Significance
Mutations in titin are a major cause of heart failure, yet the functions of large parts of titin are not understood. Here we studied titin’s I-band/A-band junction that has been proposed to be crucial for thick filament length control. We made a mouse in which titin’s IA junction was deleted. Super-resolution microscopy (structured illumination microscopy) revealed that deleting the IA junction increases the strain on titin’s molecular spring elements without altering thick filament length. Single cell biomechanical measurements showed that this increases passive stiffness while functional studies at the whole animal level revealed diastolic dysfunction, exercise intolerance, and modest concentric cardiac hypertrophy—signature features of heart failure with preserved ejection fraction. Our studies support that titin is a promising therapeutic target for treating heart failure.
Keywords: passive stiffness, molecular elasticity, hypertrophy, mechanosensing
Abstract
Titin, the largest protein known, forms a giant filament in muscle where it spans the half sarcomere from Z disk to M band. Here we genetically targeted a stretch of 14 immunoglobulin-like and fibronectin type 3 domains that comprises the I-band/A-band (IA) junction and obtained a viable mouse model. Super-resolution optical microscopy (structured illumination microscopy, SIM) and electron microscopy were used to study the thick filament length and titin’s molecular elasticity. SIM showed that the IA junction functionally belongs to the relatively stiff A-band region of titin. The stiffness of A-band titin was found to be high, relative to that of I-band titin (∼40-fold higher) but low, relative to that of the myosin-based thick filament (∼70-fold lower). Sarcomere stretch therefore results in movement of A-band titin with respect to the thick filament backbone, and this might constitute a novel length-sensing mechanism. Findings disproved that titin at the IA junction is crucial for thick filament length control, settling a long-standing hypothesis. SIM also showed that deleting the IA junction moves the attachment point of titin’s spring region away from the Z disk, increasing the strain on titin’s molecular spring elements. Functional studies from the cellular to ex vivo and in vivo left ventricular chamber levels showed that this causes diastolic dysfunction and other symptoms of heart failure with preserved ejection fraction (HFpEF). Thus, our work supports titin’s important roles in diastolic function and disease of the heart.
Titin is mostly modularly constructed of serially linked immunoglobulin-like domains (Ig) and fibronectin type 3 domains (FnIII) (1). The I-band region of titin contains exclusively Ig domains where they form the proximal tandem Ig segment near the Z disk and the distal tandem Ig segment near the A band (2) (Fig. 1A). The tandem Ig segments together with the N2B unique sequence and the PEVK comprise titin’s molecular spring segment that extends when sarcomeres are stretched (3) and develops passive force (4). The other parts of titin are poorly understood, yet they are likely to be critically important as mutations in the A-band region of titin are a major cause of heart failure (5). The A-band region contains a large number of FnIII domains that are only present in this part of the molecule (6). The FnIII and Ig domains are organized into 7-domain and 11-domain superrepeats (2) (Fig. 1A). The 11-domain super repeat is likely to span a distance that is the same as the ∼43-nm repeat distance of myosin molecules in the thick filament (7), which has contributed to the hypothesis that titin is a template for thick filament formation (8–10). It is striking that the regular domain patterns of Ig and FnIII domains is broken up near the ends of the thick filament where a stretch of 6 FnIII domains is found preceding the D zone (2)—it has been suggested that this reflects an alteration in titin–myosin interaction that is critical for the termination of the thick filament (9). Few insights exist into how length control is achieved to maintain the thick filament length at 1.6 μm (11). The degree of overlap and hence the amount of force produced is determined by the length of the thin and thick filaments (12) and the mechanisms that control filament length need to be well understood. Myosin can be easily assembled into synthetic thick filaments in vitro but they lack the precise length distribution found in vivo, suggesting the presence of a control mechanism inside the sarcomere (13). Ever since the discovery that titin spans from the Z disk to the middle of the thick filament (14), it has been proposed that titin is a protein ruler that determines thick filament length (8–10). To test this proposal, we made a mouse model in which we targeted the I-band/A-band (IA) junction of titin and deleted the 14 domains that precede the first super repeat, including the block of 6 FnIII domains. The TtnΔIAjxn model was studied with immunoelectron microscopy (IEM) and super-resolution optical microscopy (structured illumination microscopy, SIM) and a range of functional techniques from the cellular to organ levels.
Fig. 1.
Characterization of titin in WT and TtnΔIAjxn mice. (A, Upper) Location of titin in sarcomere. Titin spans from Z disk (Z) to M band (M). (Lower) Domain structure of titin (cardiac N2B isoform). Red: immunoglobulin (Ig)-like domains; white: fibronectin type III (Fn) domains; yellow: PEVK; black: titin kinase; blue unique sequence. The IA junction that was targeted for deletion is shown by the box. Also indicated is titin’s molecular spring region and its subelements that we investigated: proximal and distal tandem Ig segments and the N2B element. The horizontal short lines indicate the location of the binding sites of the antibodies that were used in this work. (B) Agarose protein gel of cardiac samples from wild-type (WT), heterozygous (Het), and homozyogous (Hom) TtnΔIAjxn mice. WT myocardium expresses two isoforms, N2B titin (dominant) and N2BA titin. T2 is a titin degradation product that forms during sample preparation. In Hom mice, the same isoforms and T2 are found but are all smaller (the IAjxn deletion is 153 kDa). (C) Western blot analysis of cardiac samples. MIR failed to detect the mutant titin isoforms in Hets (bottom-most band) and Hom mice.
Results
Generation of a Mouse Model Deficient in Titin’s IA Junction (TtnΔIAjxn).
Exons 251–269 were constitutively deleted from the mouse titin gene (SI Appendix, Fig. S1A). The deleted exons code for 3 Ig domains and 11 FnIII domains (Fig. 1A) that comprise 1,387 amino acids, representing a 153.4-kDa polypeptide. Using 1% agarose gels, the mutant titin is clearly resolved as a higher mobility band compared to wild-type (WT) titin (Fig. 1 B and C). Western blot analysis with antibodies against epitopes in the deleted region (MIR), and in regions N-terminal (Z1Z2 and I103) and C-terminal (M8/M9) of the deleted region established that the deleted region is indeed absent in homozygous TtnΔIAjxn mice but that the rest of the molecule is normally expressed (Fig. 1C). The adult heart of mammals is known to express two titin isoforms: the smaller N2B titin that dominates and the larger and more compliant N2BA titin (15) (Fig. 1B). Both isoforms are reduced in size in the TtnΔIAjxn mice, which is expected as the deleted exons are in a constitutively expressed region of titin that is present in all titin isoforms. Quantitative protein analysis revealed no differences in titin expression (SI Appendix, Fig. S1B). Our custom titin exon microarray (16) further validated only loss of expression of exons 251–269 in homozygous TtnΔIAjxn mice without adaptive changes in splicing elsewhere in titin (SI Appendix, Fig. S1C). Thus, we successfully generated a mouse model in which the IA junction has been specifically deleted.
Thick Filament Length.
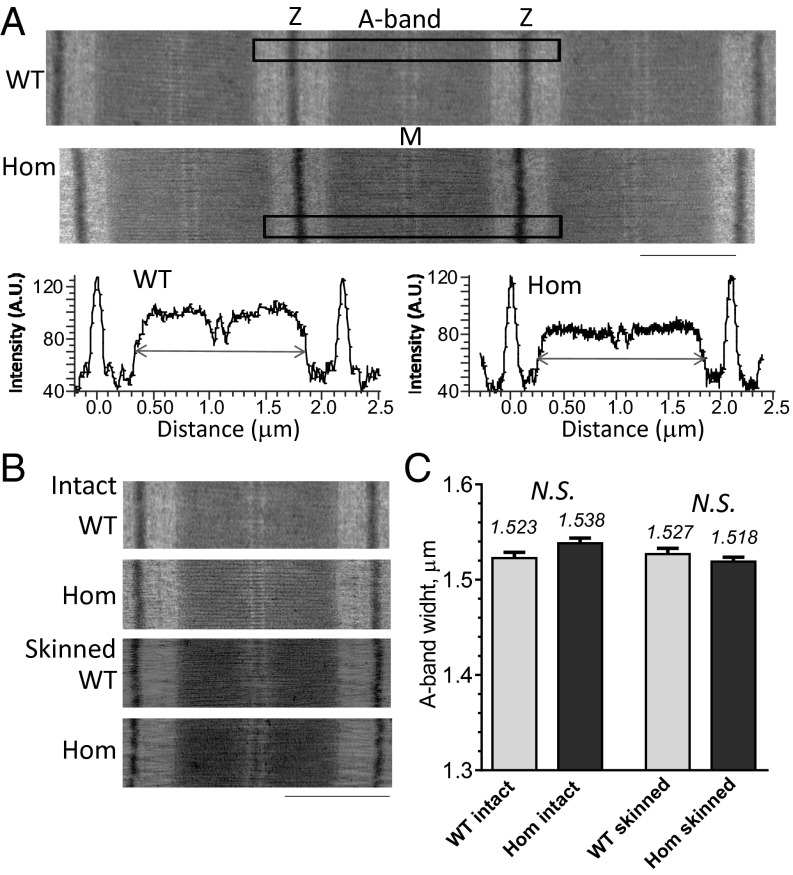
Transmission electron microscopy was performed on muscle from the left ventricular (LV) free wall of hearts that had been arrested and perfusion fixed in situ (Methods). We determined the A-band width as a measurement of thick filament length. Sarcomeres of TtnΔIAjxn mice and wild-type mice had A bands that were indistinguishable in appearance with normal M band and bare zones (Fig. 2A). We also studied muscles that were treated with Triton X-100 to remove membranes (Methods), a procedure known as skinning, and then fixed. No differences were noted in the A bands when comparing images of skinned or intact tissues from WT and TtnΔIAjxn mice (Fig. 2B). A-band width was determined from density profiles using the width at half maximal height (Fig. 2A, Bottom). Obtained values were ∼1.52 μm with no difference between the A-band width of homozygous TtnΔIAjxn mice and WT mice prepared under identical conditions, neither when using intact muscle nor when using skinned muscle (Fig. 2C). Similar findings were obtained in studies on skeletal muscle (see below).
Fig. 2.
A-band width measurement in WT and Hom TtnΔIAjxn mice. (A) Representative electron micrographs from perfusion fixed in situ left ventricular (LV) myocardium. Boxes indicate regions that were used for densitometry (Lower). Arrows indicate the width at half-maximal height of the A-band region of the sarcomere, which was taken as measurement of A-band width. (B) Comparison of tissue that was perfusion fixed in situ (intact) and tissue that has been demembranated (skinned) and then fixed. (C) A-band width measurements (mean and SEM). (Scale bars in A and B: 1.0 μm.)
Attachment Point of Titin’s Spring Region.
To study the behavior of the IA junction, we raised an antibody to the region just N-terminal of the IA junction of titin (see I103 antibody in Fig. 1A) and used it in both IEM and in SIM on cardiac LV wall muscle samples of Hom TtnΔIAjxn mice and WT mice. Both IEM and SIM revealed well-resolved epitopes (Fig. 3A, IEM, arrows; SIM, epitopes in green and SI Appendix, Fig. S2A, densitometry profiles). The distance of the I103 epitopes to the middle of the M band was determined in muscles stretched to a sarcomere length (SL) of 2.15 μm. The distance was ∼30 nm longer when measured with SIM than with IEM (in both WT and Hom mice), which is likely due to reduced shrinkage; SIM does not require dehydration, embedding, and sectioning of samples like IEM (Discussion). Importantly, with both methods the I103 epitope to M-band distance was significantly less in Hom than in WT mice (IEM, 70 nm; SIM, 67 nm) (Fig. 3B). This reduction can be explained by the deletion in the Hom of 14 domains (3 Ig and 11 FnIII domains) that each account for ∼4.5 nm distance to the M band. We also measured the sarcomere-length dependence of the I103 to M-band distance; at the full SL range that was studied, the I103 epitope to M-band distance was ∼65 nm shorter in Hom mice (Fig. 3C). The data could be fit with linear regression lines with slopes that in both genotypes are significantly different from zero, i.e. the I103 epitope moved away from the M band as SL was increased (SI Appendix, Table S1). It is unlikely that this slope is dominated by the compliance in the deleted domains as the slope would then be less in the Hom than in the WT mice, whereas the slope was significantly larger in the Hom mice (P = 0.004). Instead, this indicates a compliance in the A-band region of titin that responds to titin-based passive force (Discussion).
Fig. 3.
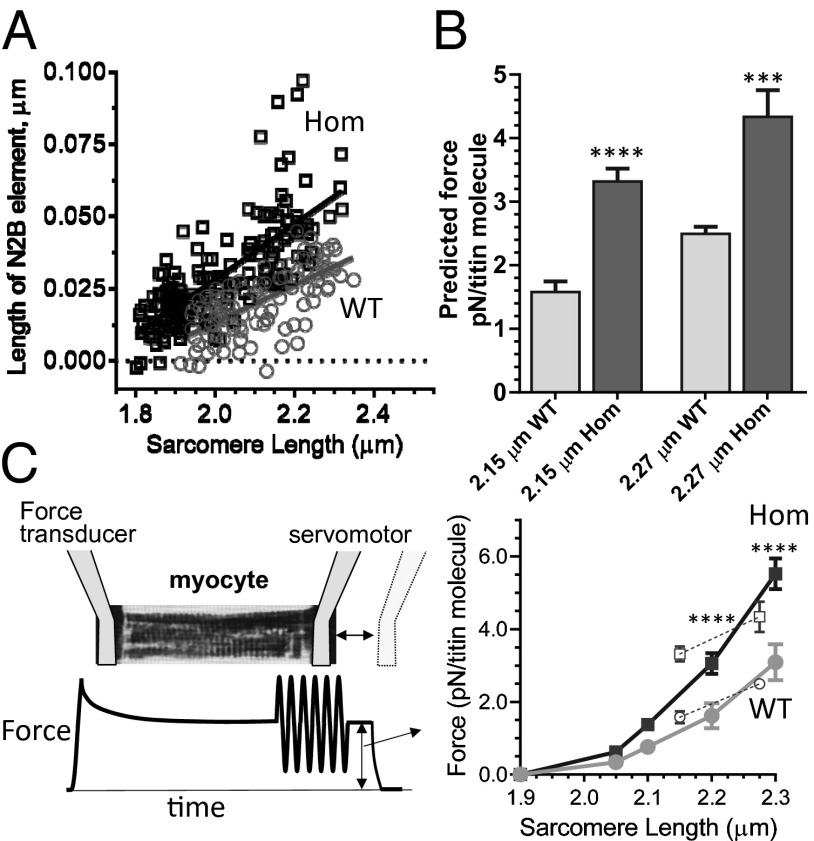
Titin’s spring region in WT and Hom TtnΔIAjxn mice. (A) Immunolabeling of skinned myocardium with the I103 antibody (labels just N-terminal of the deleted IA junction, see Fig. 1A) using IEM or structured illumination microscopy (SIM). I103 epitope indicated by arrowheads in IEM and shown in green in SIM. Note that epitopes in IEM are located just outside the A band in WT and just inside A band in Hom myocardium. (Myocardium imaged by SIM was also labeled (red) by T12 (labels N-terminal end of titin’s spring region, see Fig. 1A). (B) I103 epitope to M-band distance is significantly reduced in Hom tissue, in both IEM and SIM study. Sarcomere length (SL) is 2.15 μm in all four groups. (C) SL dependence of I103 to M band (SIM study). Lines are linear regression fits (see SI Appendix, Table S1 for equations). (D) I103 to mid Z disk and T12 to mid Z-disk data (SIM) with linear regression fits. WT and Hom T12 data overlap (for fit equations, see SI Appendix, Table S1) but I103 data are distinct—I103 epitope is further away from Z disk in Hom tissues. (Scale bars: 1.0 μm.)
The distance between the I103 epitope and the middle of the Z disk was measured in tissues that were colabeled with the T12 antibody, which has been shown (17) to bind near the N-terminal end of titin’s spring region (Fig. 3A, SIM, epitope in red and SI Appendix, Fig. S2B, densitometry scans). Results shown in Fig. 3D reveal that the T12 data of WT and Hom mice overlap. In contrast, the I103 epitope is further away from the Z disk in the Hom mice (Fig. 3D), a difference that is 65 nm at a SL of 2.15 μm. The near-zero distance between the T12 and I103 epitopes at short SLs in WT mice suggests that the spring region of titin extends from near the T12 (N-terminal end) to near the I103 epitope (C-terminal end) and comparison with the results from Hom TtnΔIAjxn mice indicates that deleting the IA junction in effect moves the C-terminal attachment point of titin’s spring region away from the Z disk. As a result, a given sarcomere stretch increases the extension of titin’s spring region to a higher degree in Hom TtnΔIAjxn than in WT mice.
Spring Element Behavior.
Using SIM, we measured the extension of the N2B element using UN and UC antibodies (Fig. 4A). As expected from the larger extension of the whole spring region (T12-I103) in TtnΔIAjxn mice, the length of the N2B WT increased steeper with SL in Hom than WT mice (Fig. 4A and SI Appendix, Table S1). The experimentally obtained extensions of the N2B element were used to calculate the force of a single titin molecule in the sarcomere. We assumed that the N2B element behaves as a worm-like chain (WLC) (18) with a persistence length of 0.5 nm and a contour length of 200 nm (Methods). The predicted single titin molecule forces were higher in TtnΔIAjxn mice (Fig. 4B). Thus, deletion of the IA junction moves the attachment point of titin’s spring region away from the Z disk, this increases extension of titin’s spring elements and this is predicted to increase the force generated by titin. Considering that titin is the main determinant of the passive force of cardiac myocytes (4) TtnΔIAjxn myocytes are predicted to be stiffer than WT cells.
Fig. 4.
Predicted and measured single titin molecule force in WT and Hom TtnΔIAjxn mice. (A) Length of N2B element (UN–UC epitopes). Results were fit with linear regression lines (SI Appendix, Table S1). The measured extensions in A at SL range 2.10–2.2 μm (mean SL 2.15 μm) and 2.25–2.35 μm (mean SL 2.27 μm) were used as input for the WLC model (see Methods for details). (B) The predicted force of mutant titin is higher than that of WT titin. (C) Passive force in cardiac myocytes in WT and Hom TtnΔIAjxn mice. (Left) Explanation of protocol to measured steady-state passive force (for sinusoidal oscillations, see SI Appendix, Fig. S3). (Right) Force–SL relation. Measured total force was converted to force per titin molecule (in piconewtons), shown with closed symbols; see text for details. Open symbols, predicted values from B.
Passive Biomechanics of Single Cardiac Myocytes.
Skinned cardiac myocytes in relaxing solution were attached at one end to a force transducer and at the other end to a servomotor that imposed a stretch–hold–release protocol on the cell. Cells were stretched from their slack length to a predetermined SL, held constant for 20 s to allow force to attain a steady state, a small amplitude (5%) sinusoidal oscillation was imposed, and then the cell was released back to the slack length (Fig. 4C, Left and Methods). Obtained steady-state passive forces were converted to force per titin molecule (assuming 3,240 titin molecules per micrometers squared of myofibril and 50% of the cell’s cross-sectional area taken up by myofibrils) (4). Results revealed increased mean passive force at all SLs in TtnΔIAjxn compared to WT myocytes and statistically significant increases at SL 2.2 and 2.3 μm (Fig. 4C, Right). We also measured dynamic stiffness from the length oscillation protocol and converted results to elastic moduli (EM) and viscous moduli (VM), see SI Appendix, Fig. S3, Left. In both genotypes EM are much larger than VM and, importantly, both EM and VM are significantly increased in Hom TtnΔIAjxn myocytes (SI Appendix, Fig. S3, Right).
LV Chamber Stiffness and Hypertrophy.
To determine whether the increased passive stiffness of TtnΔIAjxn cardiac myocytes has functional effects at the organ level, a small catheter was introduced in the LV chamber and pressure and volume were measured during both systole (contractile phase that causes ejection) and diastole (phase during which filling occurs). No differences were found in heart rate or any of the systolic parameters [systolic blood pressure (104 vs. 108 mmHg), ejection fraction (50% vs. 55%), and stroke volume (28 and 30 μL), see SI Appendix, Table S2 for details]. By transiently occluding the vena cava, we obtained pressure–volume loops (Fig. 5A, Upper) and from these loops determined the slope of the end-systolic pressure volume relation (ESPVR), a determinant of contractility. We also measured the coefficient β of the end-diastolic pressure volume relation (EDPVR), an important characteristic of diastolic stiffness. This analysis revealed no changes in contractility in TtnΔIAjxn mice but a significantly increased β, indicating increased diastolic stiffness (SI Appendix, Table S2 and Fig. 5A). As an additional assessment of diastolic stiffness, pulse-wave Doppler echocardiography was used to measure the velocity of diastolic filling at the level of the mitral valve (Methods). Filling is known to occur in two waves: early diastolic filling (E wave) and late diastolic filling due to atrial contraction (A wave) (Fig. 5B). The E-wave deceleration time (DT) (indicated by broken line in Fig 5B, Upper) varies inversely with LV diastolic stiffness (19). A significant E-wave DT reduction was found in TtnΔIAjxn mice, supporting that diastolic chamber stiffness is increased. Finally, since increased left atrial (LA) size is typically seen in patients with elevated LV stiffness (20) we measured LA weights. LA of Hom TtnΔIAjxn mice was hypertrophied, at an absolute weight level and also when normalized to either body weight or tibia length (Fig. 5C and SI Appendix, Table S3). Thus, deleting the IA junction leads to increased titin-based passive force in cardiac myocytes, and whole heart level studies support that this increases the diastolic stiffness of the heart.
Fig. 5.
Assessment of left ventricular chamber stiffness during diastole in WT and Hom TtnΔIAjxn mice. (A) Pressure–volume analysis in LV. (Upper) PV loops obtained during vena cava occlusion experiment (Methods), with the end-diastolic pressure volume curve (EDPVR) indicated. EDPVR was fit with an exponential equation and the exponent β was determined. (Lower) β is significantly increased in TtnΔIAjxn mice. (B, Upper) LV inflow E and A waves measured with pulse-wave Doppler echocardiography; the deceleration time of the E wave is indicated (DT, bracket). (Lower) E-wave deceleration time is decreased in TtnΔIAjxn mice. (C) Left atrium weight (normalized to body weight) is increased in TtnΔIAjxn mice. See text for details.
Finally, we determined LV weights of Hom TtnΔIAjxn and WT mice. The LV weight was found to be modestly increased in Hom TtnΔIAjxn mice, on an absolute weight scale as well as when normalizing LV weight to either body weight or tibia length (SI Appendix, Table S3). Consistent with LV hypertrophy, expression of the LV hypertrophy marker B-type natriuretic peptide (BNP) was significantly increased (SI Appendix, Fig. S4A). Conscious echocardiography revealed an increased LV wall thickness and concentric hypertrophy (reduced eccentricity index) in TtnΔIAjxn mice (SI Appendix, Table S4). Cell morphology measurements showed that the cross-sectional area of LV cardiac myocytes was increased in Hom mice, whereas cell length was unchanged (SI Appendix, Fig. S4 B and C), supporting that TtnΔIAjxn LV cardiac myocytes undergo mild concentric hypertrophy. Expression of the pathological hypertrophy markers, skeletal muscle actin (SI Appendix, Fig. S5A) and βMHC (SI Appendix, Fig. S5 B–E) were unaltered. Hypertrophy was absent in skeletal muscle (SI Appendix, Table S5) as were changes in skeletal muscle function and A-band width (SI Appendix, Fig. S6). Thus hypertrophy is specific to the heart. We also performed a Western blot study to evaluate expression of titin-binding proteins that have been suggested to be involved in biomechanical sensing (21) and found that only the I-band binding protein FHL2 (four-and-a-half LIM domains 2) was significantly increased at the protein (Fig. 6 and SI Appendix, Fig. S7) as well as the transcript levels (SI Appendix, Fig. S8).
Fig. 6.
Expression of titin-binding proteins in WT and Hom TtnΔIAjxn mice. FHL2 is significantly increased in Hom TtnΔIAjxn mice (see also SI Appendix, Fig. S7).
Discussion
Thick Filament Length.
The A-band width that we obtained (∼1.52 μm) is slightly less than the 1.6 μm that others have reported in cardiac and skeletal muscle (11, 22). Tissue shrinkage that occurs during sample preparation for TEM is a likely explanation (11). We can gain insights in shrinkage during the preparatory steps specific for TEM (dehydration, embedding, sectioning) by comparing the I103 epitope to M-band distances measured with transmission electron microscopy (TEM) to that measured with SIM (Fig. 3B). The estimated shrinkage is 4.4% in TtnΔIAjxn mice and 3.8% in WT mice. Using these values to correct for shrinkage yields a thick filament length in TtnΔIAjxn mice of 1.61 μm in intact tissue and 1.59 μm in skinned tissue. Importantly, these values are indistinguishable from those obtained in WT mice: intact tissue 1.60 μm and skinned tissue 1.59 μm. Lack of differences in thick filament length is supported by the lack of differences in contractile performance between WT and TtnΔIAjxn mice. In conclusion, our studies show that the IA junction does not control thick filament length.
What might be the role of the IA junction? The sequence of the IA junction is well conserved in different species (SI Appendix, Fig. S9), suggesting that it performs important functions. A comparison of the I103 epitope with the thick filament length in WT and TtnΔIAjxn mice shows that the deleted region spans in WT from ∼30 nm inside to ∼30 nm outside the edge of the A band. Considering that in the thick filament region six titin molecules are likely organized as three separate dimers on the outside of the thick filament (23), but in the distal tandem Ig segment titin appears to be organized in side-to-side hexamers (24), the IA junction might play a role in organizing the transition between these two distinct types of organization.
A-Band Titin.
Several other important conclusions can be drawn from our work with regards to the extensibility of the A-band region of titin. Titin’s A-band region in WT muscle is ∼40-fold less extensible than the I-band region of titin (SI Appendix, Table S1) and we can address whether functionally the IA junction is part of the relatively stiff A-band region or belongs to the more extensible I-band region. If the IA junction were to extend similar to I-band titin, it would have a near-zero end-to-end length in slack sarcomeres (∼1.9 um) and as sarcomeres are stretched to 2.3 μm (physiological range) it would extend away from the M band by ∼60 nm (based on the behavior of the tandem Ig segments and accounting for the size of the IA junction). Instead, the I103 epitope to M-band distance increases in WT mice by less than 10 nm (Fig. 3C). Furthermore, if this 10-nm extension were due solely to the IA junction, it would be absent in the TtnΔIAjxn mouse, but instead the extension is increased to ∼16 nm (based on the fit of Fig. 3C, Hom). Thus, the IA junction functionally belongs to the relatively inextensible A-band region of titin.
Although our work indicates that compared to the I-band region, titin in the A band is much less extensible, the slopes of the linear fits of the I103 to M-band relationship (Fig. 3C and SI Appendix, Table S1) do show that A-band titin is not completely rigid. Within the physiological SL range of 1.9 to 2.3 μm the slopes indicate that the I103 to M-band distance increases by ∼10 nm in WT sarcomeres. It is likely that this increase is due to the titin-based passive force that pulls on the thick filament region, a notion consistent with the increased passive force in the TtnΔIAjxn mouse (from 3.1 to 5.4 pN/molecule, Fig. 4C) and its increased extension (from ∼10 to ∼16 nm). Using the measured force per titin molecule (Fig. 4C) and the measured I103 to M-band extension (Fig. 3C), we can estimate the compliance of a single titin molecule in the A band of titin. The obtained values are 2,950 m/N for the WT and 2,900 m/N for the TtnΔIAjxn mouse.
It is interesting to compare the compliance of A-band titin with the compliance of myosin in the thick filament. The myosin filament compliance has been determined in studies in which simultaneous X-ray diffraction and mechanics were used on passive muscle (25), and the obtained value was 43 m/N (half of the published value for the whole thick filament), i.e., ∼70-fold less than the titin filament compliance in the A band. The 43 m/N half thick filament compliance can be used to estimate how much the half thick filament stretches when sarcomere length increases from 1.9 μm to 2.3 μm and passive force increases from 0 to 3.1 pN/titin molecule (or accounting for the six titin molecules that attach to the half thick filament, 0 to 18.6 pN per half thick filament). The obtained value for myosin thick filament elongation is 0.8 nm/half thick filament—much less than the 10 nm that A-band titin stretches. This difference might have functional consequences because within each of titin’s superrepeats there are interaction sites for myosin heads (26) as well as for myosin-binding protein C (27). Thus, as titin moves relative to the myosin-based thick filament during sarcomere stretch, alignment of interaction partners could be affected. Considering the elusive molecular basis of the length dependence of contractility (28), the compliance of A-band titin provides a possible molecular mechanism for length sensing.
Titin’s Passive Force.
Deletion of the IA junction moves the C-terminal attachment point of titin’s spring region ∼65 nm away from the Z disk (Fig. 3 B–D) and as a result an increase in sarcomere length strains titin’s spring elements to a higher degree in TtnΔIAjxn than in WT muscle. Applying the worm-like chain entropic force model to the measured extension of the N2B element predicts an increased single titin molecule force in the TtnΔIAjxn sarcomere that is close to the experimentally measured passive force of single cardiac myocytes (Fig. 4C). Thus, our work on titin’s spring elements in TtnΔIAjxn mice supports that characteristics measured on single molecules are relevant in the context of the sarcomere.
The sinusoidal analysis method revealed as expected an increased elastic modulus in the TtnΔIAjxn mice, but unexpectedly also an increased viscous modulus (SI Appendix, Fig. S3). Increased viscosity could arise from PEVK–actin interactions, [previously identified as the major viscosity source in the cardiac sarcomere (29–31)] as the increased titin-strain in the TtnΔIAjxn mouse might disrupt a greater number of PEVK–actin linkages compared to WT during sarcomere stretch. Unfolding of Ig domains might also play a role in the increased viscosity. There is a low probability of unfolding of Ig domains in WT muscle (32) and the higher titin force in the TtnΔIAjxn mice is expected to increase this probability (33).
Diastolic Dysfunction and Hypertrophy.
Multiple studies were performed at the left ventricular chamber level that focused on the filling phase (diastole) of the heart. These studies support that the increased titin-based passive force in the TtnΔIAjxn mice increases chamber stiffness during diastole: the diastolic stiffness parameter β was increased, the deceleration time of the E wave was decreased, and the weight of the left atrium was increased. The increased left atrial weight is likely to reflect the increased LV stiffness and the ensuing increase in pressure and volume of the LA. It is well recognized that increased diastolic stiffness can lead to functional deficits (20, 34), and this was confirmed in free-wheel running studies that showed that TtnΔIAjxn mice had a running deficiency (SI Appendix, Fig. S10). Thus, increased diastolic stiffness results in filling deficiency, especially during physical activity when the heart rate is elevated and the diastolic duration is reduced.
Hypertrophy signaling in the heart is tremendously complex and diverse (35) and many signaling pathways could be involved in LV hypertrophy in TtnΔIAjxn mice. However, considering that titin’s N2B element has been linked to hypertrophy signaling (36), it is worth considering that the increased strain of titin’s N2B element in TtnΔIAjxn mice (Fig. 4A) triggers hypertrophy with a role for FHL2 since it was significantly upregulated both at the protein and mRNA levels (Fig. 6 and SI Appendix, Figs. S7 and S8). FHL2 is a member of the FHL protein family and is predominantly expressed in the adult heart where it binds the N2B element of cardiac titin (37). Interestingly, a recent study in which FHL2 was upregulated through long-term chronic β-adrenergic stimulation provided experimental evidence that under those conditions, FHL2 suppressed hypertrophy (38). This response was shown to involve repression of calcineurin (a Ca2+-dependent protein phosphatase that dephosphorylates the transcription factor NFAT) leading to the inactivation of NFAT target genes. Although we do not exclude that also in the TtnΔIAjxn model FHL2 upregulation suppresses hypertrophy and that the trigger for hypertrophy has escaped detection, Occam’s razor indicates that upregulation of FHL2 in the TtnΔIAjxn model reflects hypertrophy. It is also important to note that our findings are not unprecedented. The PEVK KO and IG KO models (39, 40) both share with the TtnΔIAjxn model (i) increased N2B strain, (ii) increased FHL2 expression, and (iii) LV hypertrophy. Additionally, FHL2 expression is greatly reduced in the N2B KO mouse in which the N2B element has been deleted and hearts are atrophied (41). Hence, we propose that both global chronic isoproterenol activation (38) and local increased strain of titin’s N2B element (e.g. TtnΔIAjxn KO, PEVK KO, and IG KO models) up-regulate FHL2 but with opposite effects on hypertrophy, adding to the complex and diverse hypertrophy signaling pathways that are known to occur in the heart. Although critical future studies are clearly needed, the present work supports the emerging view that titin’s I-band region functions as a biomechanical sensor that detects sarcomere strain and together with its binding partners tunes downstream hypertrophy signaling (36, 42–44).
Summary.
A novel mouse model was made in which exons 251–269 were targeted in the Ttn gene. This specifically deleted the 14 Ig/FnIII domains that comprise the IA junction of titin without changes elsewhere in the protein. Deleting the IA junction does not affect thick filament length, indicating that the mechanism that underlies thick filament length control is elsewhere. Results show that the IA junction is not part of the extensible spring region of titin and that instead it belongs to the stiffer A-band region of titin. Deleting the IA junction moves the attachment point of titin’s spring region away from the Z disk, which results in an increase in left ventricular diastolic stiffness, exercise intolerance, and modest left ventricular hypertrophy. Increased diastolic stiffness is an important feature of heart failure with preserved ejection fraction (HFpEF), a type of heart failure that occurs in ∼50% of patients with heart failure and for which currently no effective therapies exist (20). HFpEF is accompanied by preserved systolic function, reduced exercise capacity, and hypertrophy is a comorbidity (20); all of these features are present in the TtnΔIAjxn model. Previous studies (45) have shown that titin-based passive force is greatly increased in patients with HFpEF, due to hypophosphorylation of titin, but it is unknown whether this is a cause or an effect of HFpEF. Our studies support that increased titin stiffness can cause HFpEF. Thus, our work using a novel in vivo model disproves the long-standing hypothesis that the IA junction controls thick filament length and it supports titin’s important roles in cardiac function and disease.
Methods
Generation of Mice Deficient in the IA Junction.
Mice were on a C57BL/6J background (more than eight generations) and were 4 mo of age and male, unless indicated otherwise. All animal procedures were approved by the University of Arizona Institutional Animal Care and Use Committee. See SI Appendix, Fig. S1 for details.
TEM.
We used intact and skinned muscles from the LV free wall and intact extensor digitorum longus muscle. Images were analyzed by using ImageJ and Fityk. For details, see SI Appendix.
IEM.
Ultrastructural immunolocalization of the I103 antibody was performed on LV skinned muscles.
SIM.
Muscles were frozen, cryosectioned, and labeled with one or more of the following antibodies: anti-Titin Un, anti-Titin Uc, anti-Titin I84, anti-Titin I103, anti-Titin T12, and anti–α-actinin antibody (details in SI Appendix). SIM was performed with a Zeiss ELYRA S1 (SR-SIM) microscope. Epitope distances were determined across the A band (Fig. 3 A–C) or Z disk (Figs. 3D and 4A) to obtain epitope to M-band distance or epitope to Z-disk distance, respectively. See SI Appendix.
Single Molecule Modeling.
We used the WLC model (18) with the measured extension of the N2B element (Fig. 4A). To avoid volume-exclusion and self-interaction effects, force was calculated at sarcomere lengths where the fractional extension of the N2B element was >0.1. For details, see SI Appendix.
Cardiomyocyte Studies.
Skinned myocyte biomechanical studies were used following our well-established methods (SI Appendix).
In Vivo Pressure–Volume Relationships.
In vivo pressure volume analysis was performed in mice using a SciSense Advantage Admittance Derived Volume Measurement System. See SI Appendix.
Echocardiography.
See SI Appendix for details.
Quantification of Protein Expression.
Details, including on the antibodies that were used, are in SI Appendix.
RNA Analysis.
Ttn mRNA expression was analyzed using our custom exon microarray (SI Appendix).
Quantitative RT-PCR.
See SI Appendix.
Statistics.
A one-way ANOVA with a Bonferroni post hoc analysis that calculates P-values (when needed corrected for multiple comparisons) was performed to assess differences between multiple groups. A t test was used when comparing two groups only. A two-way ANOVA was used in SI Appendix, Table S3 [chamber weights (LV, LA, RV, RA) and genotype (WT and IA KO)] with a Bonferroni post hoc test. Results are shown as mean ± SEM. P < 0.05 was defined as significant with *P < 0.05, **P < 0.01, and ***P < 0.001.
Supplementary Material
Acknowledgments
We thank our current and former laboratory members (particularly Ms. X. Luo and Dr. B. Hudson) and Dr. C. Guo (Janelia Farm Research Campus). Support was provided through the LeDucq Foundation 13CVD04 (to H.L.G.) and National Institutes of Health T32HL 07249 (to K.R.H.), HL083146 (to C.C.G.), HL062881 (to H.L.G.), and HL115988 (to H.L.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1411493111/-/DCSupplemental.
References
- 1.Labeit S, et al. A regular pattern of two types of 100-residue motif in the sequence of titin. Nature. 1990;345(6272):273–276. doi: 10.1038/345273a0. [DOI] [PubMed] [Google Scholar]
- 2.Labeit S, Kolmerer B. Titins: Giant proteins in charge of muscle ultrastructure and elasticity. Science. 1995;270(5234):293–296. doi: 10.1126/science.270.5234.293. [DOI] [PubMed] [Google Scholar]
- 3.Trombitás K, Freiburg A, Centner T, Labeit S, Granzier H. Molecular dissection of N2B cardiac titin’s extensibility. Biophys J. 1999;77(6):3189–3196. doi: 10.1016/S0006-3495(99)77149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Granzier HL, Irving TC. Passive tension in cardiac muscle: Contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J. 1995;68(3):1027–1044. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herman DS, et al. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. 2012;366(7):619–628. doi: 10.1056/NEJMoa1110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labeit S, Gautel M, Lakey A, Trinick J. Towards a molecular understanding of titin. EMBO J. 1992;11(5):1711–1716. doi: 10.1002/j.1460-2075.1992.tb05222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fürst DO, Nave R, Osborn M, Weber K. Repetitive titin epitopes with a 42 nm spacing coincide in relative position with known A band striations also identified by major myosin-associated proteins. An immunoelectron-microscopical study on myofibrils. J Cell Sci. 1989;94(Pt 1):119–125. doi: 10.1242/jcs.94.1.119. [DOI] [PubMed] [Google Scholar]
- 8.Whiting A, Wardale J, Trinick J. Does titin regulate the length of muscle thick filaments? J Mol Biol. 1989;205(1):263–268. doi: 10.1016/0022-2836(89)90381-1. [DOI] [PubMed] [Google Scholar]
- 9.Bennett PM, Gautel M. Titin domain patterns correlate with the axial disposition of myosin at the end of the thick filament. J Mol Biol. 1996;259(5):896–903. doi: 10.1006/jmbi.1996.0367. [DOI] [PubMed] [Google Scholar]
- 10.Wang K. Titin/connectin and nebulin: Giant protein rulers of muscle structure and function. Adv Biophys. 1996;33:123–134. [PubMed] [Google Scholar]
- 11.Sosa H, Popp D, Ouyang G, Huxley HE. Ultrastructure of skeletal muscle fibers studied by a plunge quick freezing method: Myofilament lengths. Biophys J. 1994;67(1):283–292. doi: 10.1016/S0006-3495(94)80479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granzier HL, Akster HA, Ter Keurs HE. Effect of thin filament length on the force-sarcomere length relation of skeletal muscle. Am J Physiol. 1991;260(5 Pt 1):C1060–C1070. doi: 10.1152/ajpcell.1991.260.5.C1060. [DOI] [PubMed] [Google Scholar]
- 13.Maw MC, Rowe AJ. The reconstruction of myosin filaments in rabbit psoas muscle from solubilized myosin. J Muscle Res Cell Motil. 1986;7(2):97–109. doi: 10.1007/BF01753410. [DOI] [PubMed] [Google Scholar]
- 14.Fürst DO, Osborn M, Nave R, Weber K. The organization of titin filaments in the half-sarcomere revealed by monoclonal antibodies in immunoelectron microscopy: A map of ten nonrepetitive epitopes starting at the Z line extends close to the M line. J Cell Biol. 1988;106(5):1563–1572. doi: 10.1083/jcb.106.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cazorla O, et al. Differential expression of cardiac titin isoforms and modulation of cellular stiffness. Circ Res. 2000;86(1):59–67. doi: 10.1161/01.res.86.1.59. [DOI] [PubMed] [Google Scholar]
- 16.Ottenheijm CA, et al. Tuning passive mechanics through differential splicing of titin during skeletal muscle development. Biophys J. 2009;97(8):2277–2286. doi: 10.1016/j.bpj.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trombitás K, Jin JP, Granzier H. The mechanically active domain of titin in cardiac muscle. Circ Res. 1995;77(4):856–861. doi: 10.1161/01.res.77.4.856. [DOI] [PubMed] [Google Scholar]
- 18.Kellermayer MS, Smith SB, Bustamante C, Granzier HL. Complete unfolding of the titin molecule under external force. J Struct Biol. 1998;122(1–2):197–205. doi: 10.1006/jsbi.1998.3988. [DOI] [PubMed] [Google Scholar]
- 19.Ohno M, Cheng CP, Little WC. Mechanism of altered patterns of left ventricular filling during the development of congestive heart failure. Circulation. 1994;89(5):2241–2250. doi: 10.1161/01.cir.89.5.2241. [DOI] [PubMed] [Google Scholar]
- 20.Butler J, et al. Developing therapies for heart failure with preserved ejection fraction: Current state and future directions. JACC Heart Fail. 2014;2(2):97–112. doi: 10.1016/j.jchf.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeWinter MM, Wu Y, Labeit S, Granzier H. Cardiac titin: Structure, functions and role in disease. Clin Chim Acta. 2007;375(1–2):1–9. doi: 10.1016/j.cca.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 22.Luther PK, et al. Understanding the organisation and role of myosin binding protein C in normal striated muscle by comparison with MyBP-C knockout cardiac muscle. J Mol Biol. 2008;384(1):60–72. doi: 10.1016/j.jmb.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Khayat HA, Kensler RW, Squire JM, Marston SB, Morris EP. Atomic model of the human cardiac muscle myosin filament. Proc Natl Acad Sci USA. 2013;110(1):318–323. doi: 10.1073/pnas.1212708110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houmeida A, et al. Evidence for the oligomeric state of ‘elastic’ titin in muscle sarcomeres. J Mol Biol. 2008;384(2):299–312. doi: 10.1016/j.jmb.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 25.Irving T, et al. Thick-filament strain and interfilament spacing in passive muscle: Effect of titin-based passive tension. Biophys J. 2011;100(6):1499–1508. doi: 10.1016/j.bpj.2011.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muhle-Goll C, et al. Structural and functional studies of titin’s fn3 modules reveal conserved surface patterns and binding to myosin S1—a possible role in the Frank-Starling mechanism of the heart. J Mol Biol. 2001;313(2):431–447. doi: 10.1006/jmbi.2001.5017. [DOI] [PubMed] [Google Scholar]
- 27.Freiburg A, Gautel M. A molecular map of the interactions between titin and myosin-binding protein C. Implications for sarcomeric assembly in familial hypertrophic cardiomyopathy. Eur J Biochem. 1996;235(1–2):317–323. doi: 10.1111/j.1432-1033.1996.00317.x. [DOI] [PubMed] [Google Scholar]
- 28.de Tombe PP, et al. Myofilament length dependent activation. J Mol Cell Cardiol. 2010;48(5):851–858. doi: 10.1016/j.yjmcc.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamasaki R, et al. Titin-actin interaction in mouse myocardium: Passive tension modulation and its regulation by calcium/S100A1. Biophys J. 2001;81(4):2297–2313. doi: 10.1016/S0006-3495(01)75876-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulke M, et al. Interaction between PEVK-titin and actin filaments: Origin of a viscous force component in cardiac myofibrils. Circ Res. 2001;89(10):874–881. doi: 10.1161/hh2201.099453. [DOI] [PubMed] [Google Scholar]
- 31.Chung CS, et al. Titin based viscosity in ventricular physiology: An integrative investigation of PEVK-actin interactions. J Mol Cell Cardiol. 2011;51(3):428–434. doi: 10.1016/j.yjmcc.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson BR, Bogomolovas J, Labeit S, Granzier H. Single molecule force spectroscopy on titin implicates immunoglobulin domain stability as a cardiac disease mechanism. J Biol Chem. 2013;288(8):5303–5315. doi: 10.1074/jbc.M112.401372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kellermayer MS, Smith SB, Granzier HL, Bustamante C. Folding-unfolding transitions in single titin molecules characterized with laser tweezers. Science. 1997;276(5315):1112–1116. doi: 10.1126/science.276.5315.1112. [DOI] [PubMed] [Google Scholar]
- 34.Kass DA, Bronzwaer JG, Paulus WJ. What mechanisms underlie diastolic dysfunction in heart failure? Circ Res. 2004;94(12):1533–1542. doi: 10.1161/01.RES.0000129254.25507.d6. [DOI] [PubMed] [Google Scholar]
- 35.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7(8):589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 36.Sheikh F, et al. An FHL1-containing complex within the cardiomyocyte sarcomere mediates hypertrophic biomechanical stress responses in mice. J Clin Invest. 2008;118(12):3870–3880. doi: 10.1172/JCI34472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lange S, et al. Subcellular targeting of metabolic enzymes to titin in heart muscle may be mediated by DRAL/FHL-2. J Cell Sci. 2002;115(Pt 24):4925–4936. doi: 10.1242/jcs.00181. [DOI] [PubMed] [Google Scholar]
- 38.Hojayev B, Rothermel BA, Gillette TG, Hill JA. FHL2 binds calcineurin and represses pathological cardiac growth. Mol Cell Biol. 2012;32(19):4025–4034. doi: 10.1128/MCB.05948-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Granzier HL, et al. Truncation of titin’s elastic PEVK region leads to cardiomyopathy with diastolic dysfunction. Circ Res. 2009;105(6):557–564. doi: 10.1161/CIRCRESAHA.109.200964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung CS, et al. Shortening of the elastic tandem immunoglobulin segment of titin leads to diastolic dysfunction. Circulation. 2013;128(1):19–28. doi: 10.1161/CIRCULATIONAHA.112.001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radke MH, et al. Targeted deletion of titin N2B region leads to diastolic dysfunction and cardiac atrophy. Proc Natl Acad Sci USA. 2007;104(9):3444–3449. doi: 10.1073/pnas.0608543104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Granzier HL, Labeit S. Titin and its associated proteins: The third myofilament system of the sarcomere. Adv Protein Chem. 2005;71:89–119. doi: 10.1016/S0065-3233(04)71003-7. [DOI] [PubMed] [Google Scholar]
- 43.LeWinter MM, Granzier HL. Titin is a major human disease gene. Circulation. 2013;127(8):938–944. doi: 10.1161/CIRCULATIONAHA.112.139717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linke WA, Hamdani N. Gigantic business: Titin properties and function through thick and thin. Circ Res. 2014;114(6):1052–1068. doi: 10.1161/CIRCRESAHA.114.301286. [DOI] [PubMed] [Google Scholar]
- 45.Borbély A, et al. Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104(6):780–786. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.