acceptor for the first dNTP of a short ε-templated oligonucleotide. This “protein-priming” reaction, which for DHBV can be reconstituted completely in vitro (7–10), establishes a 5′-phosphotyrosyl linkage between P protein and DNA which is maintained during the formation of complete minus-strand DNA (Fig. 1B), RNA-primed synthesis of usually incomplete plus-strand DNA, and circularization into RC-DNA via a short terminal redundancy (r) in the minus strand. Hence the formation of cccDNA from RC-DNA requires release of P protein and RNA, removal of r, completion of the plus strand, and eventually ligation (Fig. 1C). Ligation must occur with single-nucleotide precision because each nucleotide has a coding function in at least one ORF. Notably, because cccDNA cannot be replicated semiconservatively (11), each single cccDNA molecule must have undergone this conversion process.
To begin to decipher the conversion of RC-DNA to cccDNA, we focus here on the release of P protein from the minus-strand DNA. Notably, chemically similar protein–DNA adducts occur naturally as abortive topoisomerase (TOP) cleavage complexes. TOPs relieve DNA supercoiling (12) by incising one (TOP1) or both (TOP2) strands via the usually transient formation of tyrosyl–3′ DNA (TOP1) or tyrosyl–5′ DNA phosphodiester (TOP2) bonds. Occasionally, or induced by near-by lesions or topoisomerase poisons (13), the back-reaction fails, leaving the enzyme covalently trapped on the broken DNA (Fig. 1D). To avoid the potentially fatal consequences of this and other kinds of DNA damage, all cells are equipped with a sophisticated DNA repair machinery comprising a large network of damage sensors, signal transducers, and repair effectors (14, 15). Their concerted action usually arrests the cell cycle to allow time for repair or induces cell death if repair fails. Because of the fundamental importance of genome integrity, many, if not all, repair pathways are safeguarded by alternative systems, as is TOP cleavage complex repair. For TOP1 adducts, one pathway involves tyrosyl-DNA-phosphodiesterase 1 (TDP1; Fig. 2A), an ∼600-aa member of the phospholipase D superfamily (16) long known to cleave 3′-phosphotyrosyl bonds (17); however, yeast TDP1 (yTDP1) (18), and possibly human TDP1 (huTDP1) (19), also may possess tyrosyl-5′-DNA phosphodiesterase activity (but see also ref. 20). Alternatively, trapped TOP1 can be removed nucleolytically (21, 22). For repair of the 5′-phosphotyrosyl–linked TOP2 adducts, only nucleolytic mechanisms were considered (23, 24) until a protein known as “TRAF and TNF receptor-associated protein” (TTRAP) or “ETS1-associated protein II” (EAPII) (25) was found to have such an activity (26); that protein has been renamed “TDP2” (Fig. 2B). The preference of TDP2 for 5′ phosphotyrosyl bonds is consistent with recent X-ray data (27–29), but TDP2 also is active on 3′-substrates (20, 26, 30). Although TDP2 likely is the only enzyme of its kind (30), complete knockout of TDP2 in chicken DT40 cells and in mice was not lethal (20), possibly reflecting the power of redundancy in DNA repair. Notably, TDP2 recently also has been found to represent the “unlinkase” activity that can remove the covalently linked VPg from poliovirus RNA (31).
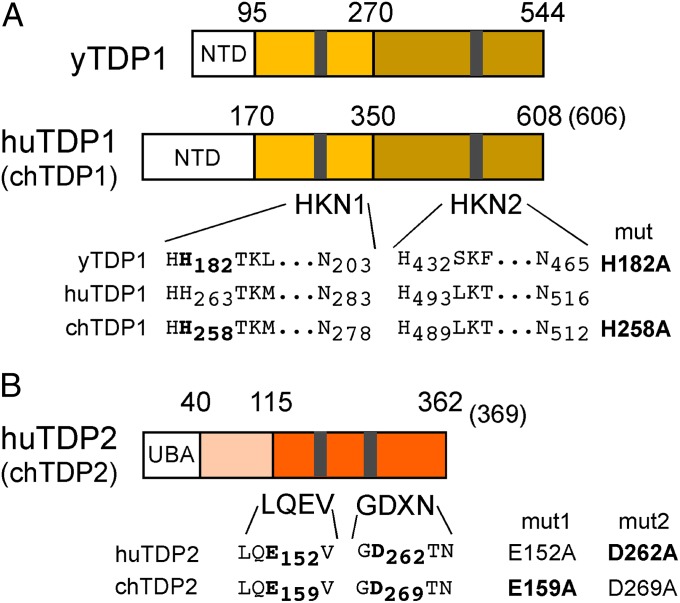
Fig. 2.
General structures of TDP1 and TDP2. (A) TDP1 contains two conserved HKN motifs; their sequences are shown for yTDP1, huTDP1, and chTDP1. (B) TDP2 contains conserved LQEV and GDXN motifs; their sequences for huTDP2 and chTDP2 are indicated. Numbers refer to amino acid positions. Catalytically important residues, for the chicken enzymes inferred by sequence homology, mutated to Ala are listed on the right. In preliminary model substrate assays, all four TDP2 mutants exerted no activity, hence only those shown in bold face were used in further experiments.
Given the structural similarity of hepadnaviral P-protein–linked RC-DNA to TOP–DNA adducts and the increasingly acknowledged fact that all viruses with a nuclear DNA phase must cope with cellular DNA repair (32), we reasoned that TDP1 and/or TDP2 might be able to release P protein from RC-DNA and thus catalyze one crucial step in cccDNA formation. As shown below, huTDP2 and chTDP2 but only yTDP1 could cleave specifically hepadnavirus-adapted Tyr-5′-DNA model substrates, covalent complexes of DHBV P protein carrying the short DNA oligonucleotide primer and, importantly, authentic RC-DNA from DHBV and HBV nucleocapsids. Last, stable shRNA knockdown of TDP2 in human hepatoma cells significantly impaired the conversion of RC-DNA to cccDNA. Together these data strongly suggest that TDP2, but not TDP1, is a first host-cell DNA-repair factor involved in hepadnaviral cccDNA biogenesis.
Results
Different Phosphodiesterase Activity Profiles of TDP1 and TDP2 from Different Organisms on Synthetic Tyr-5′-DNA and Tyr-3′-DNA Substrates.
The principal requirements in the conversion of RC-DNA to cccDNA are the same for HBV and DHBV. Because chicken as well as human hepatoma cells support efficient DHBV cccDNA formation, we investigated, in addition to huTDP1 and huTDP2 [National Center for Biotechnology Information (NCBI) reference sequences: NP_001008744.1 and NP_057698.2, respectively], their chicken orthologs (NCBI reference sequences: XP_421313.1 and NP_001263313.1, respectively). We included yTDP1 (NCBI reference sequence: NP_009782.3) because of its reported 5′-substrate activity (18). The generation of bacterial and mammalian expression vectors for these proteins, plus inactive variants with mutations of key catalytic residues (hereafter collectively termed “mut”) (Fig. 2), is described in SI Materials and Methods. All five proteins and their mutants were well expressed in Escherichia coli and could be purified with substantial yield and purity in a monomeric state (Fig. S1).
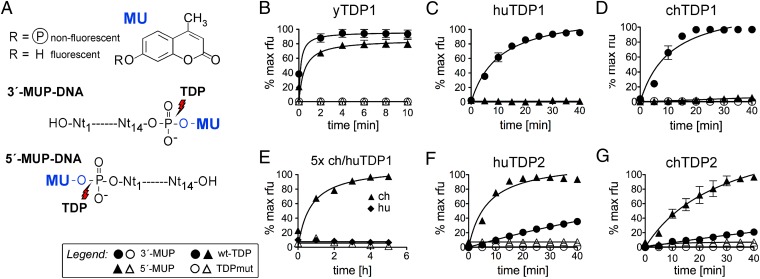
To validate their enzymatic activities, we adapted an assay reported for TDP1 activity on 3′ phosphotyrosyl bonds (33) to obtain synthetic substrates in which methylumbelliferon (MU), a Tyr mimic, is attached via a phosphodiester bond to either the 5′ or the 3′ end of a 14-mer DNA oligo (MUP-DNA); its sequence corresponded to the 5′ end of DHBV minus-strand DNA to which P protein is linked (Fig. 3A). Cleavage of the phosphotyrosyl bond releases fluorescent MU from nonfluorescent MUP-DNA and can thus be monitored in real time using a fluorescence reader. The most pertinent progress curves obtained using 0.5 µM MUP-DNA substrate and a 100-nM protein concentration are shown in Fig. 3 B–G; data on bivalent metal ion dependence are provided in Fig. S2.
Fig. 3.
Differential activities of TDP1 and TDP2 from different species on 5′- and 3′-phosphotyrosyl model substrates. (A) Assay principle. MU as a Tyr mimic was coupled to a 5′- or 3′-phosphorylated 14-mer oligonucleotide representing the P-protein–linked 5′ end of DHBV minus-strand DNA (MUP-DNA). Specific cleavage of the nonfluorescent MUP-DNA substrate releases fluorescent MU. (B–G) MU release activity of the indicated TDP proteins. Fluorescence development over time was recorded in a microplate fluorescence reader. Because of the bivalent metal ion dependence of TDP2, but not TDP1 (Fig. S2), TDP2 reactions were performed in the presence of 5 mM Mg2+. In all panels except E, 100 nM protein (filled symbols for wild-type, open symbols for the respective mutants) was reacted with 0.5 µM substrate (indicated by circles for the 3′ substrate and by triangles for the 5′ substrate). In E, protein concentrations were increased to 500 nM, and the observation time was extended to 5.5 h. Substrate conversions are expressed as the percent of the maximal fluorescence signal (in relative fluorescence units, rfu) obtained when the wild-type enzyme reaction with the optimal substrate reached a plateau. Error bars represent SD (n = 3). Formal progress curve fitting, serving only illustrative, not analytical purposes, was done by nonlinear regression using GraphPad Prism 5.
All three wild-type TDP1 orthologs, but not their mutant versions, caused a fast rise in fluorescence with the 3′ substrate (Fig. 3 B–D), independently of bivalent metal ions (Fig. S2A). In contrast, activities on the 5′ substrate differed strongly. yTDP1 was highly active (Fig. 3B), huTDP1 was inactive (Fig. 3C), and chTDP1 produced a slight increase in fluorescence (Fig. 3D) that was more evident at higher enzyme concentrations and longer incubation times (Fig. 3E). Under the same conditions, no increase occurred with chTDP1mut and wild-type huTDP1, indicating a low but specific 5′-substrate activity of the chicken but not the human enzyme (Fig. 3E). Together, these data confirmed the Tyr-3′-DNA phosphodiesterase activity of all three TDP1 orthologs and the proposed Tyr-5′-DNA activity of yTDP1 (18) but not huTDP1.
TDP2 gave a different picture (Fig. 3 F and G). Initial experiments (Fig. S2B) confirmed the strict bivalent metal ion dependence of TDP2 activity (26, 34). Accordingly, these assays were performed in the presence of 5 mM of Mg2+. Both huTDP2 and chTDP2, but not their mutant versions, exerted high activity on the 5′ substrate and a well-detectable but lower activity on the 3′ substrate. Hence huTDP2 and chTDP2 but only yTDP1 (and possibly chTDP1) possessed the proper enzymatic activity for the release of the 5′-phosphotyrosyl–linked P protein from RC-DNA.
Vertebrate TDP2 and Yeast TDP1 Can Cleave the Covalent P Protein–Oligonucleotide Bond Formed by in Vitro Protein Priming.
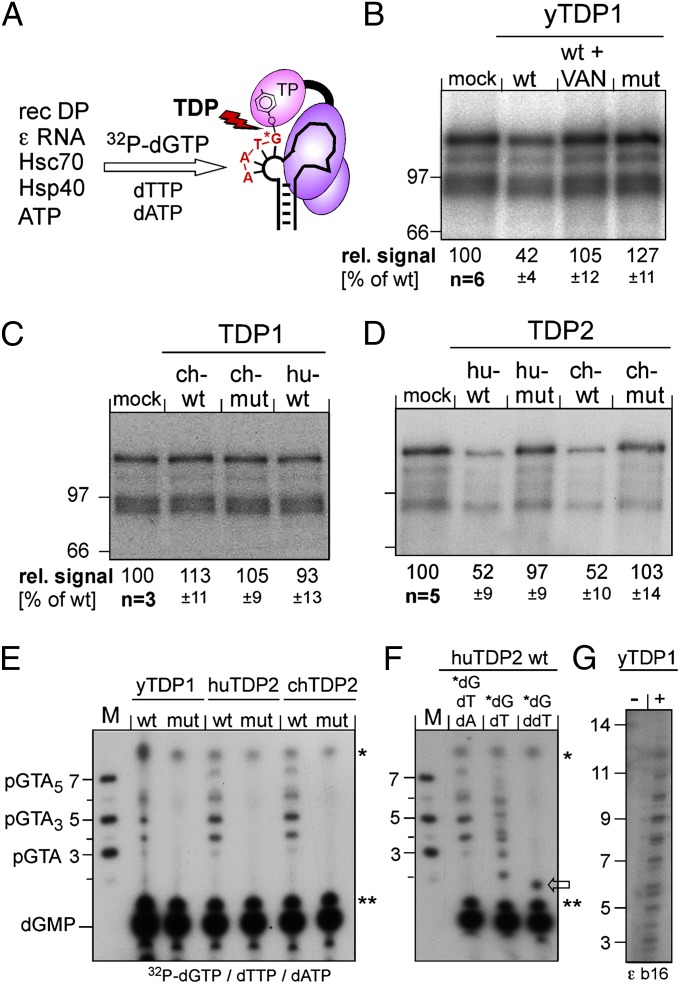
Next we reconstituted the 5′-phosphotyrosyl bond between P protein and minus-strand DNA using in vitro protein priming with recombinant DHBV P protein and ε RNA plus cellular chaperones, ATP as the energy source, and dGTP, dTTP, and dATP (10). Using [α32P]-dGTP, the 5′ residue of the DHBV oligonucleotide primer GTA or GTAA (Fig. 1B, Lower), and hence the phosphotyrosyl bond to P protein, will be radioactively labeled (Fig. 4A). TDP cleavage should reduce P-protein labeling and release the 32P-labeled oligonucleotide (35).
Fig. 4.
Both vertebrate TDP2s but only yTDP1 can cleave the P protein–DNA primer complex. (A) Assay principle. Recombinant DHBV P protein was activated in vitro to bind ε RNA, then incubated with [α32P]-dGTP plus dTTP plus dATP to initiate synthesis of the P-protein–linked primer. If P protein is a substrate for TDP, radiolabeling of P protein should be reduced by release of the labeled primer. (B–D) Impact on the radiolabeled P protein. Equal aliquots of the priming reaction were incubated with the indicated wild-type (wt) or mutant (mut) proteins or in buffer only (mock); in B, one reaction was performed in the presence of vanadate (VAN). Reactions were separated by SDS/PAGE, and labeled P protein (plus smaller degradation products) was visualized by autoradiography. The top bands were quantified by phosphorimaging. Mean values ± SD are indicated at the bottom of each panel. (E–G) Detection of released oligonucleotides. After incubation with the indicated TDP proteins, labeled nucleic acids were separated by electrophoresis in 20% polyacrylamide-7 M urea gels. M, 5′-32P–labeled marker DNA oligos of the indicated sequences and lengths. Bands marked with asterisks and double asterisks occurred in all reactions and are nonspecific. dGMP was identified by apyrase treatment of [α32P]-dGTP. In E, priming was performed with all three nucleotides required for the authentic primer. In F, [α32P]-dGTP was supplemented with only dTTP or ddTTP. In G, a variant ε RNA (ε b16) with a 16-nt bulge (5′ C in the bulge replaced by 5′ U2GU8) was used as priming template.
The priming products were incubated with the different TDP enzymes, and radiolabeled protein products were resolved by SDS/PAGE and visualized by autoradiography and/or phosphorimaging (Fig. 4 B–D). Wild-type yTDP1, but not its inactive mutant, reduced the 32P P-protein signal by about 60% compared with the mock-treated control; this reduction was prevented by vanadate, a known phosphodiesterase inhibitor (Fig. 4B). No loss of protein label was seen with chTDP1 and huTDP1 (Fig. 4C). In contrast, both vertebrate TDP2 enzymes, but not their inactive variants, also reduced P-protein labeling by about 50% (Fig. 4D).
Loss of protein labeling correlated strictly with the detection of distinct radiolabeled oligonucleotides by electrophoresis in high-percentage polyacrylamide -7 M urea gels, with yTDP1 and both vertebrate TDP2s, but not their mutants, generating very similar patterns (Fig. 4E, Left). The strongest signals appeared at the 4- and 5-nt marker positions, with weaker signals at the 3- and 6- to 7-nt marker positions. Some products thus were longer than the tri- or tetranucleotide GTA(A) that productively primes minus-strand extension in vivo. To address concerns that the released products did not originate from ε-templated primers, dATP was omitted from the priming reaction; this omission led to the appearance of a product at the expected GT dinucleotide position, but some extended products obviously containing only dG and dT were formed. However, replacement of dTTP by its dideoxy derivative ddTTP resulted exclusively in one product with slightly faster mobility than the dinucleotide marker (likely because of the modified ribose part), as expected from efficient ε-templated ddT incorporation at the second primer position (Fig. 4E, Center) and subsequent chain termination. Furthermore, using ε RNA with an extended 16-nt bulge (εb16) as a priming template, yTDP1 generated cleavage products extending up to about 13 nt in length (Fig. 4E, Right). Hence the TDP-released oligonucleotides are derived, at least in part, from authentic protein-priming events. Potential reasons for the seeming discrepancies between in vitro and in vivo priming are given in Discussion. Besides illustrating the usefulness of vertebrate TDP2s and yTDP1 for further investigation of hepadnaviral protein priming, the data showed that these enzymes were active on the authentic P protein–oligonucleotide phosphotyrosyl bond.
HuTDP2, chTDP2, and yTDP1 Can Cleave P Protein Specifically from Authentic DHBV and HBV RC-DNA.
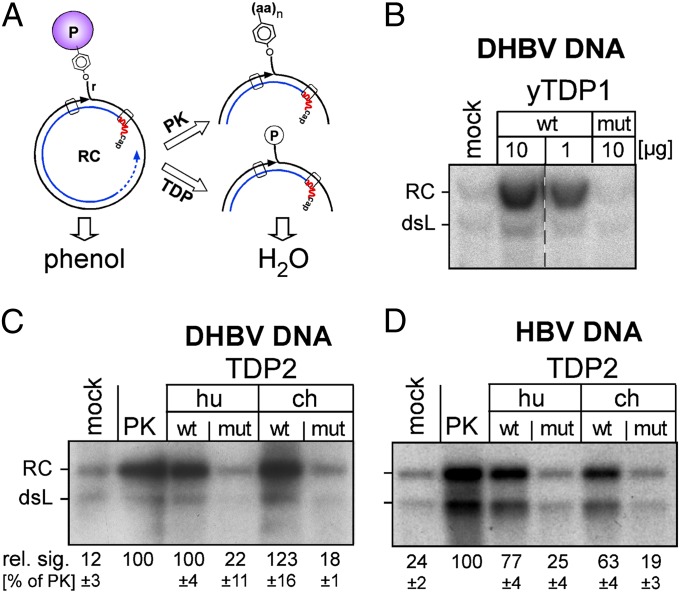
As the closest in vitro approximation to a possible in vivo role in cccDNA biogenesis, we asked whether the TDPs were able to release P protein from authentic viral RC-DNA as present in cell-derived nucleocapsids. The challenge was to make the packaged RC-DNA accessible to exogenous enzymes under conditions that maintain a soluble P protein–DNA complex and allow TDP activity. For DHBV (but not HBV) nucleocapsids we confirmed the previously reported destabilization by incubation at >500 mM NaCl (36); under these conditions all TDP enzymes retained substantial activity (Fig. S3A). We therefore incubated cytoplasmic nucleocapsids from DStet5 cells, a chicken LMH cell-derived (TetOFF) line stably transformed with an envelope-deficient (env−) DHBV genome (37), at 500 mM NaCl with yTDP1, huTDP2, and chTDP2 or their inactive mutants, or for control with proteinase K (PK) which degrades most of the P protein. Without such treatment, which is applied routinely for Southern blot analyses in HBV research, the protein-linked viral DNA partitions into the organic phase upon phenol extraction (38) and is not detected. Release of P protein by TDP cleavage should result in a signal increase similar to that seen with proteolysis (Fig. 5A).
Fig. 5.
Vertebrate TDP2 and yTDP1 release P protein from authentic RC-DNA. (A) Assay principle. P-protein–linked viral DNA partitions into phenol unless the bulk of the protein is digested by PK or is released by TDP. Only the water-soluble DNA is detected by Southern blotting. (B–D) The amount of water-soluble viral DNA is increased upon incubation with wild-type but not mutant TDP proteins. Cell-derived DHBV or HBV nucleocapsids were permeabilized (SI Materials and Methods and Fig. S3) and incubated with the indicated TDP proteins, with PK, or with buffer only (mock). After phenol extraction, DNAs were detected by Southern blotting using 32P-labeled DHBV or HBV DNA probes. The low signals in the mock reactions reflect a small fraction of RC-DNA that naturally lacks intact P protein. Numbers below panels C and D indicate mean RC-DNA signal intensities ± SD (n = 3) relative to intensities after PK treatment, which were set to 100%.
Incubation of DHBV RC-DNA with yTDP1, but not with its inactive mutant, indeed caused a strong increase in Southern blot signal as compared with the mock-treated (no PK, no TDP) control (Fig. 5B); the weak signals observed in the control represent a fraction of already “deproteinized” DNA, which routinely accounts for 10% of the total viral DNA (39). Importantly, signal enhancement similar to that of PK also was achieved by huTDP2 and chTDP2, but not by their inactive variants (Fig. 5C).
HBV nucleocapsids obtained from induced cultures of TetOFF wild-type HBV HepG2.117 cells (40) were refractory to high-salt treatment, but freezing in the presence of 0.01% (wt/vol) ethidium bromide (EB) made the RC-DNA and double-stranded linear (dsL) DNA amenable to digestion by micrococcal nuclease (Fig. S3B), possibly via their increased space requirements upon EB intercalation; in line with this notion, ssDNA remained protected (Fig. S3B). Incubation of HBV nucleocapsids destabilized in this way with huTDP2 and chTDP2, but not their mutants, caused an increase in Southern blot-detectable RC-DNA and dsL-DNA similar to that caused by PK (Fig. 5D), indicating that the vertebrate TDP2s, as well as yTDP1, can cleave P protein from authentic DHBV and HBV RC-DNA.
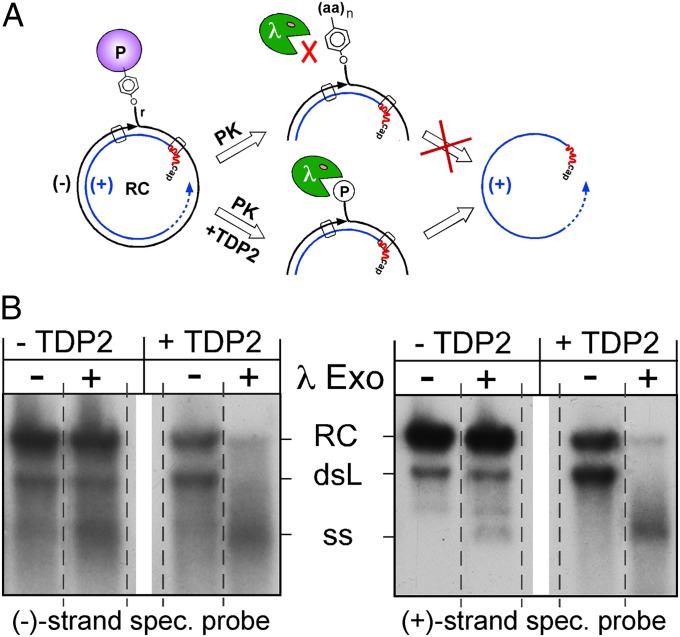
To demonstrate formally the specific cleavage of the phosphotyrosyl bond, we assessed the nature of the minus-strand DNA 5′ end after treatment with PK only and after treatment with PK plus TDP2. PK-treated DNA still contains at least the DNA-linked Tyr residue, whereas TDP cleavage should result in a free 5′ phosphoryl end. λ 5′→3′ exonuclease is highly active on dsDNA with phosphorylated 5′ ends but is much less active on ssDNA. Because the 5′ end of plus-strand DNA is at least partially protected by the RNA primer, selective digestion of minus-strand DNA in RC-DNA and dsL-DNA should lead to the accumulation of single-stranded plus strands. In contrast, 5′ aminoacylated minus-strand DNA would be resistant (Fig. 6A). The impact of λ exonuclease then was analyzed by Southern blotting using strand-specific riboprobes (Fig. 6B). Without huTDP2 treatment, at most a slight increase in single-stranded minus-strand and plus-strand DNA was detectable, the latter possibly arising from the fraction of naturally deproteinized viral DNA. In contrast, huTDP2 treatment caused a massive loss of double-stranded forms in favor of single-stranded plus-strands. Hence, as expected, huTDP2 generated minus-strand DNA ends from the specific cleavage of the tyrosyl-DNA phosphodiester bond connecting P protein and DNA.
Fig. 6.
TDP2-mediated release of P protein from RC-DNA is caused by specific phosphotyrosyl bond cleavage. (A) Assay principle. Viral DNA with 5′-phosphorylated minus-strand ends (TDP2 pathway) but not 5′-aminoacylated minus-strand ends (PK pathway) is an efficient substrate for 5′→3′ λ exonuclease; plus-strand 5′ ends are protected by the RNA primer. Specific phosphotyrosyl bond cleavage should result in the accumulation of free plus-strands. (B) TDP2-treated viral DNA is sensitive to λ exonuclease. Viral DNA from cell-derived DHBV nucleocapsids was treated with PK only or with PK and subsequently with huTDP2. Thereafter, DNAs were incubated with λ exonuclease, and the products were detected by Southern blotting, using either a minus-strand–specific (Left) or plus-strand–specific (Right) riboprobe. A marked increase in single-stranded plus-strand DNA was seen only upon TDP2 treatment. All lanes within each panel are derived from the same exposure of the same blot, but the order of lanes has been rearranged (indicated by the dotted separation lines) to facilitate a direct comparison of the impact of the nuclease on TDP2-treated versus not TDP2-treated DNA. See text for further details.
TDP2 Knockdown Impairs the Formation of Hepadnaviral cccDNA in Cells.
If the biochemically demonstrated ability of vertebrate TDP2 and yTDP1 to release P protein from RC-DNA is physiologically relevant, reducing TDP2 expression should negatively affect the conversion of RC-DNA to cccDNA in cells. Prerequisites for dependable detection of such an effect were the efficient, verifiable silencing of cellular TDP2 and sufficiently high basal levels of cccDNA so that a potential reduction could be detected unambiguously by Southern blotting. In addition, because cccDNA, once formed, appears to be highly stable (see ref. 41 and references therein), interfering with conversion should have the largest impact during the phase of maximal cccDNA production, i.e., upon synchronized induction of RC-DNA formation.
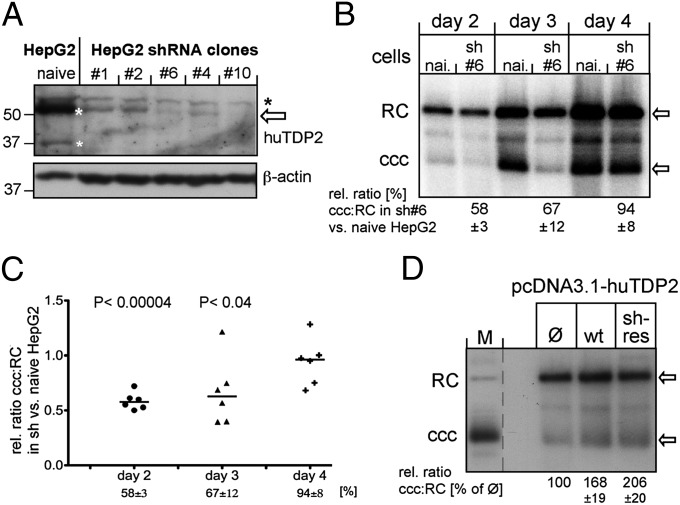
Because of equivocal immunoblot results with a commercial anti-TDP2 antibody (Sigma AV33010) and similar problems reported for antibodies from other sources (42), we first used the recombinant huTDP2 and chTDP2 proteins to obtain custom rabbit antibodies (Biogenes) which detected the two TDP2 proteins with high specificity and sensitivity (SI Materials and Methods and Fig. S4). Notably, in all anti-huTDP2 immunoblots two bands were visible, one at ∼51 kDa and one at ∼37 kDa that was more pronounced in Huh7 than in HepG2 cells; similar observations have been made recently in other cell lines 31, 43). The smaller product likely represents an N-terminally shortened but enzymatically active form (Discussion). Next, we confirmed that one of four commercial anti-huTDP2 siRNAs (Qiagen), si 9, when transfected into Huh7 cells, reduced huTDP2 levels by up to 90% (SI Materials and Methods and Fig. S5A). As a model of inducible, human cell-based conversion of RC-DNA to cccDNA, we exploited the fact that env− DHBV generates drastically more cccDNA (39) than does HBV even in human hepatoma cells and established a stable DHBVenv− Huh7 TetOFF cell line. Withdrawal of tetracycline (Tet)-initiated virus replication and RC-DNA formation, as expected and in contrast to analogous HBV lines (40), was accompanied by easily detectable accumulation of cccDNA from day 9 to day 18 postinduction (Fig. S6). However, using transient siRNA transfection, we were unable to maintain substantially decreased TDP2 levels for more than 3 d (Fig. S5B), and repeated siRNA transfections were poorly tolerated by the cells.
We therefore used stable shRNA-mediated knockdown as alternative. Screening of four anti-huTDP2 and anti-chTDP2 shRNAs predicted by i-Score (44) and transiently expressed from a nonselectable vector (45) identified one particularly efficient target site each (hu526 and ch660; Fig. S7 A and B). Incidentally, a selectable vector for a hu526-like shRNA (termed “siTTRAP_T1”) plus one for an shRNA not validated in our experiments (siTTRAP_T4) had been used successfully to knock down TTRAP (i.e., TDP2) in neuronal cells (46). Using these plasmids (kindly provided by S. Gustincich, International School of Advanced Studies, Trieste, Italy), we selected several HepG2 clones with clearly reduced TDP2 levels as compared with naive HepG2 cells (Fig. 7A); clone T4#6, showing an 80–90% reduction in TDP2, was used for most subsequent experiments. Next, T4#6 cells and naive HepG2 cells were transiently transfected with a cytomegalovirus (CMV) promoter-driven expression vector for DHBV env−; cells were harvested at days 2, 3, and 4 posttransfection, and nuclear viral DNAs were analyzed by Southern blotting. To account for potential variations in transfection efficiency and/or sample loading, the ratio of cccDNA to RC-DNA in the same lane was used to assess the efficiency of the conversion of RC-DNA to cccDNA. As already evident by visual inspection (Fig. 7B), TDP2 depletion markedly slowed the conversion kinetics, with the cccDNA:RC-DNA ratio in the shRNA line being about half that in naive HepG2 cells at day 2 and about two-thirds at day 3 posttransfection; at day 4, the difference had leveled off and did not change further. Comparable results in six independent experiments (Fig. 7C) confirmed the significance of the difference between naive and TDP2-depleted cells. Delayed cccDNA formation was not caused by a general effect of the TDP2-knockdown on the cells, because they had doubling times [1.048 d; 95% confidence interval (CI) 1.019–1.079 d] virtually identical to those of naive HepG2 cells (1.052 d; 95% CI 1.019–1.087 d). Furthermore, delayed conversion of RC-DNA to cccDNA also was seen in an siTTRAP_T1 shRNA-derived cell clone in which the shRNA targets a different site on the huTP2 mRNA. Finally, cotransfection into T4#6 cells of the DHBVenv− vector plus a huTDP2 expression vector, and more so a vector encoding a huTDP2 mRNA carrying silent mutations in the shRNA target site (SI Materials and Methods), increased the cccDNA:RC-RNA ratio as compared with cells cotransfected with empty vector, thus corroborating the TDP2-specificity of the effect (Fig. 7D). Altogether, these in-cell experiments established that reducing TDP2 by 80–90% markedly decelerated the conversion of RC-DNA to cccDNA, as expected if TDP2 is involved in the removal of P protein from RC-DNA as one of the mandatory steps in cccDNA formation.
Fig. 7.
Stable TDP2 knockdown in HepG2 cells significantly delays the conversion of RC-DNA to cccDNA. (A) Reduced huTDP2 levels in stable TDP2-knockdown HepG2 cell clones. TDP2 levels (bands marked with white asterisks) in naive HepG2 cells versus several HepG2 clones selected after transfection with a vector encoding shRNA si_TTRAP_4 were monitored by immunoblotting using rabbit anti-huTDP2 antiserum #6152 (SI Materials and Methods and Fig. S4). The black asterisk indicates a nonspecific signal. (B) TDP2 depletion slows the conversion of RC-DNA to cccDNA. Nuclear viral DNAs from naive or TDP2-depleted HepG2 cells transfected with the DHBVenv− vector harvested at the indicated days posttransfection were analyzed by Southern blotting. RC-DNA and cccDNA signals were quantified by phosphorimaging. For each time point, the ratio of cccDNA to RC-DNA in the knockdown cells was compared with that in the naive cells (set at 100%); the resulting relative ratios ± SD indicated at the bottom are derived from C. (C) Statistical significance. Individual relative cccDNA:RC-DNA ratios in TDP2 knockdown vs. naive HepG2 cells on days 2, 3, and 4 posttransfection from six independent experiments are shown as scatter plots. The horizontal bar represents the median. P values were derived by a paired two-tailed t test. (D) Ectopic TDP2 expression restores faster conversion of RC-DNA to cccDNA. TDP2-knockdown cells were cotransfected with the DHBVenv− vector plus pcDNA3.1 expression vectors for wild-type huTDP2 (wt), a variant with mutated shRNA target site (sh-res), or empty vector (Ø). RC-DNA and cccDNA were analyzed on day 3 posttransfection as in B. Relative cccDNA:RC-DNA ratios in cells transfected with theTDP2 expression vector and in cells transfected with empty vector (set at 100%), ± SD, are indicated at the bottom (n = 3).
Discussion
The central role of cccDNA in enabling productive infection by and persistence of hepadnaviruses is long established, but the mechanism by which cccDNA is formed from the incoming protein-bound RC-DNA genome remained elusive. Our in vitro data firmly establish that two distant vertebrate orthologs of TDP2, but only the yeast ortholog of TDP1, have the proper specific enzymatic activity to release P protein from DHBV and HBV RC-DNA. Furthermore, TDP2 depletion significantly decelerated the conversion of RC-DNA to cccDNA in cells; this observation is consistent with this DNA repair enzyme catalyzing an obligate step in the process. Given the biochemical data, this step is P protein release from the RC-DNA. Although we are only beginning to decipher the complete pathway from RC-DNA to cccDNA, our study provides the first experimental evidence, to our knowledge, that hepadnaviruses interact functionally with the cellular DNA repair machinery; given the multiple modifications RC-DNA must undergo for conversion into cccDNA, it is likely that this interaction is much more extensive.
Biochemical Evidence for a Role of Vertebrate TDP2 but Not TDP1 in P Protein Release from Hepadnaviral RC-DNA.
Cleavage of the P protein–RC-DNA linkage requires a 5′-phosphotyrosyl bond–specific activity. Hence TDP2 was a prime candidate (26, 27), but potential species-specific differences (18) and partly ambiguous literature data (19) did not exclude TDP1 a priori. Furthermore, the peculiarities of the hepadnaviral RC-DNA structure, including the large size of P protein (around 90 kDa), could affect whether one enzyme or the other is capable of specific P-protein release.
The fluorescence-based MUP-DNA assay allowed the 5′ and 3′ activities of numerous enzyme preparations to be compared simultaneously. Although deriving enzyme kinetic parameters from progress curves is complex (47), this straightforward analysis excluded the possibility that inactivity on one substrate was caused by general inactivity, and it revealed relative quantitative differences. All three TDP1 orthologs exerted high, bivalent metal ion–independent activity on the 3′ substrate, but, as reported (18), only yTDP1 was highly active on the 5′ substrate (Fig. 3). An obvious difference between yTDP1 and the vertebrate enzymes is the shorter N-terminal domain of yTDP1 (Fig. 2). However, deletion of the first 39 or 149 residues from recombinant huTDP1 did not unleash detectable activity in the 5′ substrate (48). The low but apparently specific 5′ activity of chTDP1 but not huTDP1 (Fig. 3E) indicates that conclusions obtained in avian systems, e.g., DT40 cells, may not apply strictly to mammalian cells. Taken together, however, these data made a role for TDP1 in HBV cccDNA formation unlikely. A remaining caveat is that in vivo TDP1 substrate specificity may be modulated by posttranslational modifications or by protein-interaction partners such as XRCC1 and PARP1 (49).
For huTDP2 and chTDP2, the assay confirmed a strictly bivalent metal ion-dependent 5′- as well as a lower 3′-phosphotyrosyl cleavage activity (Fig. 3 F and G), in accord with recent reports (20, 26, 35). Given the interest in TDP2 inhibitors for cancer therapy (13), we note that our fluorescent TDP2 assay should be easily adaptable to high-throughput applications with potential advantages over assays using low-affinity chromogenic substrates (42, 50).
All TDPs active on the 5′-model substrate also were able to cleave the covalent P protein–oligonucleotide complex formed during in vitro priming. Less than complete loss of label from the protein (Fig. 4A) might indicate that a fraction of the P-protein molecules were present as larger aggregates in which the scissile bond was sterically shielded; the notorious problems in keeping recombinant P protein soluble (9, 51) support this view. Cleavage specificity was demonstrated directly by the detectability of labeled oligonucleotides upon incubation with the wild-type but not the mutant TDP enzymes (Fig. 4 E–G). Their wider length distribution (up to six or seven residues) (Fig. 1B) compared with the 3 or 4 nt expected from genetic analyses might indicate that in vitro priming is less specific per se, although our data suggest that the authentic ε bulge template is used in vitro also. Because mutational impairment of primer transfer in cells facilitates repetitive copying of the same bulge template nucleotides (52), such polymerase slipping also may occur in vitro. Furthermore, ε-independent terminal transferase activity recently reported for HBV P protein expressed in mammalian cells (53) might contribute to the longer-than-expected priming products. Alternatively, oversized primers also may form in vivo but escape detection by assays that rely on prior transfer to DR1* (Fig. 1B). Of practical importance, yTDP1 and the vertebrate TDP2s lend themselves as tools to clarify these issues further.
More relevant here, TDP2 (and yTDP1) could accept the entire P protein–oligonucleotide complex as substrate, without prior proteasome-mediated proteolysis which appears required for efficient TDP-based repair of topoisomerase–DNA adducts (27, 54). A plausible explanation is the exposed position of the hepadnaviral phosphotyrosyl bond on the TP domain, which must be flexible enough to slide the pertinent Tyr residue into P protein's active site during priming.
Finally, the most compelling in vitro evidence for TDP2 as a potential cccDNA-relevant host factor comes from the ability of huTDP2 and chTDP2 to release P protein from authentic DHBV and HBV RC-DNA, as indicated by a similarly strong increase in water-soluble (i.e., non–protein-linked) viral DNA as that achieved by deliberate proteolysis (Fig. 5). That yeast TDP1 had the same capability is unlikely to be relevant for hepadnaviral infection. Why nucleocapsid-borne DHBV DNA was easily exposed by high-salt treatment but HBV DNA was not (Fig. S3) remains to be determined; although the two core proteins differ substantially in length and sequence, the capsids share a common architecture (55, 56). However, freezing in the presence of EB provided a novel way of permeabilizing HBV capsids. The preferential sensitivity of dsDNA containing HBV nucleocapsids and the comparable impact on DHBV nucleocapsids support the view that EB, sufficiently small to diffuse into capsids (57), intercalates into the packaged DNA, increases its space requirements, and thus destabilizes the capsid shell from within.
In-Cell Evidence for TDP2 Involvement in Hepadnaviral cccDNA Formation.
Given the multistep nature of the RC-DNA–to–cccDNA conversion (Fig. 1C), blocking any of the intermediate steps by inactivating the responsible factor(s) should prevent the accumulation of cccDNA. Consistently, in HepG2 cell clones stably transfected with the anti–huTDP2-shRNA vector and endurably expressing 80–90% lower TDP2 levels than the parental cells, the conversion of RC-DNA to cccDNA was slowed significantly (Fig. 7C); eventually, the relative and total amounts of the two DNA forms stabilized at similar levels in both cell backgrounds. Comparable results in knockdown cells with a different target site on the TDP2 mRNA and the recovery of faster conversion rates upon cotransfection with vectors for ectopic huTDP2 expression (Fig. 7D) made off-target effects unlikely. Furthermore, TDP2 depletion had no negative impact on cell viability or duplication rate.
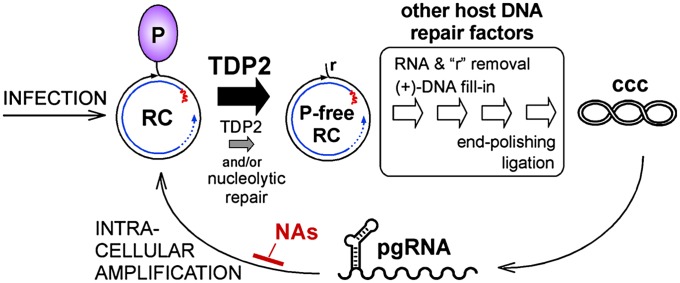
Combined with the biochemical data, a straightforward interpretation, summarized in Fig. 8, is that in the TDP2-depleted cells TDP2-dependent release of P protein from RC-DNA becomes rate limiting. Once the relative cccDNA:RC-DNA levels are equal those in naive HepG2 cells, the same unknown mechanism that limits further expansion of the cccDNA pool takes effect. These data strongly suggest that TDP2 is a host DNA-repair factor usurped by hepadnaviruses to generate cccDNA, which they need to establish and maintain infection.
Fig. 8.
Summary model and clinical implications. At normal levels, TDP2 efficiently releases P protein from RC-DNA (thick black arrow), and P-protein–free RC-DNA is processed further by other factors of the host DNA-repair machinery to yield cccDNA. P-protein release is not necessarily the first step in the pathway. In TDP2-depleted cells (smaller gray arrow), P-protein release becomes rate limiting, feeding less P-protein–free RC-DNA per time into the processing pathway and slowing the accumulation of cccDNA. Eventual formation of cccDNA levels similar to those in normal cells could be caused by residual TDP2 or, alternatively, by nucleolytic pathways. Clinically used NAs inhibit reverse transcription of cccDNA-derived pgRNA but not cccDNA formation and pgRNA production. Inhibition of TDP2 or other cccDNA-relevant host factors should provide a strategy for targeting the HBV persistence reservoir directly. A critical unresolved issue is whether the reported longevity of cccDNA holds on the individual-molecule level or reflects turnover and replenishment via intracellular amplification or infection.
However, the data leave room for more complex scenarios. First, the pleiotropic functions of TDP2 (25) could affect the conversion of RC-DNA to cccDNA indirectly; we consider this possibility unlikely, because RC-DNA is a proven substrate for TDP2's tyrosyl-DNA phosphodiesterase activity. A second issue is whether alternative nucleolytic pathways such as those safeguarding cells from TDP2 failure (58) could step in when TDP2 is limiting (Fig. 8). Slow formation of cccDNA in our TDP2-knockdown cells could be caused by residual TDP2 but also could reflect a contribution by alternative pathway(s); one candidate would be the MRE11/RAD50/NBS (MRN) complex, which is implicated in both TOP1- and TOP2-DNA adduct resolution (23) and (detrimentally to the virus) in removing the TP from the double-stranded adenovirus genome (32). To distinguish among these possibilities, we attempted to knock out TDP2 in Huh7 cells, using transcription activator-like effector nucleases targeting the region immediately downstream of the initiator ATG specified by the TDP2 mRNA (RefSeq accession no. NM_016614.2). However, in corresponding cell clones only the larger of the two anti-TDP2 reactive bands (Figs. S4B, S5 B and C, and S6A) was deleted, as is consistent with the smaller form representing an N-terminally truncated protein. In line with the absence of catalytic residues in the deleted region (Fig. 2B), a corresponding recombinant protein was active in the MUP-DNA assay. Hence the knockout cells were not functionally TDP2 deficient.
In any case, the multiple structural peculiarities of RC-DNA (Fig. 1C) indicate that additional host DNA-repair factors are involved in cccDNA conversion (Fig. 8). Examples are structure-specific endonucleases (59) involved in the removal of the terminal redundancy from minus-strand DNA or the RNA primer from plus-strand DNA, or the involvement of host DNA ligases in catalyzing the final step of cccDNA formation. Other such factors may be identified only by more comprehensive approaches, such as RNAi-based screening. Even small RNAs recently have been directly linked to DNA repair (60). Hence it is more than likely the interaction between hepadnaviruses and cellular DNA repair involves more than TDP2 alone.
Virological Implications.
Although the release of P protein from RC-DNA is evidently beneficial to hepadnaviruses, TDP's ability to clip off the short oligonucleotide generated during protein priming (Fig. 4) also poses the threat of premature termination of replication, especially because TDP2 is not a strictly nuclear protein (25). The typical hepadnavirus feature of restricting the bulk of reverse transcription to the inside of intact cytoplasmic nucleocapsids which then deliver the RC-DNA to the nuclear pore for release into the nucleoplasm (61) appears to be an appropriate strategy for avoiding such untimely encounters. If so, the most dangerous period would be the period immediately after P protein has bound to ε, triggering both pgRNA encapsidation and replication initiation (5, 6). The order of these events is unknown, but initiating assembly before reverse transcription would protect the replication–initiation complex from inappropriate TDP2 activity.
Medical Implications.
The persistence of cccDNA under current anti-HBV treatments is the key obstacle for eventually curative therapies. The most potent NAs achieve several-log reductions in circulating virions by inhibiting the reformation of RC-DNA (3), but transcription from cccDNA continues (Fig. 8). Completely purging cccDNA from a patient´s liver by this approach could take >50 y (62). Silencing the transcriptional activity of cccDNA (63) may suffer the same limitations. Our data suggest inactivation of TDP2, or any other host DNA-repair factor involved in the conversion of RC-DNA to cccDNA, as a means of interrupting the formation of the HBV persistence reservoir (Fig. 8). Targeting host factors has become a valid option in drug development (64), and TDP2 inhibitors already are in development to enhance the efficacy of TOP poisons in cancer therapy (13). However, several issues must be resolved before the conversion of RC-DNA to cccDNA can be considered a valid target for chronic hepatitis B treatment. One is the redundancy in DNA repair; if the redundancy is fully exploitable by HBV, inhibiting a single repair factor would have little impact. Conversely, if the virus were restricted to just one of several repair pathways, redundancy should minimize adverse effects on the cell. A second key issue is whether the longevity of cccDNA (ref. 41 and references therein) holds on the single cccDNA molecule level or instead results from replenishment by low-level intracellular amplification or infection (Fig. 8) because suppression of reverse transcription is not absolute. Without any turnover, blocking the conversion of RC-DNA to cccDNA would be restricted to prophylactic applications; if turnover occurs, such a blockade could be turned into a relevant complement to current therapies. As recently reported, high doses of IFN-α or, more efficiently, activation of the lymphotoxin-β receptor can induce cccDNA degradation experimentally (65), but about one third of the cccDNA remained refractory, available for later reamplification. Hence further investigation into how cccDNA is formed appears highly warranted.
Materials and Methods
Cell Culture and Transfection.
Huh7, HepG2, and LMH cells were maintained in DMEM F12 (PAA Laboratories) supplemented with 10% FCS, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37 °C and 5% CO2. Media for the TetOFF Dstet5 DHBVenv− (37) and HBV HepG2.117 lines (40) contained in addition 200 µg/mL G418 and 80 µg/mL hygromycin to maintain the integrated Tet-transactivator (tTA) and Tet-responsive element (TRE) promoter-controlled virus expression cassettes. Expression was repressed by 1 µg/mL of doxycycline (DOX) and was induced by its withdrawal. Transfections of plasmid DNA and siRNA were performed using TransIT-LT1 (Mirus) and HiPerFect transfection reagent (Qiagen), respectively, as recommended by the manufacturers.
Expression Vectors.
The DHBVenv− vector (39) and plasmid pET23b-yTDP1 (18), kindly provided by J. Nitiss (University of Illinois at Chicago, Chicago), have been described previously. Coding sequences for huTDP1 (NM_001008744.1) and huTDP2 (NM_016614.2) were amplified from sequence-verified cDNA clones (Source Bioscience); those for chTDP1 (XM_421313.4) and chTDP2 (NM_001276384.1) were from an LMH cell cDNA library. PCR primers provided terminal restriction sites for cloning into the prokaryotic expression vector pET30a(+) or the eukaryotic vector pcDNA3.1, as detailed in SI Materials and Methods. Enzymatically inactivated mutants (Fig. 2) were obtained by conventional mutagenic PCR.
Recombinant TDP Expression.
Wild-type and mutant TDP proteins were expressed in E. coli BL21-CodonPlus cells (Stratagene) and purified by sequential immobilized metal ion affinity chromatography and size-exclusion chromatography, as detailed in SI Materials and Methods. Representative purifications are shown in Fig. S1.
Fluorescent TDP Activity Assay.
MUP-DNA substrates were obtained from a commercial service (IBA GmbH) by carbodiimid-mediated condensation of 4-MU to a 3′-phosphorylated (33) or a 5′-phosphorylated (this work) DNA oligo of the sequence 5′-GTAATTCTTAAGTTG. Cleavage assays (total volume, 200 µL) were performed in 96-well flat-bottom plates in MUP-DNA buffer [50 mM Tris⋅HCl (pH 8.0), 80 mM KCl, 2 mM EDTA, 1 mM DTT, 40 µg/mL BSA, 10% DMSO] and 0.5 µM MUP-DNA, usually plus 100 nM TDP. For TDP2, the buffer was supplemented with 5 mM Mg2+ (Fig. S2). Time-resolved fluorescence was monitored in a Genios Pro Microplate Reader (Tecan) at 37 °C using a 355-nm excitation filter and a 460-nm emission filter.
P Protein–Oligonucleotide Primer Cleavage Assays.
In vitro priming was performed as previously described (9, 10) using recombinant GrpE-fused DHBV P protein and Hsc70 and Hsp40; in vitro transcribed DHBV ε RNA or a mutant containing an extended bulge region (ε b16) served as templates. Primer synthesis was induced by adding [α32P]-dGTP (3,000 Ci/mMol; Perkin-Elmer) and various combinations of other dNTPs. Equal aliquots of the reactions were incubated with the respective TDP proteins at 1.5-µM concentration at 37 °C for 30 min; thereafter, one-half of each sample was incubated with SDS sample buffer for SDS/PAGE and detection of 32P-labeled P protein. The other half was incubated with formamide gel buffer (95% formamide, 20 mM EDTA) for detection of clipped-off 32P-labeled oligonucleotides by urea-PAGE [7M urea, 20% acrylamide-bisacrylamide (19:1), 0.5× Tris-borate-EDTA]. Radiolabeled products were visualized by autoradiography or phosphorimaging (Typhoon FLA 7000, GE Healthcare) and quantified using ImageQuant software.
RC-DNA Susceptibility for TDP.
Conditions for exposing packaged viral DNAs were established by incubating cytoplasmic nucleocapsids from induced cultures of Dstet5 or HepG2.117 cells under various conditions and assessing their resistance or sensitivity toward micrococcal nuclease, as detailed in SI Materials and Methods and Fig. S3. The TDP-mediated release of P protein from RC-DNA was assessed by incubation with 50 nM wild-type or mutant TDP2 or 30 nM yTDP1 for 1 h at 37 °C, followed by phenol extraction and ethanol precipitation of the water-soluble DNA and subsequent Southern blotting.
Susceptibility of huTDP2-Treated RC-DNA for λ 5′→3′ Exonuclease.
Cytoplasmic DHBV nucleocapsids from Dstet5 cells were treated with 0.5 mg/mL PK for 1 h at 37 °C. After phenol extraction and ethanol precipitation, DNA from the aqueous phase was incubated in Mg2+-supplemented MUP-DNA buffer with 25 nM huTDP2 for 2 h at 37 °C. After a second round of phenol extraction and ethanol precipitation, the DNA was dissolved in 10 µL water plus 1 µL 10× λ-exonuclease-buffer and was treated with 2.5 U λ exonuclease (New England Biolabs) for 2 h at 37 °C. Products were analyzed by Southern blotting using minus-strand– or plus-strand–specific DHBV riboprobes.
Nuclear Viral DNA.
Transfected cells were lysed in Nonidet P-40 lysis buffer, and the nuclei were separated by low-speed centrifugation as previously described (39). Nuclear DNAs were prepared using the QiaAmp Blood Mini Kit (Qiagen) according to the manufacturer´s tissue protocol for subsequent Southern blotting.
Southern Blotting.
Southern blotting was performed as previously described (39). 32P-labeled DNA probes were obtained by random priming using cloned full-length DHBV or HBV genomes as templates. Strand-specific riboprobes were obtained by in vitro transcription from a pBluescript plasmid containing the complete DHBV genome between T7 and T3 RNA polymerase promoters.
Validation of TDP2 Knockdown Efficiency.
Rabbit polyclonal anti-TDP2 antisera were obtained from a commercial service (Biogenes) using recombinant huTDP2 and chTDP2 as immunogens. Specificity and sensitivity of TDP2 detection were determined as detailed in Fig. S4. Using these antisera, TDP2 levels in Huh7 cells transiently transfected with four commercial huTDP2-specific and two control siRNAs (Qiagen) were evaluated as detailed in Fig. S5. Efficient target sites for shRNAs on huTDP2 and chTDP2 mRNA were identified analogously after transient transfection with human H1 promoter-driven shRNA vectors (45), as detailed in Fig. S7.
Stable Cell Lines.
A Tet-regulatable DHBVenv−-expressing Huh7 TetOFF cell clone was obtained by transfection of the tTA-containing line Huh7.TA61 (40) with a pTRE2hyg–based (Clontech) vector producing DHBVenv− pgRNA under the control of a Tet-responsive promoter and selection with hygromycin. RC-DNA and cccDNA induction by DOX withdrawal are shown in Fig. S6. Stably huTDP2-silenced HepG2 cell clones were obtained by transfection of pSuperior.neo+GFP–based vectors (Oligoengine) encoding the shRNAs siTTRAP_1 and siTTRAP_4 (46), enrichment of transfected cells by sorting for GFP+, and G418 selection.
Supplementary Material
Acknowledgments
We thank Dr. S. Gustincich for selectable pSuperior vectors encoding shRNAs siTTRAP_1 and siTTRAP_4; Dr. J. Nitiss for vector pET23b-yTDP1; and Prof. Dr. Hubert E. Blum for his continuous support. This work was supported by the Deutsche Forschungsgemeinschaft Grant Na154/12-1,2 within the Collaborative Research Unit 1202 (Persistence of Hepatotropic Viruses).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1409986111/-/DCSupplemental.
References
- 1.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: New estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30(12):2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 2.Ganem D, Prince AM. Hepatitis B virus infection—natural history and clinical consequences. N Engl J Med. 2004;350(11):1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 3.Zoulim F, Locarnini S. Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology. 2009;137(5):1593–1608 e1591-1592. doi: 10.1053/j.gastro.2009.08.063. [DOI] [PubMed] [Google Scholar]
- 4.Scaglione SJ, Lok AS. Effectiveness of hepatitis B treatment in clinical practice. Gastroenterology. 2012;142(6):1360–1368 e1361. doi: 10.1053/j.gastro.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 5.Beck J, Nassal M. Hepatitis B virus replication. World J Gastroenterol. 2007;13(1):48–64. doi: 10.3748/wjg.v13.i1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nassal M. Hepatitis B viruses: Reverse transcription a different way. Virus Res. 2008;134(1-2):235–249. doi: 10.1016/j.virusres.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 7.Hu J, Anselmo D. In vitro reconstitution of a functional duck hepatitis B virus reverse transcriptase: Posttranslational activation by Hsp90. J Virol. 2000;74(24):11447–11455. doi: 10.1128/jvi.74.24.11447-11455.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck J, Nassal M. Reconstitution of a functional duck hepatitis B virus replication initiation complex from separate reverse transcriptase domains expressed in Escherichia coli. J Virol. 2001;75(16):7410–7419. doi: 10.1128/JVI.75.16.7410-7419.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck J, Nassal M. Efficient Hsp90-independent in vitro activation by Hsc70 and Hsp40 of duck hepatitis B virus reverse transcriptase, an assumed Hsp90 client protein. J Biol Chem. 2003;278(38):36128–36138. doi: 10.1074/jbc.M301069200. [DOI] [PubMed] [Google Scholar]
- 10.Stahl M, Retzlaff M, Nassal M, Beck J. Chaperone activation of the hepadnaviral reverse transcriptase for template RNA binding is established by the Hsp70 and stimulated by the Hsp90 system. Nucleic Acids Res. 2007;35(18):6124–6136. doi: 10.1093/nar/gkm628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuttleman JS, Pourcel C, Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell. 1986;47(3):451–460. doi: 10.1016/0092-8674(86)90602-1. [DOI] [PubMed] [Google Scholar]
- 12.Chen SH, Chan NL, Hsieh TS. New mechanistic and functional insights into DNA topoisomerases. Annu Rev Biochem. 2013;82:139–170. doi: 10.1146/annurev-biochem-061809-100002. [DOI] [PubMed] [Google Scholar]
- 13.Pommier Y. Drugging topoisomerases: Lessons and challenges. ACS Chem Biol. 2013;8(1):82–95. doi: 10.1021/cb300648v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 15.Deriano L, Roth DB. Modernizing the nonhomologous end-joining repertoire: Alternative and classical NHEJ share the stage. Annu Rev Genet. 2013;47:433–455. doi: 10.1146/annurev-genet-110711-155540. [DOI] [PubMed] [Google Scholar]
- 16.Huang SN, Pommier Y, Marchand C. Tyrosyl-DNA Phosphodiesterase 1 (Tdp1) inhibitors. Expert Opin Ther Pat. 2011;21(9):1285–1292. doi: 10.1517/13543776.2011.604314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang SW, et al. A eukaryotic enzyme that can disjoin dead-end covalent complexes between DNA and type I topoisomerases. Proc Natl Acad Sci USA. 1996;93(21):11534–11539. doi: 10.1073/pnas.93.21.11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nitiss KC, Malik M, He X, White SW, Nitiss JL. Tyrosyl-DNA phosphodiesterase (Tdp1) participates in the repair of Top2-mediated DNA damage. Proc Natl Acad Sci USA. 2006;103(24):8953–8958. doi: 10.1073/pnas.0603455103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murai J, et al. Tyrosyl-DNA phosphodiesterase 1 (TDP1) repairs DNA damage induced by topoisomerases I and II and base alkylation in vertebrate cells. J Biol Chem. 2012;287(16):12848–12857. doi: 10.1074/jbc.M111.333963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng Z, et al. TDP2 promotes repair of topoisomerase I-mediated DNA damage in the absence of TDP1. Nucleic Acids Res. 2012;40(17):8371–8380. doi: 10.1093/nar/gks622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pommier Y, et al. Repair of topoisomerase I-mediated DNA damage. Prog Nucleic Acid Res Mol Biol. 2006;81:179–229. doi: 10.1016/S0079-6603(06)81005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maede Y, et al. Differential and common DNA repair pathways for topoisomerase I- and II-targeted drugs in a genetic DT40 repair cell screen panel. Mol Cancer Ther. 2014;13(1):214–220. doi: 10.1158/1535-7163.MCT-13-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartsuiker E, Neale MJ, Carr AM. Distinct requirements for the Rad32(Mre11) nuclease and Ctp1(CtIP) in the removal of covalently bound topoisomerase I and II from DNA. Mol Cell. 2009;33(1):117–123. doi: 10.1016/j.molcel.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura K, et al. Collaborative action of Brca1 and CtIP in elimination of covalent modifications from double-strand breaks to facilitate subsequent break repair. PLoS Genet. 2010;6(1):e1000828. doi: 10.1371/journal.pgen.1000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, Sun SY, Khuri FR, Li R. Pleiotropic functions of EAPII/TTRAP/TDP2: Cancer development, chemoresistance and beyond. Cell Cycle. 2011;10(19):3274–3283. doi: 10.4161/cc.10.19.17763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortes Ledesma F, El Khamisy SF, Zuma MC, Osborn K, Caldecott KW. A human 5′-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature. 2009;461(7264):674–678. doi: 10.1038/nature08444. [DOI] [PubMed] [Google Scholar]
- 27.Caldecott KW. Tyrosyl DNA phosphodiesterase 2, an enzyme fit for purpose. Nat Struct Mol Biol. 2012;19(12):1212–1213. doi: 10.1038/nsmb.2455. [DOI] [PubMed] [Google Scholar]
- 28.Schellenberg MJ, et al. Mechanism of repair of 5′-topoisomerase II-DNA adducts by mammalian tyrosyl-DNA phosphodiesterase 2. Nat Struct Mol Biol. 2012;19(12):1363–1371. doi: 10.1038/nsmb.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi K, et al. Structural basis for recognition of 5′-phosphotyrosine adducts by Tdp2. Nat Struct Mol Biol. 2012;19(12):1372–1377. doi: 10.1038/nsmb.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng Z, Cortés-Ledesma F, El Khamisy SF, Caldecott KW. TDP2/TTRAP is the major 5′-tyrosyl DNA phosphodiesterase activity in vertebrate cells and is critical for cellular resistance to topoisomerase II-induced DNA damage. J Biol Chem. 2011;286(1):403–409. doi: 10.1074/jbc.M110.181016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Virgen-Slane R, et al. An RNA virus hijacks an incognito function of a DNA repair enzyme. Proc Natl Acad Sci USA. 2012;109(36):14634–14639. doi: 10.1073/pnas.1208096109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weitzman MD, Lilley CE, Chaurushiya MS. Genomes in conflict: Maintaining genome integrity during virus infection. Annu Rev Microbiol. 2010;64:61–81. doi: 10.1146/annurev.micro.112408.134016. [DOI] [PubMed] [Google Scholar]
- 33.Rideout MC, Raymond AC, Burgin AB., Jr Design and synthesis of fluorescent substrates for human tyrosyl-DNA phosphodiesterase I. Nucleic Acids Res. 2004;32(15):4657–4664. doi: 10.1093/nar/gkh796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao R, Huang SY, Marchand C, Pommier Y. Biochemical characterization of human tyrosyl-DNA phosphodiesterase 2 (TDP2/TTRAP): A Mg(2+)/Mn(2+)-dependent phosphodiesterase specific for the repair of topoisomerase cleavage complexes. J Biol Chem. 2012;287(36):30842–30852. doi: 10.1074/jbc.M112.393983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones SA, Boregowda R, Spratt TE, Hu J. In vitro epsilon RNA-dependent protein priming activity of human hepatitis B virus polymerase. J Virol. 2012;86(9):5134–5150. doi: 10.1128/JVI.07137-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radziwill G, Zentgraf H, Schaller H, Bosch V. The duck hepatitis B virus DNA polymerase is tightly associated with the viral core structure and unable to switch to an exogenous template. Virology. 1988;163(1):123–132. doi: 10.1016/0042-6822(88)90239-5. [DOI] [PubMed] [Google Scholar]
- 37.Guo JT, et al. Conditional replication of duck hepatitis B virus in hepatoma cells. J Virol. 2003;77(3):1885–1893. doi: 10.1128/JVI.77.3.1885-1893.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerlich WH, Robinson WS. Hepatitis B virus contains protein attached to the 5′ terminus of its complete DNA strand. Cell. 1980;21(3):801–809. doi: 10.1016/0092-8674(80)90443-2. [DOI] [PubMed] [Google Scholar]
- 39.Köck J, et al. Generation of covalently closed circular DNA of hepatitis B viruses via intracellular recycling is regulated in a virus specific manner. PLoS Pathog. 2010;6(9):e1001082. doi: 10.1371/journal.ppat.1001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun D, Nassal M. Stable HepG2- and Huh7-based human hepatoma cell lines for efficient regulated expression of infectious hepatitis B virus. J Hepatol. 2006;45(5):636–645. doi: 10.1016/j.jhep.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 41.Reaiche-Miller GY, et al. Duck hepatitis B virus covalently closed circular DNA appears to survive hepatocyte mitosis in the growing liver. Virology. 2013;446(1-2):357–364. doi: 10.1016/j.virol.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Thomson G, et al. Generation of assays and antibodies to facilitate the study of human 5′-tyrosyl DNA phosphodiesterase. Anal Biochem. 2013;436(2):145–150. doi: 10.1016/j.ab.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Li C, et al. Oncogenic role of EAPII in lung cancer development and its activation of the MAPK-ERK pathway. Oncogene. 2011;30(35):3802–3812. doi: 10.1038/onc.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ichihara M, et al. Thermodynamic instability of siRNA duplex is a prerequisite for dependable prediction of siRNA activities. Nucleic Acids Res. 2007;35(18):e123. doi: 10.1093/nar/gkm699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun D, Rösler C, Kidd-Ljunggren K, Nassal M. Quantitative assessment of the antiviral potencies of 21 shRNA vectors targeting conserved, including structured, hepatitis B virus sites. J Hepatol. 2010;52(6):817–826. doi: 10.1016/j.jhep.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 46.Zucchelli S, et al. Aggresome-forming TTRAP mediates pro-apoptotic properties of Parkinson’s disease-associated DJ-1 missense mutations. Cell Death Differ. 2009;16(3):428–438. doi: 10.1038/cdd.2008.169. [DOI] [PubMed] [Google Scholar]
- 47.Nikolova N, Tenekedjiev K, Kolev K. Uses and misuses of progress curve analysis in enzyme kinetics. Cent Eur J Biol. 2008;3(4):345–350. doi: 10.2478/s11535-008-0035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Königer C (2014) Impact of cellular DNA repair factors on formation of the covalently closed circular (ccc) DNA of hepatitis B viruses. PhD thesis (University of Freiburg, Freiburg, Germany)
- 49.Das BB, et al. PARP1-TDP1 coupling for the repair of topoisomerase I-induced DNA damage. Nucleic Acids Res. 2014;42(7):4435–4449. doi: 10.1093/nar/gku088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adhikari S, et al. Development of a novel assay for human tyrosyl DNA phosphodiesterase 2. Anal Biochem. 2011;416(1):112–116. doi: 10.1016/j.ab.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vörös J, et al. Large-scale production and structural and biophysical characterizations of the human hepatitis B virus polymerase. J Virol. 2014;88(5):2584–2599. doi: 10.1128/JVI.02575-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nassal M, Rieger A. A bulged region of the hepatitis B virus RNA encapsidation signal contains the replication origin for discontinuous first-strand DNA synthesis. J Virol. 1996;70(5):2764–2773. doi: 10.1128/jvi.70.5.2764-2773.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones SA, Hu J. Protein-primed terminal transferase activity of hepatitis B virus polymerase. J Virol. 2013;87(5):2563–2576. doi: 10.1128/JVI.02786-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Interthal H, Champoux JJ. Effects of DNA and protein size on substrate cleavage by human tyrosyl-DNA phosphodiesterase 1. Biochem J. 2011;436(3):559–566. doi: 10.1042/BJ20101841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kenney JM, von Bonsdorff CH, Nassal M, Fuller SD. Evolutionary conservation in the hepatitis B virus core structure: Comparison of human and duck cores. Structure. 1995;3(10):1009–1019. doi: 10.1016/s0969-2126(01)00237-4. [DOI] [PubMed] [Google Scholar]
- 56.Nassal M, et al. A structural model for duck hepatitis B virus core protein derived by extensive mutagenesis. J Virol. 2007;81(23):13218–13229. doi: 10.1128/JVI.00846-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Birnbaum F, Nassal M. Hepatitis B virus nucleocapsid assembly: Primary structure requirements in the core protein. J Virol. 1990;64(7):3319–3330. doi: 10.1128/jvi.64.7.3319-3330.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nitiss JL, Nitiss KC. Tdp2: A means to fixing the ends. PLoS Genet. 2013;9(3):e1003370. doi: 10.1371/journal.pgen.1003370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsutakawa SE, Lafrance-Vanasse J, Tainer JA. The cutting edges in DNA repair, licensing, and fidelity: DNA and RNA repair nucleases sculpt DNA to measure twice, cut once. DNA Repair (Amst) 2014;19:95–107. doi: 10.1016/j.dnarep.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.d’Adda di Fagagna F. A direct role for small non-coding RNAs in DNA damage response. Trends Cell Biol. 2014;24(3):171–178. doi: 10.1016/j.tcb.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 61.Kann M, Schmitz A, Rabe B. Intracellular transport of hepatitis B virus. World J Gastroenterol. 2007;13(1):39–47. doi: 10.3748/wjg.v13.i1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chevaliez S, Hézode C, Bahrami S, Grare M, Pawlotsky JM. Long-term hepatitis B surface antigen (HBsAg) kinetics during nucleoside/nucleotide analogue therapy: Finite treatment duration unlikely. J Hepatol. 2013;58(4):676–683. doi: 10.1016/j.jhep.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 63.Levrero M, et al. Control of cccDNA function in hepatitis B virus infection. J Hepatol. 2009;51(3):581–592. doi: 10.1016/j.jhep.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 64.Zeisel MB, Lupberger J, Fofana I, Baumert TF. Host-targeting agents for prevention and treatment of chronic hepatitis C - perspectives and challenges. J Hepatol. 2013;58(2):375–384. doi: 10.1016/j.jhep.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 65.Lucifora J, et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343(6176):1221–1228. doi: 10.1126/science.1243462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.