Significance
Obesity is one of the most common health concerns today. Herbal-based food supplements are widely used to treat obesity. Among them, the African cactiform Hoodia gordonii supplements are extremely popular. The development of Hoodia and P57, the purported active ingredient, has been problematic due to controversy about intellectual property rights and limited natural resources. To date, the true active components and molecular targets of Hoodia remain unclear. Here, we demonstrate that Gordonoside F, a compound from Hoodia, activates GPR119, a receptor critically involved in metabolic homeostasis, and leads to increased insulin secretion and reduced food intake. The successful synthesis of Gordonoside F described here will provide an opportunity for developing new drugs in treating metabolic diseases.
Abstract
African cactiform Hoodia gordonii (Asclepiadaceae) has been used for thousands of years by Xhomani Bushmen as an anorexant during hunting trips and has been proposed as a new agent for the management of body weight. However, its in vivo targets and molecular mechanisms remain elusive. GPR119, a G protein-coupled receptor highly expressed in pancreatic β cells and intestinal L cells, has been demonstrated to facilitate glucose-stimulated insulin secretion (GSIS) and represents a novel and attractive target for the therapy of metabolic disorders. Here, we disclose that Gordonoside F (a steroid glycoside isolated from H. gordonii), but not the widely known P57, activates specifically GPR119. Successful synthesis of Gordonoside F facilitates further characterization of this compound. Gordonoside F promotes GSIS both in vitro and in vivo and reduces food intake in mice. These effects are mediated by GPR119 because GPR119 knockout prevents the therapeutic effects of Gordonoside F. Interestingly, the appetite-suppressing effect of Hoodia extract was also partially blocked by GPR119 knockout. Our results demonstrate for the first time, to our knowledge, that GPR119 is a direct target and one of the major mechanisms underlying the therapeutic effect of the popular “weight loss” herb H. gordonii. Given the long history of safe application of this herb in weight control, it is foreseeable that the novel scaffold of Gordonoside F provides a promising opportunity to develop new drugs in treating metabolic diseases.
Obesity is one of the most common health concerns, affecting more than 300 million people worldwide (1). Obesity is associated with and can lead to a number of diseases, including type 2 diabetes (T2D), cardiovascular disease, osteoarthritis, dyslipidemia, and sleep apnea, many of which can be prevented with a reduction in body weight (1). Current strategies for the management of body weight include calorie restriction, regular exercise, and behavior modification. Although effective in the short term, diet and exercise alone are difficult to maintain over the long term for the majority of patients. Thus, medications and alternative treatments have been sought. The commercial market for antiobesity preparations is enormous, but unfortunately, there are only two FDA-approved drugs available, phentermine and orlistat, both with limited efficacy but significant side effects (2).
Herbal-based food supplements are among the most widely used alternative treatments for obesity. However, their effectiveness, safety, and mechanism of actions largely remain unknown. Among them, the Hoodia gordonii supplements are extremely popular. The African cactiform H. gordonii (Asclepiadaceae) is a succulent plant growing in the Kalahari Deseret in South Africa, Namibia, and Botswana (3). The plant has been used for thousands of years by Xhomani Bushmen as a hunger and thirst suppressant during hunting trips, and the natural antiobesity agent from the plant has attracted great attention and led to many commercial preparations (4).
The plant is rich in pregnane glycosides containing 6-deoxy- and 2,6-dideoxy-sugars. To date, P57AS3 (P57) is the only biologically active constituent from H. gordonii that has been reported to have anorexigenic activity (5). There is no evidence of P57 binding to or altering activity of known receptors or proteins, but intracerebroventricular injections of P57 in rats has been reported to increase ATP content in the hypothalamus, which might represent a signal of satiety and suppress appetitive responses (6). However, a recent study claimed that P57 was not detectable in the brain upon oral administration in mice, which makes the mechanisms of action of P57 and H. gordonii more elusive (7). The development and commercialization of H. gordonii and P57 have been problematic due to controversy about intellectual property rights and limited natural resources, in addition to the lack of a clear mechanism, resulting in the withdrawal of several major pharmaceutical companies from Hoodia projects (3). Since the isolation of P57, more new glycosides, including Gordonoside A-L (8), have been disclosed in the literature although their bioactivities remain unclear.
Meanwhile, many new potential drug targets related to metabolic disorders have been identified. Compounds targeting a number of G protein-coupled receptors (GPCRs), including GLP-1R, GPR40, GPR120, CB1, GCGR, and β2-adenoceptor (9), have been proposed to treat T2D and obesity. Recently, GPR119, a GPCR highly expressed in pancreatic β cells and intestinal L cells (10, 11), has been demonstrated to facilitate glucose-stimulated insulin secretion (GSIS) (12). The endogenous ligands LPC and OEA promote GSIS, directly by activating GPR119 on the β cells and indirectly by activating GPR119 on the L cells, and induce GLP-1 secretion (13). Oral administration of AR231453, a synthetic agonist of GPR119, significantly improves circulating insulin, GLP-1, and GIP levels, and lowers the blood glucose concentration in glucose tolerance tests in mice (11, 14). Another GPR119 agonist, PSN632408, has been shown to suppress food intake and reduce body weight gain in rats, in addition to its blood glucose-lowering effect (15). Therefore, GPR119 represents an attractive target for the therapy of diabetes and obesity.
Here, we disclose that Gordonoside F (a steroid glycoside isolated from H. gordonii), but not the widely known P57, activates GPR119 potently and selectively, which consequently promotes GSIS both in vitro and in vivo and reduces food intake in animals. In addition, chemical synthesis of Gordonoside F is reported. The present results not only demonstrate unambiguously that the activation of GPR119 receptor is an important mechanism underlying H. gordonii’s therapeutic effect but also suggest that Gordonoside F or its congeners could be developed into new drugs in treating metabolic disorders.
Materials and Methods
Chemicals and Reagents.
Gordonoside F and its natural congeners were purchased from AnalytiCon Discovery and/or synthesized chemically (see SI Appendix, Materials and Methods for details). Crude extract of H. gordonii (Ref: EA149464) was provided by Naturex. Mammalian expression vectors encoding various GPCRs and Gα16 were purchased from the UMR cDNA Resource Center.
Reporter Assay.
Cells expressing GPR119 (or other GPCRs) and CRE-luc were plated at a density of 10,000 cells per well in a 96-well plate. After 24 h culture, compounds at various concentrations were added. DMSO (1%) was used as negative control. Another 24 h later, luciferase activities were measured using the Steady-Glo luciferase assay system (Promega) and an EnVision (PerkinElmer) multiplate reader according to the manufacturer’s instructions.
Insulin Secretion from Isolated Islets.
All animal experiments were approved by the Animal Ethics Committee of the Shanghai Institute of Materia Medica. Islets were isolated as previously described (16) from anesthetized male C57BL/6 mice or Sprague–Dawley rats (8 wk old). In brief, 5 mL of collagenase XI (Sigma, 0.25 mg/mL in HBSS) solution was injected into the pancreas via the bile duct, and the pancreas was digested at 37 °C for 15 min. The detached islets were collected and cultured overnight in RPMI 1640 supplemented with 11.1 mM glucose and 10% (vol/vol) FBS. The islets were washed with Hepes-balanced KRBB containing 0.5% fatty acid-free BSA (KRBB buffer) and 2.8 mM glucose, and then hand-selected under a stereomicroscope and moved to 24-well plates. After preincubation for 30 min at 37 °C in the KRBB buffer (2.8 mM glucose), islets were incubated with various compounds for 2 h at 37 °C in KRBB buffers containing 2.8 mM or 16.8 mM glucose. Supernatants were collected, and the insulin concentrations were measured using a Cisbio HTRF Insulin kit and an EnVision multiplate reader according to the manufacturer’s instructions.
In Vivo Experiments.
For the oral glucose tolerance test (OGTT), male C57BL/6 mice were fasted overnight and then given either 0.5% methyl-cellulose (vehicle) or test compounds (n = 8 per treatment group) at desired doses via oral gavage. A glucose bolus was then delivered (2 g/kg orally). Blood was collected from a tail nick at designated time points, and plasma glucose levels were determined with a glucose meter. For insulin and GLP-1 measurement, compounds and glucose were given to fasted mice just like the OGTT. Blood was collected in heparinized tubes containing a DPP-IV Inhibitor (DPP4-010; Millipore) 10 min after glucose administration. Plasma samples were obtained via centrifugation at 500 × g for 20 min. Insulin and active GLP-1 were detected with ELISA.
Acute Feeding Experiments.
Male C57BL/6 mice were maintained on a reverse-phase light-dark cycle and had free access to standard powdered mice diet. Mice (n = 8 per treatment group) were fasted overnight, and the test compound or 0.5% methyl-cellulose (vehicle) was given via oral gavage. Food intake was monitored by weighing feeding jars at 2 h, 4 h, 8 h, and 24 h after drug administration.
Data Analysis.
Data were analyzed with GraphPad Prism software. Nonlinear regression analysis was performed to generate dose–response curves and EC50 values. Data are presented as means ± SEM. Two-tailed Student t tests were performed, and P < 0.05 was considered to be statistically significant.
Results
Gordonoside F Activates GPR119-Mediated Signal Transduction.
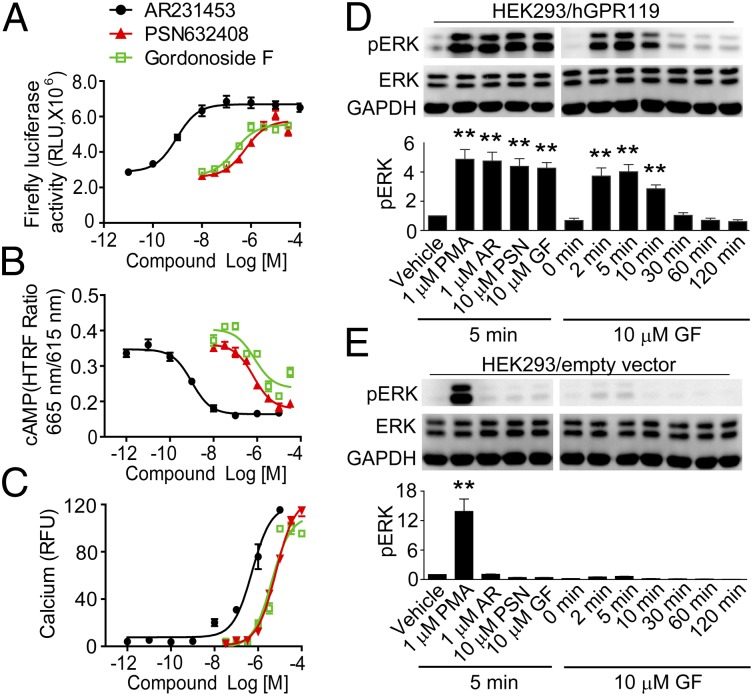
To search for new GPR119 agonists, we screened a library of ∼4,400 natural products with a HEK293 cell line stably expressing human GPR119 and the pCRE-luc reporter (HEK293/GPR119/pCRE-luc). Several hits were identified to activate the luciferase reporter expression in GPR119-expressing cells. Among them was a natural compound isolated from the African cactiform H. gordonii, Gordonoside F (structure in Table 1). Gordonoside F was found to induce CRE-driven luciferase expression (Fig. 1A), cAMP accumulation (Fig. 1B), and intracellular calcium mobilization (Fig. 1C) in GPR119-expressing HEK293 cells in a dose-dependent manner, with EC50 values of 0.23 μM, 0.76 μM, and 6.6 μM, respectively. Gordonoside F showed similar efficacy and potency as PSN632408 (EC50 = 0.61 μM, 0.69 μM, and 4.1 μM in luciferase, cAMP, and calcium assays, respectively) whereas another reported GPR119 agonist, AR231453, showed higher potency (EC50 = 0.94 nM, 0.99 nM, and 0.53 μM in luciferase, cAMP, and calcium assays, respectively).
Table 1.
Biological activities of the naturally occurring Hoodigosides/Gordonosides (1–6) and synthetic Gordonoside F on the human GPR119 receptor
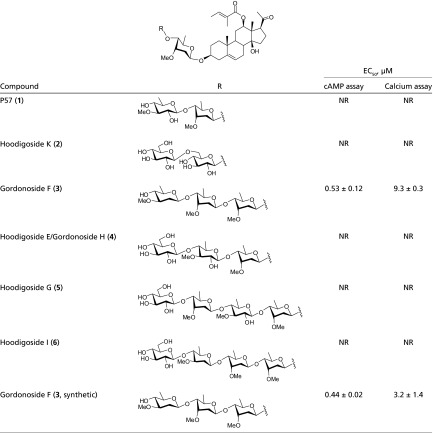 |
NR, no response at concentrations up to 100 μM.
Fig. 1.
Gordonoside F activates GPR119-mediated signaling. (A) Gordonoside F induces luciferase expression in HEK293/GPR119/pCRE-luc cells. (B) Gordonoside F triggers intracellular cAMP accumulation in HEK293/GPR119 cells. (C) Gordonoside F activates calcium mobilization in HEK293 cells cotransfected with hGPR119 and Gα16. (D and E) HEK293 cells transfected with GPR119 (D) or empty vector (E) were stimulated with compounds at indicated concentration and time at 37 °C, and ERK1/2 phosphorylation was detected with Western blot. Data are means ± SEM (n = 3). **P < 0.01, versus vehicle control.
Many GPCRs couple to the ERK1/2 pathway. Similar to the known GPR119 agonists AR231453 and PSN632408, Gordonoside F (10 μM) induced phosphorylation of ERK1/2 in a time-dependent manner in HEK293 cells expressing GPR119 (Fig. 1D). In contrast, in HEK293 cells transfected with empty vector, only the PKC activator phorbol-12-myristate-13-acetate (PMA) induced phosphorylation of ERK1/2 whereas all of the other compounds did not have any effect (Fig. 1E).
Chemical Synthesis and Receptor Selectivity of Gordonoside F.
Interestingly, among the naturally occurring Hoodigosides/Gordonosides tested (1–6 in Table 1), only the tetrasaccharide Gordonoside F (3) evoked intracellular cAMP accumulation and calcium response in HEK293 cells expressing GPR119; other compounds showed no activity at concentrations up to 100 μM. All these compounds share the same aglycone, namely Hoodigogenin A, which also displayed no activity. The glycan thus contributes critically to the activity. Nevertheless, the glycans in these congeners are structurally relevant, differing in only the terminal di- or trisaccharide residues in 4-6. Comparing Gordonoside F (3) and Hoodigoside I (6), an additional glucose residue (in 6) abolishes the activity completely. It was also surprising to find that P57 (1), which was previously believed to be the active compound in the plant, was not active at all.
To rule out the possibility that the very limited number of purchased natural products were incorrect in structures and quality and to further study the in vivo activities, we embarked on chemical syntheses of Gordonoside F (3). Adopting a modification of our recently developed approach to the synthesis of P57 (1) (17, 18, 19), we managed to synthesize Gordonoside F (3) (see SI Appendix, Materials and Methods for details). Gratifyingly, the synthetic Gordonoside F (3) did activate GPR119 in the cAMP assay with an EC50 of 0.44 μM, consistent with the previous results with the natural product (EC50 = 0.53 μM, Table 1). In addition, Gordonoside F (3) did not evoke calcium response in HEK293 cells transfected with empty vector or 28 other GPCRs, including GPR40, GPR41, GPR120, CB1, CB2, GLP-1R, β2AR, etc., at concentrations up to 100 μM (SI Appendix, Table S1). These results indicate that Gordonoside F is a highly selective GPR119 agonist. We then synthesized Gordonoside F (3) in gram scale and subjected it to further characterization.
Gordonoside F Induces Desensitization and Internalization of GPR119.
Receptor desensitization is a common mechanism that leads to the loss of function in GPCRs after agonist stimulation. To assess whether Gordonoside F induces GPR119 desensitization, HEK293 cells stably expressing GPR119 were first stimulated with either PSN632408 (30 μM), Gordonoside F (30 μM), or 0.1% DMSO (Fig. 2A, first arrow). Both PSN632408 and Gordonoside F induced robust calcium responses, confirming again that they are agonists of GPR119. After 10 min incubation, cells were washed and restimulated with 30 μM PSN632408 (Fig. 2A, second arrow). Cells prestimulated with 0.1% DMSO responded normally toward PSN632408 whereas prestimulation with both PSN632408 and Gordonoside F led to receptor desensitization: i.e., reduced calcium response at the second stimulation with PSN632408.
Fig. 2.
Gordonoside F induces desensitization and internalization of GPR119. (A) HEK293/GPR119/Gα16 cells were loaded with Fluo-4:00 AM and stimulated (first arrow) with 30 μM Gordonoside F, 30 μM PSN632408 (positive control), or DMSO (negative control). After 10 min incubation, cells were washed and restimulated (second arrow) with 30 μM PSN632408. Intracellular calcium level was measured. (B) Kinetics of GPR119 endocytosis. HEK293 cells expressing HA-GPR119 were incubated with 10 μM Gordonoside F for 0–120 min at 37 °C, and the cell-surface level of GPR119 was determined by measuring surface HA immunoreactivity with flow cytometry. Data are means ± SEM (n = 3). *P < 0.05, **P < 0.01, versus vehicle control.
Receptor internalization is another common phenomenon following GPCR stimulation. To determine the ability of Gordonoside F to induce GPR119 internalization, HEK293 cells expressing HA-tagged GPR119 were incubated with Gordonoside F (10 μM) at 37 °C for various durations. The reaction was stopped, and the cell-surface GPR119 was labeled and analyzed with flow cytometry. As demonstrated in Fig. 2B, Gordonoside F induced a significant and time-dependent decrease of cell-surface GPR119. Taken together, these data confirm that Gordonoside F is able to activate a full spectrum of signaling mediated by GPR119 in vitro, including cAMP accumulation, calcium mobilization, ERK phosphorylation, and receptor desensitization and internalization.
Gordonoside F Induces Insulin Secretion from Isolated Islets.
Activation of GPR119 has been reported to enhance GSIS from β cells (11). Thus, we examined the effect of Gordonoside F on GSIS in isolated rat islets (Fig. 3A). High glucose (16.8 mM) induced substantial insulin release from rat islets whereas GLP-1 (0.1 μM) and AR231453 (0.5 μM) significantly enhanced GSIS. Gordonoside F significantly and dose-dependently stimulated insulin secretion in high-glucose conditions. All these stimulants were less effective or had no effect in low-glucose media (Fig. 3A).
Fig. 3.
Gordonoside F induces insulin release via GPR119 from isolated islets. (A) Rat islets were isolated and incubated in medium supplemented with 2.8 mM or 16.8 mM glucose and indicated compounds for 2 h, and insulin in the supernatants were measured. (B) Gene-targeting strategy in generating GPR119 KO mice. The ORF of GPR119 was replaced by a neomycin cassette via homologous recombination. P1–P6 indicate the primers used to characterize the GPR119 KO animals. (C) Representative PCR analysis of the genomic DNA of embryonic stem (ES) cells with targeted homologous recombination. (D) Representative PCR genotyping of DNA samples from tail clips of WT and GPR119 KO mice. (E) Immunofluorescent analysis of insulin and glucagon in pancreatic sections from 8-wk-old male GPR119 KO mice and age-matched WT litters. (F) Islets isolated from GPR119 KO or WT mice were incubated in medium supplemented with 2.8 mM or 16.8 mM glucose and indicated compounds for 2 h, and insulin in the supernatants was measured. Data are means ± SEM (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle control.
To investigate whether the insulinotropic effect of Gordonoside F was indeed via the activation of GPR119, we generated GPR119-deficient mice. As in human, the gene encoding mouse GPR119 is also located on the X chromosome (Unigene Cluster Mm.34953). A segment of this gene containing a neomycin cassette, which replaces the ORF of GPR119, was targeted by homologous recombination (Fig. 3B). Obtained targeted ES cells were identified by PCR (Fig. 3C), and the targeted clone was further used for creation of GPR119 KO mice. The genotypes of the obtained mice were determined to be wild type (WT) and homozygote (GPR119 KO) by PCR (Fig. 3D). The GPR119 KO mice appeared generally normal, reproduced successfully, and showed Mendelian inheritance characteristics. These mice also had normal size, body weight, and fed/fasted blood-glucose levels, similar to those reported elsewhere (20). There was no gross difference in islet morphology between WT and GPR119 KO mice as demonstrated by glucagon/insulin staining patterns (Fig. 3E). Islets isolated from both WT and KO mice displayed normal GSIS. GLP-1 was able to further stimulate insulin release in the presence of high glucose (16.8 mM) (Fig. 3F) from all islets. However, neither AR231453 nor Gordonoside F could further stimulate insulin secretion in high-glucose conditions from the GPR119 KO islets whereas both enhanced GSIS in the WT islets (Fig. 3F). These results indicate that the insulinotropic effect of Gordonoside F is indeed from the activation of GPR119 and that the effect is glucose-dependent.
Gordonoside F Enhances GSIS in Vivo and Improves Glucose Tolerance in Mice.
Next, we explored the function of Gordonoside F and the crude extract of H. gordonii on glucose homeostasis in vivo. The authenticity of the Hoodia extract was confirmed by HPLC, and both P57 and Gordonoside F were detected (SI Appendix, Figs. S1 and S2). An oral glucose tolerance test was performed after overnight fasting. In WT mice, pretreatment with AR231453 (20 mg/kg, oral), Gordonoside F (200 mg/kg, oral), or Hoodia extract (1,000 mg/kg, oral) significantly improved oral glucose tolerance (Fig. 4A). GPR119 KO almost completely abolished the glucose-lowering effect of AR231453 and Gordonoside F (Fig. 4B), indicating that the efficacy of these compounds was mediated by GPR119 in vivo. It was interesting to notice that GPR119 KO also blocked the glucose-lowering effect of Hoodia extract (P < 0.05 in WT mice versus P = 0.22 in KO mice) (Fig. 4 A and B) although the impact was not as dramatic as with AR231453 and Gordonoside F. Blood collected 10 min after glucose administration was used to analyze the levels of plasma insulin and active GLP-1. WT mice treated with Gordonoside F had markedly elevated insulin and GLP-1 levels 10 min after glucose bolus, but not the GPR119-deficient mice (Fig. 4 C and D). Hoodia extract significantly increased insulin level and slightly increased GLP-1 level (P = 0.055) in WT mice. These effects were also reduced in GPR119 KO mice, especially the GLP-1 promoting effect (Fig. 4 C and D). These data suggest that Gordonoside F acts primarily by activating GPR119 on β cells and intestine cells and thus enhances glucose-dependent GLP-1 and insulin release that subsequently lead to enhanced glucose disposal. The Hoodia extract also regulates glucose homeostasis by oral administration, and the effect is at least partially mediated via GPR119.
Fig. 4.
Gordonoside F induces insulin and GLP-1 release via GPR119 in vivo. (A and B) Oral glucose tolerant test (OGTT). Age-matched WT (A) or GPR119 KO (B) male mice were fasted overnight. AR231453 (20 mg/kg), Gordonoside F (200 mg/kg), Hoodia extract (1,000 mg/kg), or vehicle (0.5% methyl-cellulose) was administered orally 30 min before a glucose bolus (2 g/kg orally). Blood glucose level was measured at indicated time points, and the areas under the curves (AUC) were plotted (Inset). (C and D) In another set of experiments, plasma was obtained 10 min after oral glucose administration to measure the levels of insulin (C) and active GLP-1 (D). Data are means ± SEM (n = 8). *P < 0.05, **P < 0.01 versus vehicle control.
Gordonoside F Acutely Reduces Food Intake in Mice by Activating GPR119.
It has been reported that GPR119 agonist suppressed food intake and reduced body-weight gain in a rat model (15). So we tested whether Gordonoside F and Hoodia extract affect food intake in free-feeding C57BL/6 mice. Gordonoside F (200 mg/kg, oral) significantly reduced cumulative food intake at all time points tested (2 h, 4 h, 8 h, and 24 h postdosing) (Fig. 5A). However, in GPR119 KO mice, Gordonoside F failed to suppress food intake (Fig. 5B). It was not surprising to find that Hoodia extract reduced food consumption in WT mice (Fig. 5A). However, it was extremely interesting to notice that the appetite-suppressing effect of Hoodia was significantly reduced in GPR119 KO mice although the extract could still block food intake significantly in these KO mice (Fig. 5 A and B). At 2 h postdosing, Hoodia reduced 42.5 ± 5.6% of the food intake in WT mice whereas, in KO mice, it reduced only 20.9 ± 2.7%, demonstrating a statistically significant reduction compared with the WT mice (P = 0.03). Similar phenomena could also be observed at 4 h (50.1 ± 4.2% reduction in WT vs. 34.3 ± 5.4% in KO, P = 0.06) and 8 h (42.0 ± 2.3% reduction in WT vs. 17.2 ± 2.0% in KO, P = 0.006) postdosing.
Fig. 5.
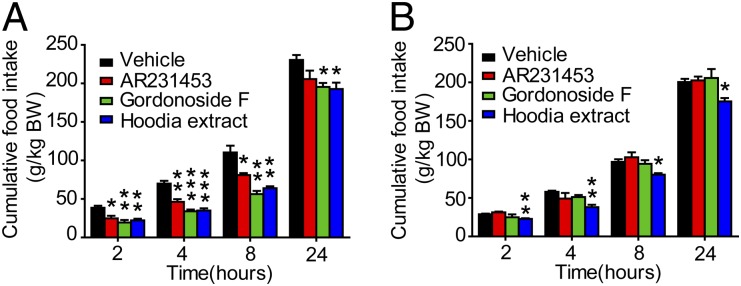
Gordonoside F reduces food intake via GPR119. Age-matched WT (A) or GPR119 KO (B) male mice were fasted overnight, and AR231453 (20 mg/kg), Gordonoside F (200 mg/kg), Hoodia extract (1,000 mg/kg), or vehicle (0.5% methyl-cellulose) was administered orally. Cumulative food intake upon refeeding was recorded at 2 h, 4 h, 8 h, and 24 h. Data are means ± SEM (n = 8). *P < 0.05, **P < 0.01, *** P < 0.001 versus vehicle control.
Dose effects of Gordonoside F and Hoodia extract on food intake were further evaluated. At 100 mg/kg and 200 mg/kg, Gordonoside F significantly reduced food intake in WT mice but had no effect in GPR119 KO mice (SI Appendix, Fig. S3 A and B). At 400 mg/kg, Gordonoside F reduced food intake in both the WT and GPR119 KO mice. However, the appetite-suppressing effect was significantly reduced in GPR119 KO mice (SI Appendix, Fig. S3 A–C). Similarly, low-dose Hoodia extract (500 mg/kg) significantly reduced food intake in WT mice at 2 h and 4 h after dosing whereas, in GPR119 KO mice, it had no effect at any time point (SI Appendix, Fig. S4 A and B). At 1,000 mg/kg, Hoodia extract reduced food intake in both the WT and GPR119 KO mice at all time points, but the appetite-suppressing effect was significantly lower in GPR119 KO mice (SI Appendix, Fig. S4 A–C). However, at high dose (2,000 mg/kg), the difference between WT and GPR119 KO groups was much less significant even though Hoodia was slightly less effective in GPR119 KO mice (SI Appendix, Fig. S4D). Although the exact mechanisms remain unclear, high-dose Gordonoside F or Hoodia extract might cause adverse effects or activate other pathways to reduce food intake in GPR119 KO animals.
Taken together, our data demonstrate that the hypophagic action of Hoodia is at least partially mediated via GPR119 and that Gordonoside F is one of the active components conveying such biological activities of this folkloric plant.
Discussion
The stem of the H. gordonii has traditionally been chewed by the native bushmen of the South African deserts to suppress hunger (4, 21). There are 13 species in the Hoodia genus. At present, only H. gordonii has been used in commercial formulations marketed as dietary supplements. As a weight loss herb, it has gained popularity in Western countries and has been investigated since the 1980s (22). Acute toxicity studies were described in a patent application by Van Heerden et al (23). An H. gordonii extract administered orally to mice in doses up to 3,028.5 mg/kg revealed no clinical signs of toxicity (23).
The majority of the secondary metabolites isolated from H. gordonii have been characterized as pregnane glycosides containing 6-deoxy and 2,6-dideoxy sugars (4). The only active appetite suppressant component of H. gordonii identified so far is a trisaccharide of 12β-tigloyloxy-14β-hydroxypregn-5-en-20-one (referred to as P57AS3 or P57) (5, 23). There is no evidence that P57 binds to or alters the activity of known receptors or proteins, including Na/K-ATPase, the putative target of cardiac glycosides. Intracerebroventricular injections of P57 in rats increases ATP content/production in the hypothalamus, which might represent a signal of satiety and suppress appetitive responses (6). However, in a recent study, P57 was not detected in the brain upon oral administration in mice, and it was found at a low concentration in the intestine, kidney, and liver (7). Therefore, the effect in the central nervous system is not likely the main mechanism underlying H. gordonii’s anorexigenic effect.
In the present study, we discovered that Gordonoside F, a pregnane steroidal glycoside isolated from H. gordonii (8), is a novel and highly selective agonist of GPR119. Interestingly, the natural congeners (1, 2, and 4–6) of Gordonoside F occurring in the same plant, including P57 (1), displayed no such activity. GPR119 is expressed predominantly in pancreatic β cells and in enteroendocrine cells. It has become a major target for the development of antidiabetic/obesity drugs because GPR119 activation can stimulate both insulin release from the pancreas and GLP-1 release from the intestine (9, 24, 25). GLP-1 is a potent insulin-releasing and appetite-suppressing hormone, and GLP-1 analogs have been used to treat type 2 diabetes in clinics (26, 27). GPR119 is activated predominantly by fatty acid ethanolamide derivatives, the phospholipid lysophosphatidylcholine (28), and 5-hydroxy eicosapentanoic acid (5-HEPE) (29). In addition to these endogenous ligands, a number of the synthetic agonists of GPR119 have been developed, including AR231453, PSN632408, and, more recently, AS1269574, AS1907417, AS1535907, GSK-1292263, and JNJ-38431055 (28). GSK-1292263 has completed phase II clinical trials and was reported as well-tolerated. Finding safe and effective GPR119 agonists continues to be a major effort in developing antidiabetic drugs.
Further characterization of Gordonoside F and the animal tests were facilitated by chemical synthesis. Synthesis of Gordonoside F, a complex pregnane tetrasaccharide, turned out to be a challenging task (17, 18). Taking advantage of our recently developed approach to the synthesis of P57 (19), we achieved the syntheses of Gordonoside F in a longest linear sequence of 26 steps and in overall 10% yield. This approach would enable us to synthesis congeners of Gordonoside F in a flexible manner in the future and warrant further structural activity relationship study.
Gordonoside F displayed extremely high selectivity for GPR119; in fact, it showed no activity for all of the other 28 GPCRs tested (SI Appendix, Table S1). The gram-scale synthesis of Gordonoside F enabled us to test the in vivo effects of this compound. It promoted GSIS both in vitro and in vivo and reduced food intake by oral administration in WT mice but not in GPR119-deficient mice. More interestingly, the appetite-suppressing effect of Hoodia extract was also partially blocked by GPR119 knockout. Because GPR119 is mainly expressed in the intestine and pancreatic β cells, it is surely more accessible to these compounds than the targets in the central nervous system.
Although we have demonstrated that the biological activity of Hoodia extract was partially mediated by GPR119 and that Gordonoside F was the only active compound among the six natural compounds that we tested in this study, we still can’t rule out the possibility of the existence of other GPR119-activating compounds in Hoodia. As demonstrated in the food-intake experiment, 200 mg/kg Gordonoside F showed similar appetite-suppressing effect as 1,000 mg/kg Hoodia extract (Fig. 5). Although the exact amount of Gordonoside F within Hoodia remains unclear, it can obviously not reach 10–20% of the dry weight. Thus, the in vivo study suggested that a group of GPR119-activating compounds might exist in Hoodia extract, some of which might have higher potency for GPR119 than the Gordonoside F. The partial reduction of the biological effects of Hoodia extract by GPR119 KO also indicated the existence of mechanisms other than GPR119 activation.
Taken together, we demonstrate for the first time, to our knowledge, that the activation of GPR119 receptor is an important mechanism underlying H. gordonii’s therapeutic effect. Given the present results on homogeneous compounds and the long history of safe application of H. gordonii in body-weight control, we believe that the novel scaffold of Gordonoside F provides a promising opportunity for developing new drugs in treating metabolic diseases.
Supplementary Material
Acknowledgments
This project was supported by Ministry of Science and Technology of China Grants 2013ZX09507001, 2014CB541906, and 2012ZX09301001-005, National Natural Science Foundation of China Grant 91213301, and Shanghai Commission of Science and Technology Grants 12XD1402100 and 11ZR1408000.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1324130111/-/DCSupplemental.
References
- 1.Jain R, et al. Implications of obesity for drug therapy: Limitations and challenges. Clin Pharmacol Ther. 2011;90(1):77–89. doi: 10.1038/clpt.2011.104. [DOI] [PubMed] [Google Scholar]
- 2.Powell AG, Apovian CM, Aronne LJ. New drug targets for the treatment of obesity. Clin Pharmacol Ther. 2011;90(1):40–51. doi: 10.1038/clpt.2011.82. [DOI] [PubMed] [Google Scholar]
- 3.Vermaak I, Hamman JH, Viljoen AM. Hoodia gordonii: An up-to-date review of a commercially important anti-obesity plant. Planta Med. 2011;77(11):1149–1160. doi: 10.1055/s-0030-1250643. [DOI] [PubMed] [Google Scholar]
- 4.van Heerden FR. Hoodia gordonii: A natural appetite suppressant. J Ethnopharmacol. 2008;119(3):434–437. doi: 10.1016/j.jep.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 5.van Heerden FR, et al. An appetite suppressant from Hoodia species. Phytochemistry. 2007;68(20):2545–2553. doi: 10.1016/j.phytochem.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 6.MacLean DB, Luo LG. Increased ATP content/production in the hypothalamus may be a signal for energy-sensing of satiety: Studies of the anorectic mechanism of a plant steroidal glycoside. Brain Res. 2004;1020(1-2):1–11. doi: 10.1016/j.brainres.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 7.Madgula VL, et al. Bioavailability, pharmacokinetics, and tissue distribution of the oxypregnane steroidal glycoside P57AS3 (P57) from Hoodia gordonii in mouse model. Planta Med. 2010;76(14):1582–1586. doi: 10.1055/s-0030-1249818. [DOI] [PubMed] [Google Scholar]
- 8.Dall’Acqua S, Innocenti G. Steroidal glycosides from Hoodia gordonii. Steroids. 2007;72(6-7):559–568. doi: 10.1016/j.steroids.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Ahrén B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8(5):369–385. doi: 10.1038/nrd2782. [DOI] [PubMed] [Google Scholar]
- 10.Sakamoto Y, et al. Expression and distribution of Gpr119 in the pancreatic islets of mice and rats: Predominant localization in pancreatic polypeptide-secreting PP-cells. Biochem Biophys Res Commun. 2006;351(2):474–480. doi: 10.1016/j.bbrc.2006.10.076. [DOI] [PubMed] [Google Scholar]
- 11.Chu ZL, et al. A role for beta-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology. 2007;148(6):2601–2609. doi: 10.1210/en.2006-1608. [DOI] [PubMed] [Google Scholar]
- 12.Soga T, et al. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem Biophys Res Commun. 2005;326(4):744–751. doi: 10.1016/j.bbrc.2004.11.120. [DOI] [PubMed] [Google Scholar]
- 13.Lauffer LM, Iakoubov R, Brubaker PL. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes. 2009;58(5):1058–1066. doi: 10.2337/db08-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu ZL, et al. A role for intestinal endocrine cell-expressed g protein-coupled receptor 119 in glycemic control by enhancing glucagon-like Peptide-1 and glucose-dependent insulinotropic Peptide release. Endocrinology. 2008;149(5):2038–2047. doi: 10.1210/en.2007-0966. [DOI] [PubMed] [Google Scholar]
- 15.Overton HA, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3(3):167–175. doi: 10.1016/j.cmet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Li DS, Yuan YH, Tu HJ, Liang QL, Dai LJ. A protocol for islet isolation from mouse pancreas. Nat Protoc. 2009;4(11):1649–1652. doi: 10.1038/nprot.2009.150. [DOI] [PubMed] [Google Scholar]
- 17.Yu B, Zhang Y, Tang P. Carbohydrate chemistry in the total synthesis of saponins. Eur J Org Chem. 2007;(31):5145–5161. [Google Scholar]
- 18.Yu B, Sun J, Yang X. Assembly of naturally occurring glycosides, evolved tactics, and glycosylation methods. Acc Chem Res. 2012;45(8):1227–1236. doi: 10.1021/ar200296m. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Shi H, Ma Y, Yu B. Expeditious synthesis of saponin P57, an appetite suppressant from Hoodia plants. Chem Commun (Camb) 2012;48(69):8679–8681. doi: 10.1039/c2cc34404a. [DOI] [PubMed] [Google Scholar]
- 20.Lan H, et al. GPR119 is required for physiological regulation of glucagon-like peptide-1 secretion but not for metabolic homeostasis. J Endocrinol. 2009;201(2):219–230. doi: 10.1677/JOE-08-0453. [DOI] [PubMed] [Google Scholar]
- 21.Vermaak I, Viljoen AM, Chen W, Hamman JH. In vitro transport of the steroidal glycoside P57 from Hoodia gordonii across excised porcine intestinal and buccal tissue. Phytomedicine. 2011;18(8-9):783–787. doi: 10.1016/j.phymed.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Knight TL, et al. Cultivation practices and manufacturing processes to produce Hoodia gordonii extract for weight management products. Food Chem Toxicol. 2012;50(Suppl 1):S1–S5. doi: 10.1016/j.fct.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 23.Van Heerden FR, Vleggaar R, Horak RM, Learmonth RA, Maharaj V, Whittal RD. 2002. Pharmaceutical compositions having appetite-suppressant activity. US Patent 6376657 B1.
- 24.Lauffer L, Iakoubov R, Brubaker PL. GPR119: “Double-dipping” for better glycemic control. Endocrinology. 2008;149(5):2035–2037. doi: 10.1210/en.2008-0182. [DOI] [PubMed] [Google Scholar]
- 25.Semple G, et al. Discovery of the first potent and orally efficacious agonist of the orphan G-protein coupled receptor 119. J Med Chem. 2008;51(17):5172–5175. doi: 10.1021/jm8006867. [DOI] [PubMed] [Google Scholar]
- 26.Drucker DJ, Nauck MA. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 27.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: Systematic review and meta-analysis. JAMA. 2007;298(2):194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 28.Cornall LMMM, Mathai ML, Hryciw DH, McAinch AJ. Is GPR119 agonism an appropriate treatment modality for the safe amelioration of metabolic diseases? Expert Opin Investig Drugs. 2013;22(4):487–498. doi: 10.1517/13543784.2013.775245. [DOI] [PubMed] [Google Scholar]
- 29.Kogure R, Toyama K, Hiyamuta S, Kojima I, Takeda S. 5-Hydroxy-eicosapentaenoic acid is an endogenous GPR119 agonist and enhances glucose-dependent insulin secretion. Biochem Biophys Res Commun. 2011;416(1-2):58–63. doi: 10.1016/j.bbrc.2011.10.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.