Significance
Antarctic notothenioid fishes are protected from freezing by antifreeze proteins (AFPs) that bind to invading ice crystals and inhibit their growth. Paradoxically, accumulation of AFP-stabilized ice could be lethal. Whether and how fishes eliminate internal ice is unknown; one hypothesis is that it melts during summer warming episodes. However, prior in vitro evidence indicates that AFPs also inhibit melting. Our study establishes that pronounced melting inhibition occurs in vivo (i.e., superheated ice occurs inside notothenioid fishes). Our long-term temperature record of a high-latitude Antarctic fish habitat indicates that summer warming does not overcome AFP-induced superheating to reliably rid fishes of ice. Evolution of the life-saving AFPs exacts a cost: the risk of lifelong accumulation of damaging internal ice crystals.
Keywords: antagonistic pleiotropy, antifreeze glycoprotein, antifreeze potentiating protein, McMurdo Sound temperature, melting hysteresis
Abstract
Antifreeze proteins (AFPs) of polar marine teleost fishes are widely recognized as an evolutionary innovation of vast adaptive value in that, by adsorbing to and inhibiting the growth of internalized environmental ice crystals, they prevent death by inoculative freezing. Paradoxically, systemic accumulation of AFP-stabilized ice could also be lethal. Whether or how fishes eliminate internal ice is unknown. To investigate if ice inside high-latitude Antarctic notothenioid fishes could melt seasonally, we measured its melting point and obtained a decadal temperature record from a shallow benthic fish habitat in McMurdo Sound, Antarctica. We found that AFP-stabilized ice resists melting at temperatures above the expected equilibrium freezing/melting point (eqFMP), both in vitro and in vivo. Superheated ice was directly observed in notothenioid serum samples and in solutions of purified AFPs, and ice was found to persist inside live fishes at temperatures more than 1 °C above their eqFMP for at least 24 h, and at a lower temperature for at least several days. Field experiments confirmed that superheated ice occurs naturally inside wild fishes. Over the long-term record (1999–2012), seawater temperature surpassed the fish eqFMP in most summers, but never exceeded the highest temperature at which ice persisted inside experimental fishes. Thus, because of the effects of AFP-induced melting inhibition, summer warming may not reliably eliminate internal ice. Our results expose a potentially antagonistic pleiotropic effect of AFPs: beneficial freezing avoidance is accompanied by melting inhibition that may contribute to lifelong accumulation of detrimental internal ice crystals.
Various polar teleost fishes rely on the presence of antifreeze proteins (AFPs) in their blood and other body fluids to survive in the freezing seawater (−1.9 °C) of the world’s polar oceans. These special proteins irreversibly bind to ice crystals that enter the body, depressing the temperature at which ice will grow below the equilibrium freezing/melting point (eqFMP) of body fluids (approximately −0.7 to −1.0 °C) to a lower nonequilibrium hysteresis freezing point (hFP) (1–3). This departure of the actual from the expected freezing temperature, termed freezing hysteresis (FH = eqFMP − hFP), prevents the rapid onset of organismal freezing that occurs upon the entry of ice into unprotected fishes (Fig. S1). Paradoxically, the evolution of this adsorption-inhibition mechanism of freezing avoidance has created another problem: AFP-stabilized ice crystals could accrue within the body (4, 5) to the extent that they may occlude blood vessels and interfere with essential physiological functions (6).
Fishes inhabiting the high-latitude coastal regions of Antarctica, where ice is prevalent and low temperatures occur year-round (7, 8), risk accumulating ice within the body. By virtue of their two AFPs, antifreeze glycoprotein (AFGP) (1) and antifreeze potentiating protein (AFPP) (9), members of the perciform suborder, Notothenioidei, thrive in and overwhelmingly dominate these environments (10, 11). In ice-laden McMurdo Sound in the southwestern Ross Sea (approximately 78°S) (Fig. 1), the common notothenioid species include shallow benthic members of the genus Trematomus and the cryopelagic Pagothenia borchgrevinki (12). Systemic invasion of environmental ice is common in these fishes, which endure routine contact with ice on the sea floor (anchor ice) (13) and on the surface of the ocean, and ingest ice crystals during feeding and drinking in freezing seawater (12). Ice is regularly found associated with their superficial structures, such as the integument, gill epithelium, and gastrointestinal tract, and in the spleen (4, 5, 10). The presence of ice in the deep-seated spleen indicates that it can penetrate the ice-resistant epithelial barrier (14, 15) and transit the circulation. Notably, adult notothenioids require only a few hours to days of exposure to icy seawater to acquire splenic ice (4), indicating that they could accumulate a substantial ice load over time.
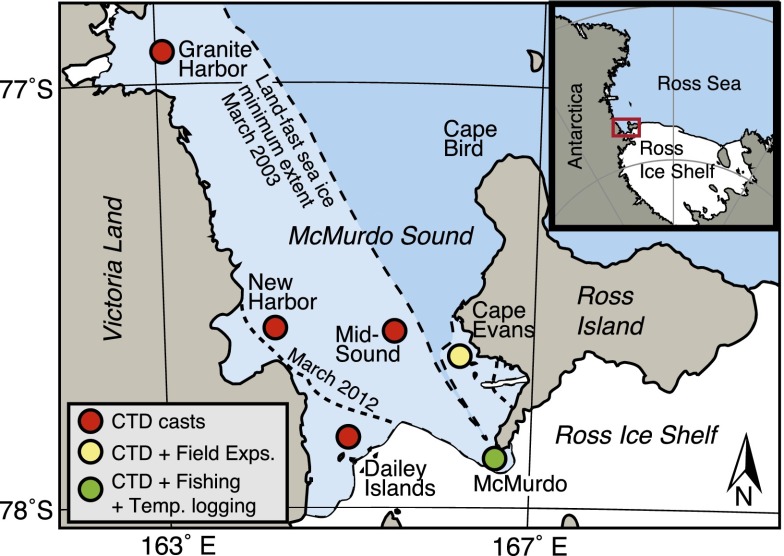
Fig. 1.
Locations of study sites in McMurdo Sound, Antarctica. Colored circles indicate approximate locations of conductivity (salinity), temperature, and depth (CTD) profiler casts, field experiments, collection sites, and a long-term temperature record. McMurdo Sound is covered by sea ice for much of each year. Dashed lines indicate the annual minimum extent of land-fast sea ice in 2 y of the study (estimated from satellite imagery). GPS coordinates are given in Table S3.
Whether and how fishes eliminate potentially problematic internal ice is unknown. One study of McMurdo Sound notothenioids suggested that macrophages in the spleen play a role in immobilizing AFP-stabilized ice crystals (demonstrated with AFP-conjugated silica nanoparticles) that have invaded the circulatory system (6). However, whether internal ice is then removed by physiological or biochemical means remains unresolved. In another study, one experiment suggested that Antarctic notothenioids might in fact lack such a mechanism, in that no specimens of the McMurdo Sound P. borchgrevinki eliminated their naturally acquired splenic ice during a lengthy stay (6 wk) in a frigid but ice-free aquarium (4).
An obvious alternate mechanism for the elimination of internal ice is by passive thermal melting when fish are exposed to temperatures above their eqFMP. Experimentally warming notothenioids to 4 °C for 1 h completely melts internal ice from these ectothermic fishes (4), but this is far above the maximum seawater temperature expected for much of coastal Antarctica (8). For fishes in McMurdo Sound, thermal melting of internal ice has historically been considered impossible on the belief that seawater there is perennially at its freezing point, approximately 1 °C below the eqFMP of fish body fluids (16, 17). More recently, continuous logging in McMurdo Sound over 2 y revealed temperatures slightly above the notothenioid fish eqFMP (approximately −1.0 °C) during brief summer warming episodes (tmax = −0.35 and −0.65 °C for 1999–2000 and 2000–2001, respectively) (17). In principle therefore, ice crystals that have accumulated internally over the many months of the year when the seawater is at its freezing point might be melted on at least an annual basis. However, whether internal ice melts readily at temperatures above the fish eqFMP is unknown.
It has been observed in vitro that AFPs can inhibit the melting of ice, thereby allowing it to persist in a superheated state at temperatures above the eqFMP. This underrecognized property of AFPs was first demonstrated 25 y ago by Knight and DeVries using purified notothenioid AFGPs (18). The authors reasoned that melting hysteresis (MH = hMP – eqFMP) was a consequence of the irreversible binding of AFPs required for the beneficial prevention of ice crystal growth through FH (18, 19). A more recent study further documented MH by directly observing superheated ice in solutions of AFPs from several insects, non-Antarctic fishes, and a sea-ice bacterium (20), reporting a maximum superheating of 0.44 °C. Accounting for AFP-induced melting inhibition would further diminish thermal melting as a mechanism for fishes to eliminate internal ice. However, no studies have demonstrated the occurrence of superheated ice within an organism or in nature, nor addressed the associated physiological or organismal ramifications of melting inhibition in AFP-bearing organisms. A conceptual framework for the effects of AFPs and temperature on the fate of internal ice in polar fishes is presented in Fig. S1.
The in vitro observation of MH portends unfavorable in vivo consequences for AFP-bearing fishes. In perennially frigid high-Antarctic environments, even moderate superheating of internal ice could substantially restrict opportunities for fishes to reduce or eliminate their ice load by melting during the brief and attenuated summer warming episodes. In this study, we investigated the occurrence of AFP-induced ice superheating in vitro and in vivo, and how this phenomenon may affect the melting of ice that occurs naturally inside McMurdo Sound fishes. We measured MH in blood sera of notothenioid fishes and determined the contribution of their AFPs to the superheating effect. We tested the magnitude and duration of heating above the eqFMP that ice could withstand in vivo and whether superheated ice occurs naturally in notothenioid fishes in the wild. Finally, to assess the physiological implications of MH in the high-latitude members of this dominant Antarctic fish lineage, we related our experimental results to oceanographic data, including a long-term seawater temperature record that we obtained from a shallow benthic fish habitat in McMurdo Sound.
Results
Ice Crystals Resist Melting in Notothenioid Sera and Solutions of Their Purified AFPs.
In the absence of AFPs, the ice growth-to-melting transition occurs precisely at the eqFMP, a colligative property dependent on the osmotic concentration of the solution (21). Because MH complicates measuring the eqFMP of AFP-containing solutions by direct observation of ice crystal melting (20), we calculated the eqFMP of samples from their osmotic concentration determined with a vapor pressure osmometer (eqFMP = −1.858 × osmolality in Osm/kg) (1).
The mean serum eqFMP of five common notothenioid species from McMurdo Sound was −1.04 °C (range −1.11 to −0.93 °C) (Fig. 2A). We used this value to represent the nototothenioid fish eqFMP: the temperature at which internal ice should grow or melt in the absence of AFP-induced FH or MH, respectively. As shown for Trematomus pennellii, the eqFMPs of other body fluids are similar to that of serum (range −1.21 to −0.89 °C) (Fig. 2A).
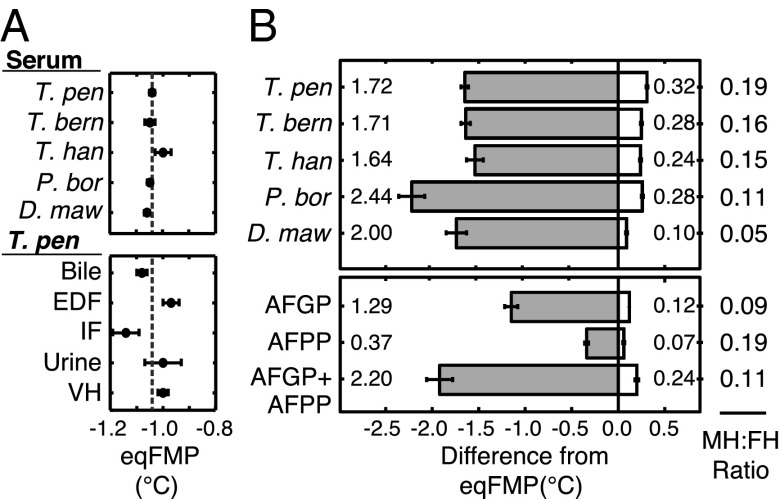
Fig. 2.
Superheating of ice in fish serum is caused by AFPs. (A) The eqFMPs of the blood sera of various notothenioid species (Upper) and of other fluids of T. pennellii (Lower) were determined with a vapor pressure osmometer (mean ± SE; n = 3–15 individuals for each point). The mean serum eqFMP for all species tested (−1.04 °C) is indicated by a dashed line; we used this value as a proxy for the eqFMP of McMurdo Sound notothenioid fishes. EDF, extradural fluid; IF, intestinal fluid; and VH, vitreous humor. Species names shortened from T. pennellii, T. bernacchii, T. hansoni, P. borchgrevinki, and D. mawsoni. (B) Ice persisted in vitro at temperatures both above and below solution eqFMPs. Nonequilibrium hFPs (shaded bars) and hMPs (white bars) shown as the difference from the eqFMPs of serum (Upper) and of purified AFPs at physiological concentration in buffer (Lower). Values (mean ± SE) for a single sample assayed three to four times. The greatest observed values of MH and FH are enumerated, with the corresponding MH:FH ratio shown at right.
To determine the hFPs and hMPs of solutions, we used a light microscope and a freezing-point osmometer modified to allow precise control of sample temperature and rate of change (0.002 °C resolution). For AFP solutions, the hFP is commonly defined as the lowest temperature at which a single small ice crystal (2–10 µm) overcomes inhibition from adsorbed AFPs and grows uncontrollably (14). We measured hFP in this manner. Serum hFP values for all fishes assayed were more than 1.5 °C below their eqFMPs (Fig. 2B), thus these species are protected against freezing in icy seawater (approximately −1.9 °C).
We measured hMPs of samples under conditions simulating those experienced by shallow-living Antarctic fishes in nature. We began each measurement with a population of ice crystals (approximately 20–200) of a size that could conceivably enter a fish and circulate through the vasculature (approximately 2- to 100-µm long). Following a period of annealing to allow the ice-AFP interaction to stabilize as it would in nature under winter conditions (10 min at 0.32 °C below the eqFMP), we warmed samples slowly at a rate (0.03 °C/min) comparable to the maximum warming rates recorded in McMurdo Sound (0.01–0.09 °C/min) (Table S2). Ice crystals melted sporadically as samples were warmed above the eqFMP (Movie S1); we took the temperature at which the last visible ice crystal disappeared as the hMP.
We observed ice crystals superheated above the eqFMP in the sera of all species tested, with the greatest MH observed in T. pennellii serum (0.32 °C) (Fig. 2B). In these experiments, the magnitude of MH was never more than 20% of FH (Fig. 2B). However, smaller ice crystals that are invisible using our techniques must persist to even higher temperatures than the observed hMP, because it was often impossible to undercool samples below the hFP (approximately −3.5 to −2.5 °C) (Fig. 2) following the disappearance of the last visible crystal, whereas ice-free serum did not freeze spontaneously at temperatures warmer than −10 °C. Thus, the maximum MH that we directly observed in vitro is almost certainly an underestimate of the maximum possible superheating of ice in serum. We confirmed that the two endogenous notothenioid AFPs were responsible for the observed superheating of ice. When purified and reconstituted in buffer at concentrations typical of the sera of McMurdo Sound trematomin fishes (9), AFGP and AFPP each exhibited MH that was not observed in buffer alone. When reconstituted together in buffer, these AFPs acted synergistically to account for the majority (75%) of the greatest MH observed in serum (Fig. 2B).
Superheated Ice Occurs Inside Live Notothenioid Fishes.
Given the pronounced melting inhibition observed in vitro, we expected that superheated ice would also occur in vivo in AFP-endowed fishes. We hypothesized that the magnitude of MH observed in vitro could be augmented or diminished for ice crystals entrained within the dynamic physiological system of a living fish, and would differ for crystals formed and acquired naturally in the environment compared with those created experimentally. We thus performed a series of experiments to assess the long-term stability of naturally acquired and experimentally introduced ice inside notothenioid fishes held at temperatures above their eqFMP.
Notothenioid fishes were collected from wintertime conditions in McMurdo Sound and transported to and maintained in the laboratory at seawater temperatures (−1.8 to −1.5 °C) within the FH interval, so that internal ice could neither grow nor melt (Fig. S1). Under these conditions, all individuals test positive for internal ice (4, 5, 10). Fishes were then either (i) maintained under conditions that did not melt naturally occurring internal ice, or (ii) warmed to 4 °C for 1 h to melt ice acquired in the environment and then cooled to −1.5 °C before introducing ice by briefly spray-freezing a small patch of skin. Spray-freeze inoculated ice unequivocally becomes systemic in all specimens, because it appears rapidly in the circulation and is quickly (<1 h) distributed throughout the body (4). In both treatments, following a 12-h period at temperatures (−1.8 to −1.5 °C) within the FH interval to allow ice to disperse throughout the body (4), fishes were transferred to a temperature-controlled aquarium and maintained for a prescribed period at a set temperature (the experimental warming treatment). We then performed undercooling assays in which we tested the ability of fishes to avoid freezing during a short-term immersion (10 min) in a glycerol-seawater solution at a temperature (−6 °C) substantially below their hFP (approximately −3.5 to −2.5 °C) (Fig. 2) (4, 22). Ice-free fishes lack nuclei that could initiate ice growth within the fish at this temperature, and therefore do not freeze spontaneously even after >1-h immersion (4). Thus, the outcome of this assay reveals whether at least a single internal ice crystal withstood the warming treatment.
Individuals from all tested species retained internal ice after long periods (24–72 h) of exposure to temperatures above their eqFMP (i.e., ice was stably superheated in vivo) (Fig. 3). Superheated ice must be harbored inside fishes and not loosely associated with external surfaces, because superficial crystals would be bathed in seawater and melt at its melting point, approximately −1.9 °C. Moreover, we confirmed that experimental fishes quickly equilibrate to the aquarium temperature (<10–30 min, depending on body size) by using implanted microthermocouples to record deep body temperature following their transfer to an elevated temperature aquarium (SI Results and Fig. S2).
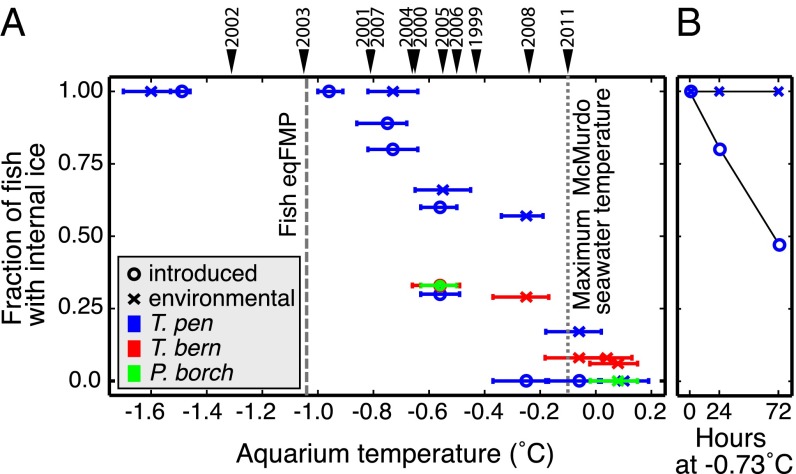
Fig. 3.
Superheated ice occurs inside live notothenioid fishes. (A) Fraction of McMurdo Sound notothenioid fishes harboring experimentally introduced (circles) or naturally acquired (crosses) internal ice after a 24-h experimental warming treatment at the indicated aquarium temperature (mean with range error bars). Peak annual seawater temperatures for the McMurdo site are indicated by arrowheads and labeled by oceanographic year (Table S1). The fish eqFMP (−1.04 °C) and the maximum temperature ever recorded at the McMurdo site (−0.10 °C) are shown by dashed and dotted lines, respectively. (B) Fraction of T. pennellii harboring internal ice as a function of time at −0.73 °C (mean, range −0.82 to −0.65 °C), 0.31 °C above their eqFMP. In both experiments, aquarium temperature was measured within 30 cm of the fishes every 2 s with a high-resolution logger. n = 8–15 individuals for each datapoint.
The proportion of fishes with internal ice crystals declined as a function of increasing temperature but, in multiple replicates, a fraction of individuals from two species (T. pennellii and Trematomus bernacchii) continued to harbor internal ice following 24 h at temperatures near or above 0 °C, roughly 1 °C above their eqFMP (Fig. 3A). One T. bernacchii individual maintained naturally acquired ice following 24 h at 0.08 °C (mean treatment temperature, range: −0.02 to 0.15 °C); that is, internal ice was superheated about 1.13 °C above the fish eqFMP (Table 1). When held at the same temperature or for the same amount of time above their eqFMP, the proportion of T. pennellii specimens retaining ice was greater for those with naturally acquired internal ice than for those with experimentally introduced ice (Fig. 3 and Fig. S3). This quantitative difference suggests that ice crystals acquired from the environment are perhaps exceptionally small, lacking defects, or optimally bound with AFPs. The remarkable stability of superheated ice in vivo was shown for T. pennellii, in which internal ice persisted after 3 d (72 h) of being superheated by about 0.31 °C (Fig. 3B).
Table 1.
Greatest observed in vivo superheating of ice
| Species | Fish eqFMP (mean ± SD) (°C)* | Highest temperature at which internal ice persisted in vivo (mean, range) (°C)† | Greatest observed in vivo superheating of ice (°C)‡ |
| T. bernacchii | −1.05 ± 0.03 | +0.08, −0.02 to +0.15 | 1.13 |
| T. pennellii | −1.04 ± 0.03 | −0.06, −0.18 to +0.02 | 0.98 |
| P. borchgrevinki | −1.05 ± 0.02 | −0.56, −0.63 to −0.45 | 0.49 |
For T. bernacchii and T. pennellii, internal ice was naturally acquired from the environment; for P. borchgrevinki, ice was experimentally introduced.
Fish eqFMP = serum eqFMP, n = 3–15 (Fig. 2A).
Fishes held for 24 h in an aquarium, temperature monitored every 2 s (Fig. 3A).
In vivo superheating = mean aquarium temperature − fish eqFMP.
Superheated Ice Occurs in Nature.
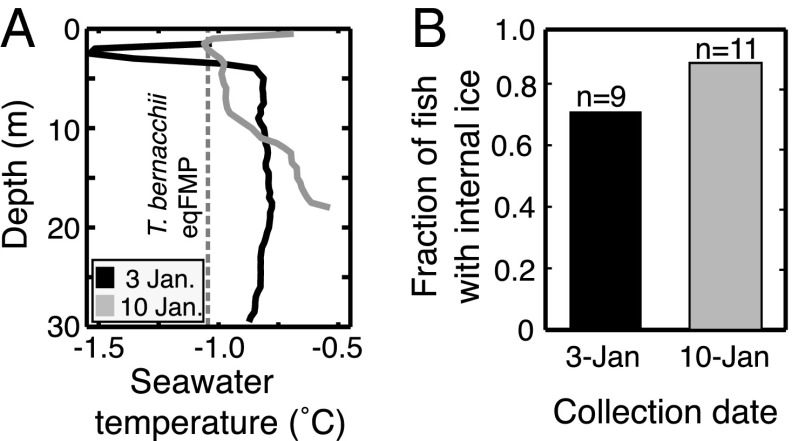
Results from in vivo laboratory experiments strongly suggest that the superheating of ice could occur inside fishes in the wild. To test this hypothesis, we assayed specimens in the field for the presence of ice during a summer warming episode in McMurdo Sound. On January 3 and 10, 2013, we recorded vertical temperature profiles (Fig. 4A) at sites near Cape Evans (Fig. 1) and collected T. bernacchii from the bottom at 17–30 m. Despite seawater temperatures at the collection depth having risen substantially above the fish eqFMP, the majority of individuals collected on both dates harbored internal ice (Fig. 4B). On January 10, fishes were collected from −0.53 °C seawater, which is 0.52 °C above the mean eqFMP of their serum (Table 1), yet about 90% of individuals possessed internal ice. Ice-free control fishes that we suspended in the water column (15 min at 10 m) and then retrieved through the same colder surface layer of water did not test ice-positive; thus, crystals were not inadvertently introduced during capture. We observed during earlier diving operations that anchor ice had melted by December 9 and, apart from the rapidly deteriorating surface ice cover, ice would not be present elsewhere in the water column at these elevated temperatures. The simplest explanation for the presence of ice inside these fishes is that it was acquired before the onset of seawater warming in early December and, because of AFP-induced superheating, it resisted melting despite subsequent warming episodes with temperatures substantially above the fish eqFMP.
Fig. 4.
Superheated ice occurs in nature. (A) Profiles of the water column, from underneath surface ice to the sea floor, at collection sites near Cape Evans, Ross Island in January 2013 revealed seawater temperatures above the T. bernacchii eqFMP (mean −1.05 °C, dashed line). (B) Nevertheless, internal ice persisted in a large fraction of individuals collected from the bottom at these sites. Control experiments verified that fishes did not acquire internal ice during the brief transit through the colder surface layer during capture.
Superheating Further Restricts Infrequent Opportunities for Melting Internal Ice.
Interpreting the organismal and physiological relevance of AFP-induced superheating requires an understanding of the thermal history of the environment over the lifespan of the fishes, because the acquisition and loss of internal ice depends on seawater temperature (Fig. 3A and Fig. S1). Thus, we considerably extended the existing temperature record (1999–2001) (17) for a shallow benthic fish habitat (25- to 40-m depth) adjacent to McMurdo Station (Fig. 1), so that it now covers about half of the approximately 20-y maximum lifespan of the resident Trematomus species (23). The result is an unprecedented, nearly continuous, long-term (1999–2012) record of temperature of one of the highest-latitude accessible marine environments in the Southern Hemisphere (Fig. 5A and Tables S1 and S2).
Fig. 5.
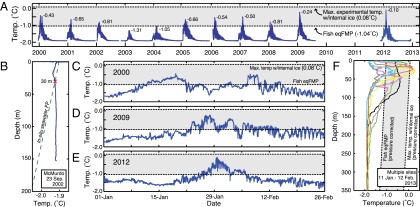
Superheating further restricts infrequent opportunities for melting internal ice. (A) Temperature record for the McMurdo site. Peak annual temperatures are enumerated. Seawater temperature occasionally exceeded the fish eqFMP (lower dashed line). However, in over a decade of logging at this shallow benthic (25–40 m) fish habitat, it never exceeded the highest temperature to which internal ice persisted in vivo under laboratory conditions (+0.08 °C, upper dashed line). Experimental results from this study strongly support the inference that superheated internal ice could persist under the thermal conditions indicated in the shaded areas throughout the figure. (B) Vertical seawater temperature profile (solid line) representative of winter conditions. Seawater in the upper 30 m is below the pressure- and salinity-dependent in situ freezing point (dashed line), resulting in a high probability of shallow-living fishes contacting and internalizing abundant environmental ice. (C–E) Period of peak annual temperatures shown for years with (C) the longest continuous period above the fish eqFMP (9.79 d in 2000), (D) the greatest cumulative time above the eqFMP (18.76 d in 2009), and (E) the highest temperature (−0.10 °C in 2012). (F) Summertime seawater temperature profiles from multiple sites in McMurdo Sound. Temperatures above the fish eqFMP occur only in the top 100 m of the water column, deeper waters are perennially frigid. Profile sites: January 11, mid-Sound (yellow); January 17, Cape Evans (pink), Dailey Islands (gray), Granite Harbor (orange), McMurdo (green), New Harbor (blue); February 12, mid-Sound (black).
Our temperature record confirms that the fishes of McMurdo Sound inhabit one of the coldest and most thermally stable near-surface marine habitats on earth (8). Over the 11-y record, temperature at the McMurdo site varied less than 2 °C, ranging from −1.96 to −0.10 °C, and the long-term mean temperature was −1.79 °C (±0.22 °C, SD) (Fig. S4 and Table S1). Furthermore, vertical profiles during the coldest period often revealed near-surface waters undercooled below the in situ seawater freezing point (Fig. 5B). Despite the frigid temperatures and low overall thermal variability, McMurdo Sound habitats shallower than about 200 m are distinctly seasonal (17, 24). At our McMurdo site, a long winter of stable, near-freezing temperatures (7 d running mean ≤ −1.90, July 3 ± 33 d to December 18 ± 17 d) is briefly interrupted by a summer period with slightly warmer water, annually variable peak temperatures, and relatively large and rapid temperature fluctuations (Fig. 5 and Tables S1 and S2).
Ice accumulated by fishes during the protracted winter periods when seawater temperature is nearly 1 °C below the fish eqFMP would presumably melt if their body temperature could rise sufficiently during summer warming episodes. Our long-term record of the McMurdo site reveals that peak annual temperatures indeed exceeded the fish eqFMP (−1.04 °C) in most years from 1999 to 2012 (Fig. 5A and Table S2), but opportunities for melting internal ice are nevertheless severely spatiotemporally restricted. First, temperature excursions above the fish eqFMP occurred only intermittently (Fig. 5 A and C–E), and were restricted to a single 3- to 4-wk period of each year (January 22 ± 9 d to February 16 ± 8 d, means ± SD) (Table S2). Second, the maximum summer seawater temperature did not reliably exceed the fish eqFMP each year; low peak temperatures in two consecutive summers resulted in a 3-y period (February 19. 2002 to February 23, 2005) with temperatures continuously below the notothenioid eqFMP (Fig. 5A and Table S2). Finally, vertical seawater temperature profiles indicate that only fishes present in the top approximately 100 m of the water column during summer warming episodes could chance encountering temperatures above their eqFMP, because deeper waters remain frigid year-round (Fig. 5F) (17, 24). Taken together, these observations demonstrate that notothenioid fishes in McMurdo Sound must routinely cope with accumulated internal ice crystals for months or years before opportunities for melting them arise.
Furthermore, because of AFP-induced ice superheating, temperatures substantially above the fish eqFMP would be required to eliminate internal ice. Besides temperature, the melting of internal ice might also be affected by the duration of exposure to warm temperatures and the rate of temperature increase, as we observed for experimentally created ice crystals in vivo (Fig. 3B) and in vitro (SI Methods), respectively. However, we do not expect the duration of the warming episode to be a primary factor in the persistence of superheated internal ice in wild fishes. Instead, given the effectively irreversible binding of AFPs to ice (3), if an ice crystal can tolerate superheating to a given temperature at all it should be stable at that temperature indefinitely. This supposition is supported by the remarkable persistence of naturally acquired internal ice for at least 72 h at 0.31 °C above the fish eqFMP in laboratory experiments (Fig. 3B), and at higher temperatures for at least 7 d in our field experiments (Fig. 4). Moreover, the long-term temperature record revealed that warm summer temperatures are not continuous. Indeed, most episodes with temperatures above the fish eqFMP were substantially shorter in duration than our experimental warming treatments. For example, the median duration of these episodes ranged from only 0.5–3.25 h in all years, the longest individual episode was less than 10 d, and the greatest cumulative time above the eqFMP in any year was only about 19 d (Table S2). Furthermore, in our in vivo superheating experiments, fishes were directly transferred from −1.5 °C to the experimental warming treatment, resulting in a rapid change in body temperature. Because MH appears to be somewhat rate-dependent and warming necessarily occurs more slowly in nature (maximum rate observed = 0.09 °C/min) (Table S2), the greatest in vivo superheating observed in our experiments may actually underestimate the maximum amount of superheating of internal ice that could occur in the wild.
Given the high likelihood that substantial superheating of internal ice occurs in nature, we reevaluated our oceanographic observations, taking the highest temperature at which internal ice persisted in our in vivo experiments (0.08 °C) (Fig. 3A and Table 1) as the minimum temperature necessary to completely eliminate internal ice from the resident fishes. Remarkably, over the 11 y of the record—a substantial portion of their approximately 20-y maximum expected life span—the greatest temperature experienced by the shallow benthic fishes near McMurdo Station was only −0.10 °C (January 29, 2012); that is, it never exceeded the highest temperature to which internal ice persisted in vivo under laboratory conditions (Fig. 5A, Table 1, and Table S1).
Discussion
We directly observed ice crystals superheated by up to 0.32 °C in notothenioid fish serum in vitro, and the endogenous AFPs alone were largely responsible for this effect. In vivo experiments demonstrated that internal ice could resist melting at temperatures at least 1 °C above the fish eqFMP, and for at least 3 d at a lower superheating. Field experiments indicated that superheated internal ice occurs under natural conditions, where it could persist for long periods at temperatures substantially above the fish eqFMP. Finally, our long-term temperature record of a shallow benthic fish habitat in McMurdo Sound provided the environmental context for the physiological impact of melting inhibition in notothenioid fishes. Taken together, our results reveal the considerable challenge faced by AFP-bearing Antarctic fishes at frigid high latitudes: opportunities for fishes to acquire internal ice are abundant, yet those for melting it arise only infrequently and do not reliably occur on even an annual basis. Furthermore, the occurrence of AFP-induced superheating considerably diminishes—and may even eliminate—the prospect of melting internal ice over a fish’s lifetime.
Although the superheating of ice in solutions of AFPs was first demonstrated 25-y ago (18), the biological implications of superheating for AFP-bearing organisms have not been addressed. Additionally, although there is a longstanding literature on FH in the body fluids of notothenioids, MH has escaped recognition. These omissions likely stem from the common focus on freezing-related events when studying AFPs. To our knowledge, this study therefore provides the first investigations and insights into these issues.
It remains unclear whether notothenioid fishes experience adverse physiological consequences resulting from the retention of ice within their bodies. Although ice crystals lodged in tissues or organs could promote detrimental inflammatory responses and ice emboli in the circulation could occlude essential blood vessels, whether these potential outcomes occur is unknown. The regular occurrence of ice in the spleens of notothenioid fishes (4, 5, 10) might indicate this organ’s involvement in a physiological response to internal ice. Indeed, there is evidence that splenic macrophages engulf AFP-coated silica nanoparticles (a proxy for ice) (6). If the same occurs for internal ice crystals, this mechanism might mitigate their harmful effects by clearing them from circulation, despite the possibility that AFP-stabilized sequestered ice could persist indefinitely.
Our study demonstrates that superheated ice occurs naturally in the environment. Although the transient superheating of ice with respect to the liquid phase has been accomplished using physical manipulations in the laboratory (e.g., focused internal heating and pressure jumps) (25–27), until the present study, natural superheating of ice was apparently unknown (18, 28, 29). Because MH has also been reported for purified AFPs of other fishes (types I–III) (20) and northern hemisphere cods (family Gadidae) possess AFGPs nearly identical to those of the Antarctic notothenioids (30), we posit that naturally occurring superheated ice is in fact common (i.e., inside AFP-endowed fishes in icy locales where seawater temperature fluctuates around the fish eqFMP).
The evolution of AFPs for freezing prevention in Antarctic notothenioid fishes has become one of the best examples of a molecular adaptation evolved in response to a drastic environmental change: the cooling of Antarctica and the development of icy, freezing waters in the Southern Ocean over the past 30 million y (31). We have shown that this crucial adaptive trait, however, exacts a cost: AFPs also prevent melting to a small but important extent in vivo, and thereby limit opportunities to eliminate ice from the body. This pleiotropic effect of AFPs apparently opposes the key role these proteins play in enabling the survival of notothenioid fishes in freezing seawater, because the accumulation of AFP-stabilized ice crystals could ultimately be just as lethal as the wholesale freezing of body fluids that occurs in AFP-deficient fishes. We suggest that the evolution of AFPs alone was insufficient to permit survival in the harshest of polar environments, necessitating complementary protection by highly resistant epithelia to reduce ice entry and the evolution of mechanisms to sequester internalized ice crystals from the circulation. These additional adaptations would offset the potentially antagonistic pleiotropic effects of melting inhibition presented by the evolution of novel AFPs as notothenioid fishes adapted to their cooling environment.
Methods
Experimental details are presented in the SI Methods and SI Results. Briefly, melting and freezing points were determined for fluids sampled from adult fishes collected from wintertime conditions in McMurdo Sound, including shallow-living T. pennellii, T. bernacchii, Trematomus hansoni, and P. borchgrevinki and the deeper living Dissostichus mawsoni. Total AFGPs and AFPP were isolated from T. pennellii and P. borchgrevinki serum, respectively, as previously described (9). To assess in vivo superheating of ice, McMurdo Sound fishes were collected from the bottom at 15- to 30-m depth (Trematomus spp.) and from directly beneath the approximately 2-m-thick sea ice (P. borchgrevinki) and returned to the aquarium at Scott Base or, for field experiments, to a suitably equipped tent erected on location. Fishes were maintained at temperatures within the FH interval during transport and before undercooling assays so that internal ice could neither melt nor grow. Fishes were found to quickly equilibrate to the ambient temperature (SI Results and Fig. S2). To determine the fraction of fishes maintaining internal ice during natural warming episodes, we collected fishes and assayed them at the field site after a brief hold (<10 min) in ambient seawater pumped from 5-m below surface ice cover. All animal care and use was performed as per approved institutional guidelines (University of Illinois). The multiyear benthic temperature record was obtained with high-resolution loggers deployed by divers at 25- or 40-m depth within 100 m of the McMurdo Station seawater intake jetty. Vertical conductivity (salinity), temperature, and depth profiles were obtained through holes drilled in sea ice or from aboard the research vessel Nathaniel B. Palmer. Oceanographic data were analyzed using custom-written routines in MATLAB (MathWorks). GPS coordinates of study sites are presented in Table S3.
Supplementary Material
Acknowledgments
We thank the staff at McMurdo Station and Scott Base; E. DeVries, B. Evans, L. Fields, K. Hoefling, B. Hunt, L. Hunt, K. Meister, K. Murphy, B. Palmintier, R. Robbins, S. Rupp, and R. Tien for assistance in the field; members of the J. W. Thornton laboratory for their support; A. Rempel for helpful discussion; and C. Brooks for providing a conductivity, temperature, and depth cast from the research vessel Nathaniel B. Palmer. This work was supported by US National Science Foundation Awards OPP 0231006 (to A.L.D. and C.-H.C.C.) and ANT 1142158 (to C.-H.C.C. and A.L.D.) and a National Science Foundation Graduate Research fellowship (to P.A.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The McMurdo temperature record has been deposited in the Integrated Earth Data Applications (IEDA) repository, www.iedadata.org (doi:10.1594/IEDA/321474).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1410256111/-/DCSupplemental.
References
- 1.DeVries AL. Antifreeze glycopeptides and peptides: Interactions with ice and water. Methods Enzymol. 1986;127:293–303. doi: 10.1016/0076-6879(86)27024-x. [DOI] [PubMed] [Google Scholar]
- 2.DeVries AL. Glycoproteins as biological antifreeze agents in Antarctic fishes. Science. 1971;172(3988):1152–1155. doi: 10.1126/science.172.3988.1152. [DOI] [PubMed] [Google Scholar]
- 3.Celik Y, et al. Microfluidic experiments reveal that antifreeze proteins bound to ice crystals suffice to prevent their growth. Proc Natl Acad Sci USA. 2013;110(4):1309–1314. doi: 10.1073/pnas.1213603110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Praebel K, Hunt B, Hunt LH, DeVries AL. The presence and quantification of splenic ice in the McMurdo Sound notothenioid fish, Pagothenia borchgrevinki (Boulenger, 1902) Comp Biochem Physiol A Mol Integr Physiol. 2009;154(4):564–569. doi: 10.1016/j.cbpa.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Tien R. 1995. Freezing avoidance and the presence of ice in shallow water Antarctic fishes. PhD dissertation. (Univ of Illinois–Urbana, Champaign, IL)
- 6.Evans CW, et al. How do Antarctic notothenioid fishes cope with internal ice? A novel function for antifreeze glycoproteins. Antarct Sci. 2010;23(1):57–64. [Google Scholar]
- 7.DeVries AL, Steffensen JF. In: The Physiology of Polar Fishes. Farrell AP, Steffensen JF, editors. San Diego: Elsevier Academic; 2005. pp. 1–24. [Google Scholar]
- 8.Barnes DK, Fuentes V, Clarke A, Schloss IR, Wallace MI. Spatial and temporal variation in shallow seawater temperatures around Antarctica. Deep Sea Res Part II Top Stud Oceanogr. 2006;53(8):853–865. [Google Scholar]
- 9.Jin Y. 2003. Freezing avoidance of Antarctic fishes: The role of a novel antifreeze potentiating protein and the antifreeze glycoproteins. PhD dissertation. (Univ of Illinois–Urbana, Champaign, IL)
- 10.DeVries AL, Cheng C-HC. In: The Physiology of Polar Fishes. Farrell AP, Steffensen JF, editors. San Diego: Elsevier; 2005. pp. 155–201. [Google Scholar]
- 11.Eastman JT. The nature of the diversity of Antarctic fishes. Polar Biol. 2004;28(2):93–107. [Google Scholar]
- 12.Eastman JT. Antarctic Fish Biology. New York: Academic; 1993. [Google Scholar]
- 13.Dayton PK, Robilliard GA, Devries AL. Anchor ice formation in McMurdo Sound, Antarctica, and its biological effects. Science. 1969;163(3864):273–274. doi: 10.1126/science.163.3864.273. [DOI] [PubMed] [Google Scholar]
- 14.Cziko PA, Evans CW, Cheng C-HC, DeVries AL. Freezing resistance of antifreeze-deficient larval Antarctic fish. J Exp Biol. 2006;209(3):407–420. doi: 10.1242/jeb.02008. [DOI] [PubMed] [Google Scholar]
- 15.Valerio PF, Kao MH, Fletcher GL. Fish skin: An effective barrier to ice crystal propagation. J Exp Biol. 1992;164(1):135–151. [Google Scholar]
- 16.Littlepage JL. Oceanographic investigations in McMurdo Sound, Antarctica. Antarctic Research Series. 1965;5:1–37. [Google Scholar]
- 17.Hunt BM, Hoefling K, Cheng CHC. Annual warming episodes in seawater temperatures in McMurdo Sound in relationship to endogenous ice in notothenioid fish. Antarct Sci. 2003;15(3):333–338. [Google Scholar]
- 18.Knight CA, Devries AL. Melting inhibition and superheating of ice by an antifreeze glycopeptide. Science. 1989;245(4917):505–507. doi: 10.1126/science.245.4917.505. [DOI] [PubMed] [Google Scholar]
- 19.Knight CA, Wierzbicki A. Adsorption of biomolecules to ice and their effects upon Ice growth. 2. A discussion of the basic mechanism of “antifreeze” phenomena. Cryst Growth Des. 2001;1(6):439–446. [Google Scholar]
- 20.Celik Y, et al. Superheating of ice crystals in antifreeze protein solutions. Proc Natl Acad Sci USA. 2010;107(12):5423–5428. doi: 10.1073/pnas.0909456107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeVries AL. The role of antifreeze glycopeptides and peptides in the freezing avoidance of Antarctic fishes. Comp Biochem Physiol B. 1988;90(3):611–621. [Google Scholar]
- 22.Scholander PF, Van Dam L, Kanwisher JW, Hammel HT, Gordon MS. Supercooling and osmoregulation in Arctic fish. J Cell Comp Physiol. 1957;49(1):5–24. [Google Scholar]
- 23.La Mesa M, Vacchi M. Age and growth of high Antarctic notothenioid fish. Antarct Sci. 2001;13(3):227–235. [Google Scholar]
- 24.Mahoney AR, et al. The seasonal appearance of ice shelf water in coastal Antarctica and its effect on sea ice growth. J Geophys Res. 2011;116(C11032):1–16. [Google Scholar]
- 25.Schmeisser M, Iglev H, Laubereau A. Bulk melting of ice at the limit of superheating. J Phys Chem B. 2007;111(38):11271–11275. doi: 10.1021/jp0736802. [DOI] [PubMed] [Google Scholar]
- 26.Käss M, Magun S. Zur Überhitzung am Phasenübergang fest-flüssig. Z Kristallogr. 1961;116(1-6):354–370. [Google Scholar]
- 27.Baumann K, Bilgram JH, Känzig W. Superheated ice. Z Phys B Con Mat. 1984;56(4):315–325. [Google Scholar]
- 28.Dash J, Rempel A, Wettlaufer J. The physics of premelted ice and its geophysical consequences. Rev Mod Phys. 2006;78(3):695–741. [Google Scholar]
- 29.Kamb B. Superheated ice. Science. 1970;169(3952):1343–1344. [Google Scholar]
- 30.Chen L, DeVries AL, Cheng C-HC. Convergent evolution of antifreeze glycoproteins in Antarctic notothenioid fish and Arctic cod. Proc Natl Acad Sci USA. 1997;94(8):3817–3822. doi: 10.1073/pnas.94.8.3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, DeVries AL, Cheng CH. Evolution of antifreeze glycoprotein gene from a trypsinogen gene in Antarctic notothenioid fish. Proc Natl Acad Sci USA. 1997;94(8):3811–3816. doi: 10.1073/pnas.94.8.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.