Significance
Ecosystem functioning is more strongly affected by biodiversity loss when multiple functions are considered because different species affect different functions. To quantify these biodiversity-functioning relationships, the emerging multifunctionality framework advocates calculation of indices that aggregate responses of individual functions. Data aggregation, however, is notorious for providing misleading information by obscuring true relationships between explanatory and response variables. We test the ability of common multifunctionality indices to reveal effects on key ecosystem functions of changes in soil communities. The multifunctionality indices all decrease with soil animal loss, but the responses of individual functions diverge markedly from these aggregated metrics. Application of the multifunctionality framework for landscape provision of multiple ecosystem services should therefore emphasize understanding relationships between communities and individual functions.
Keywords: aboveground–belowground interactions, ecosystem functioning, plant-soil feedbacks, soil biodiversity, soil fauna
Abstract
Ecosystem management policies increasingly emphasize provision of multiple, as opposed to single, ecosystem services. Management for such “multifunctionality” has stimulated research into the role that biodiversity plays in providing desired rates of multiple ecosystem processes. Positive effects of biodiversity on indices of multifunctionality are consistently found, primarily because species that are redundant for one ecosystem process under a given set of environmental conditions play a distinct role under different conditions or in the provision of another ecosystem process. Here we show that the positive effects of diversity (specifically community composition) on multifunctionality indices can also arise from a statistical fallacy analogous to Simpson’s paradox (where aggregating data obscures causal relationships). We manipulated soil faunal community composition in combination with nitrogen fertilization of model grassland ecosystems and repeatedly measured five ecosystem processes related to plant productivity, carbon storage, and nutrient turnover. We calculated three common multifunctionality indices based on these processes and found that the functional complexity of the soil communities had a consistent positive effect on the indices. However, only two of the five ecosystem processes also responded positively to increasing complexity, whereas the other three responded neutrally or negatively. Furthermore, none of the individual processes responded to both the complexity and the nitrogen manipulations in a manner consistent with the indices. Our data show that multifunctionality indices can obscure relationships that exist between communities and key ecosystem processes, leading us to question their use in advancing theoretical understanding—and in management decisions—about how biodiversity is related to the provision of multiple ecosystem services.
Biodiversity contributes to the functioning of ecosystems by controlling both the rate and the variance of ecosystem processes, making understanding the consequences of biodiversity loss crucial to ecosystem management (1–3). Elucidating the likely impacts of belowground biodiversity loss is particularly important, as soil taxa play key roles in nearly every biogeochemical process that makes Earth an inhabitable planet (4, 5). However, the general relationship between soil biodiversity and ecosystem functioning remains largely unknown because positive, negative, and neutral effects of soil diversity on ecosystem processes are reported (6–10). Similarly idiosyncratic responses of individual ecosystem processes to loss of plant diversity prompted consideration of how biodiversity loss simultaneously affects multiple ecosystem processes, termed “ecosystem multifunctionality” (11). Recent studies on multifunctionality appear to suggest strong and consistent negative effects of plant diversity loss on ecosystem functioning because species that do not contribute to one ecosystem process may play an important role in a separate process and/or under different conditions (11, 12). Similar assessments for soil biodiversity are in their infancy, but appear to lend support to the idea that the study of single ecosystem processes underestimates the importance of species and functional diversity for ecosystem functioning (13–16).
Soils contain a huge diversity of cryptic organisms living in an opaque environment, complicating direct assessment of the taxonomic, phylogenetic, and functional diversity of soil taxa and, in turn, the consequences of diversity loss in soil. Body size, however, presents a trait by which to manipulate the functional complexity of soil communities that is experimentally tractable as well as relevant to community change (6, 16, 17). First, larger body size is positively associated with susceptibility to mortality from human activities such as forest conversion to cropland and soil tillage (18, 19). Second, body size strongly affects ecosystem processes (2) because (i) it correlates with metabolic rate, generation time, population density, and food size (20); (ii) the physical structure of the soil habitat constrains access to resources for certain body sizes and hence modulates interactions between organisms (21); and (iii) the relationship of fauna with microflora shifts from predation to mutualism with increasing body size (22). As such, body size is a trait expected to be strongly related to ecosystem functioning (23).
We simulated the loss of soil functional complexity by excluding larger-bodied soil fauna from model grassland ecosystems. We compared treatments with only microorganisms and microfauna (nematodes and protists; low functional complexity) to those that also contained common larger-bodied meso- and macrofauna, such as springtails, mites, and earthworms (high functional complexity treatment). Taxonomic diversity therefore likely differed little among our experimental grasslands because we did not directly manipulate the microflora, which harbors the majority of soil taxonomic richness (5). We crossed the functional complexity treatment with inorganic nitrogen fertilizer, which is typically applied to more intensively managed grasslands and so is a management that typically accompanies the loss of larger-bodied fauna. Given that a central argument to the adoption of multifunctionality approaches (24, 25) is that different species maintain functions in different environmental contexts, the nitrogen fertilizer treatment allowed us to additionally ask how the effects of the functional complexity treatments were influenced by context. We assessed the responses of five ecosystem processes related to plant productivity, decomposition, ecosystem carbon storage, and respiration.
Loss of soil taxa has divergent effects on individual ecosystem processes, often within the same experimental system (10). Some of these idiosyncrasies may arise because direct effects of the loss of taxa are counterbalanced through indirect effects of soil organisms on plant community composition and diversity (6, 7, 14). Earlier work, for example, in the experimental systems studied here showed that reductions in soil functional complexity decreased litter decomposition, but increased legume abundances (6). The N2-fixing ability of legumes might then have compensated for reductions in nitrogen resupply via organic matter breakdown and so sustained similar plant productivity rates in the low- and high-complexity treatments. We therefore hypothesized that two phenomena might diminish the usefulness of multifunctionality indices for understanding soil community relationships with ecosystem functioning: (i) contrasting responses of individual processes to functional complexity are common and arise through a combination of direct and indirect (via the plant community) effects of soil organisms (6, 14); and (ii) the combination of direct and indirect effects alters the soil environment, and hence relationships between complexity and ecosystem processes shift as the context changes in space and time (24, 25). For example, differences in legume abundance between low- and high-complexity treatments might mean that nitrogen fertilization stimulates plant productivity only where legume abundances are low. Such phenomena would mean that amalgamating individual process data to yield aggregated multifunctionality indices could alter, and potentially even reverse, our inferences about causal relationships between community change and ecosystem functioning (26).
Results and Discussion
Multifunctionality.
We first examined the relationship between soil functional complexity and ecosystem multifunctionality. We measured rates of ecosystem processes that are either directly or indirectly mediated by soil biota through their effects on nutrient cycling and turnover of organic matter. The processes that we measured were net primary productivity (NPP), net ecosystem productivity (NEP), ecosystem respiration, and litter decomposition. Our approach reflects that of recent research into multifunctionality where investigators focus on the rates of a defined set of biogeochemical processes that together influence the quality of services that an ecosystem provides (27). We calculated multifunctionality using three distinct methods (28), given that a standard approach has not yet been adopted and all of the proposed metrics have pros and cons (28). Regardless of the approach used, we find that the loss of functional complexity in soil significantly decreases ecosystem multifunctionality (Fig. 1 and Table 1).
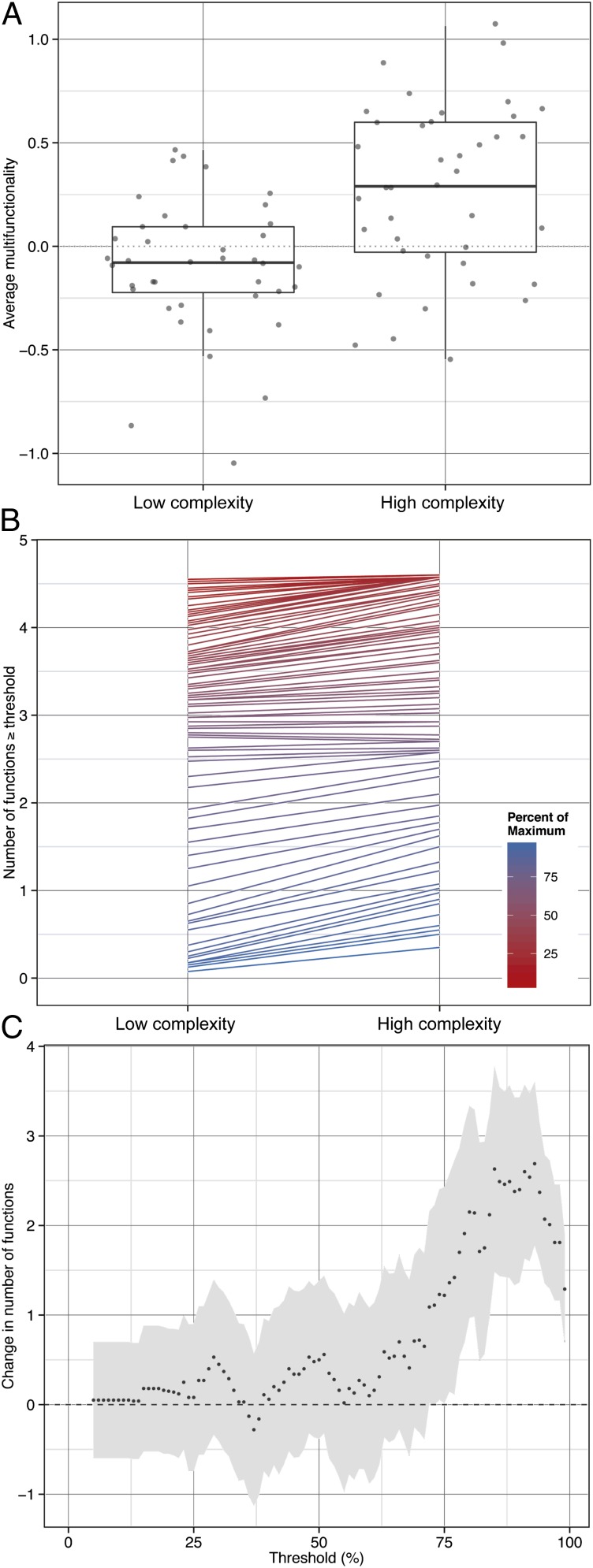
Fig. 1.
Loss of soil community functional complexity decreases multifunctionality when measured as (A) average multifunctionality or (B) the number of functions exceeding threshold levels of maximum process rates. Data points in A are jittered to visualize vertical spread, and represent average multifunctionality of each replicate at each of the four time points at which measures were taken. The horizontal line shows the median, the box the 25th and 75th percentiles of the data, and the extent of the whiskers 1.5 times the interquartile range. (B) Lines represent the slope between soil fauna loss and the number of functions greater than or equal to a threshold value. Separate statistical models were fit at each threshold ranging from 5 to 99% of maximum functioning, with blue lines indicating high percentages of maximum functioning and red lines indicating low percentages of maximum functioning. The curve in C indicates the change in the slope of the relationship described in B across all thresholds with the gray area showing SE. When the error does not cross the zero line (starting at 72%), the loss of soil community functional complexity is associated with a statistically significant decrease in ecosystem multifunctionality.
Table 1.
Soil community functional complexity and nitrogen fertilization effects on three different estimates of ecosystem multifunctionality and the five underlying ecosystem processes
| Multifunctionality | Single functions | |||||||
| Factor | Average multifunctionality | Functioning ≥80% threshold | Functioning ≥20% threshold | NPP | NEP | Respiration | Standard litter decomposition | Returned litter decomposition |
| Complexity | 0.37*** | 0.40* | 0.15 | 42.50*** | 0.35 | −0.29*** | 0.08*** | 0.01 |
| (0.18, 0.57) | (−0.02, 0.83) | (−0.13, 0.42) | (28.97, 55.36) | (−0.25, 0.97) | (−0.42, −0.15) | (0.05, 0.11) | (−0.02, 0.05) | |
| Nitrogen (N) | −0.11 | −0.37† | −0.25* | 13.03* | −1.10*** | 0.13* | −0.02 | −0.02 |
| (−0.27, 0.05) | (−0.75, 0.01) | (−0.45, −0.04) | (1.71, 24.41) | (−1.63, −0.55) | (0.00, 0.24) | (−0.05, 0.01) | (−0.05, 0.01) | |
| Moisture | 0.26** | 0.57** | −0.25* | 30.43*** | −0.89** | 0.37*** | 0.00 | 0.01 |
| (0.09, 0.41) | (0.18, 0.96) | (−0.45, −0.05) | (19.52, 42.07) | (−1.44, −0.35) | (0.26, 0.49) | (−0.02, 0.03) | (−0.02, 0.04) | |
| Time | −0.03 | -0.33† | 0.25* | −6.76 | 1.24*** | −0.20** | −0.02 | −0.01 |
| (−0.18, 0.13) | (−0.70, 0.07) | (0.05, 0.45) | (−17.89 4.47) | (0.70, 1.78) | (−0.31, −0.08) | (−0.04, 0.01) | (−0.04, 0.02) | |
| Complexity: N | 0.14 | 0.50 | −0.50* | 21.63† | −0.72 | 0.24* | −0.00 | 0.00 |
| (−0.18, 0.43) | (−0.24, 1.27) | (−0.90, 0.10) | (−0.43, 43.58) | (−1.76, 0.38) | (0.00, 0.46) | (−0.5, 0.05) | (−0.06, 0.06) | |
| Intercept | 0.08† | 1.90*** | 4.50*** | 87.58*** | 0.11 | 1.71*** | 0.75*** | 0.67*** |
| (−0.01, 0.18) | (1.68, 2.10) | (4.36, 4.64) | (81.04, 94.10) | (−0.20, 0.42) | (1.64, 1.77) | (0.73, 0.76) | (0.66, 0.69) | |
Values are coefficients with statistical significance from the linear models with the lower and upper bounds shown in parenetheses. Coefficients are centered to permit comparison of community and fertilization main effects that are also part of a significant community × nitrogen interaction. Moisture and time are included in the analysis only to ensure that they do not contribute to the community complexity and fertilization effects (Materials and Methods). †P < 0.1, *P < 0.05, **P < 0.01, ***P < 0.001.
The reduction in multifunctionality with the loss of functional complexity is shown most clearly by the averaging approach (15), where the mean index of average function is approximately five times less in the low- vs. high-complexity treatment (Table 1 and Fig. 1A). The effects of functional complexity loss on average multifunctionality were not influenced by nitrogen fertilization (Table 1). It is possible, however, that the averaging approach might give multifunctionality values that are driven by the response of a single ecosystem process (28). A threshold approach overcomes this limitation by scoring how many functions exceed a specified threshold of functioning (12). We explored two thresholds representing high and low functioning—80 and 20% of maximum observed functioning—and found that the soil community manipulations affected multifunctionality significantly only at higher levels of functioning (i.e., the 80% threshold; Table 1). That is, loss of functional complexity impaired multifunctionality only when higher threshold levels of functioning were considered.
One limitation of the single-threshold approach is that it does not necessarily quantify the extent to which functioning is affected, and so a multiple threshold approach has been proposed (28). We used this approach to simultaneously assess the relationship between functional complexity and multifunctionality for all thresholds between 5 and 99%. The effect (i.e., slope) of the loss of functional complexity increased with the threshold value, confirming that the consequences of the soil community manipulations were significant only when considering high levels of functioning (Fig. 1 B and C). Specifically, loss of larger-bodied soil fauna began to significantly affect multifunctionality at a threshold value of 72%, peaked at 93%, and remained significantly lower in the low-complexity treatment at the 99% threshold value (Fig. 1C). Thus, loss of functional complexity from the soil communities reduced multifunctionality to about three-fourths of that observed in the high-complexity treatment (i.e., given that effects were evident at thresholds ≥72%).
Individual Functions.
We hypothesized that contrasting responses of individual processes to loss of functional complexity, mediated in part by indirect effects of functional complexity on environmental context, would obscure true relationships between community composition and functioning when aggregated metrics of ecosystem multifunctionality were determined. Despite consistent effects of the functional complexity treatments on the three multifunctionality indices that we calculated (Fig. 1 and Table 1), only two of the individual processes showed significant positive relationships with functional complexity (Table 1). Furthermore, the magnitude and/or direction of the community effect typically strongly contrasted between mutlifunctionality and all of the individual processes (Fig. 2). We note that relationships between consumer and decomposer diversity on ecosystem multifunctionality, in both aquatic and terrestrial systems, were also consistently positive, but that the underlying process responses were not (16, 27). Our data, therefore, show that whereas functional community complexity has a positive effect on multifunctionality indices, the act of aggregating data to estimate these indices can reverse the direction and/or alter the magnitude of the relationship between community composition and individual process rates (as shown in Fig. 2). As such, multifunctionality indices may obscure insights into the mechanistic relationships required to understand and manage the influence of community change on ecosystem service provision.
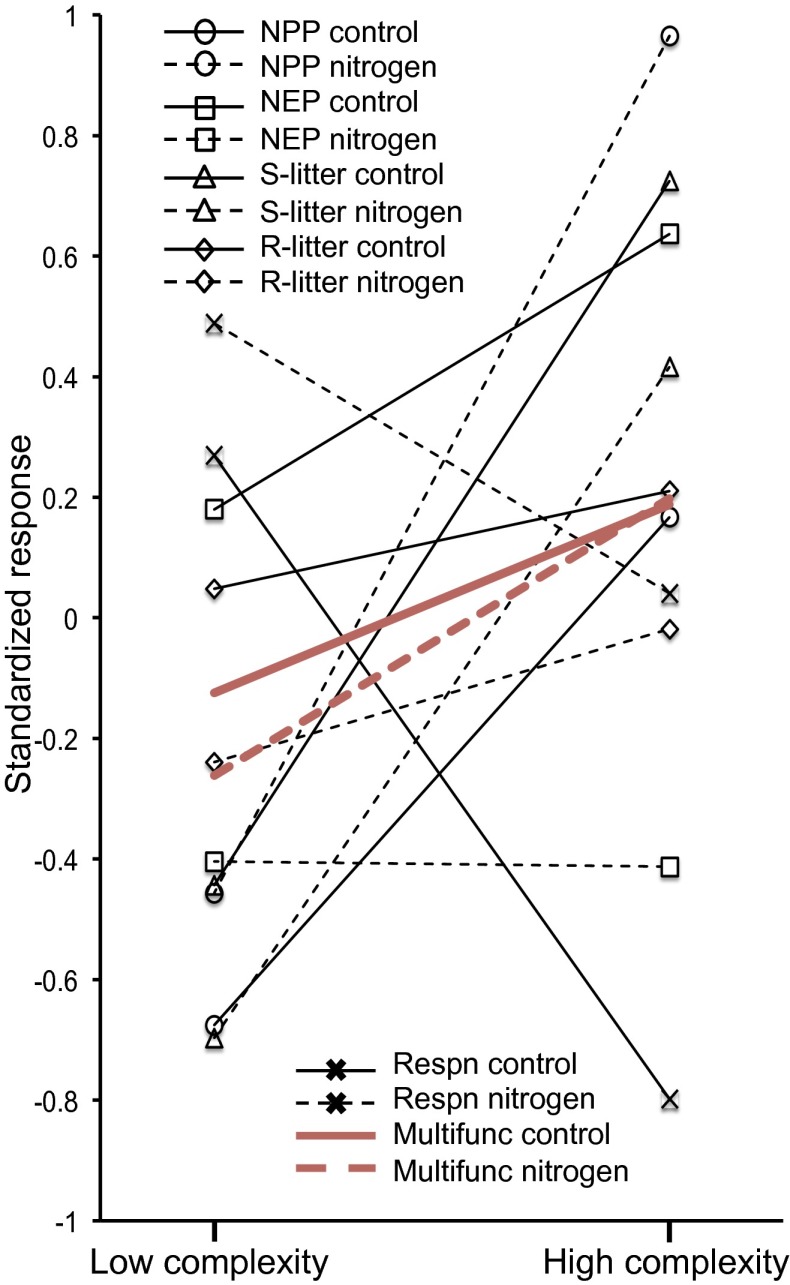
Fig. 2.
Loss of soil community functional complexity and nitrogen fertilization effects on the rates of five biogeochemical processes, compared with the response of average multifunctionality (Multifunc). The biogeochemical processes are net primary productivity (NPP); net ecosystem productivity (NEP); decomposition of a standard litter (S-litter); decomposition of litter returned within each replicate community (R-litter); and total community respiration (Respn). Each symbol represents the mean response to the complexity by fertilization treatments, and lines connect low- and high-complexity plots within control or nitrogen-fertilized subplots to facilitate the visualization of community effects. Values are standardized using a z-score transformation to permit comparison with the response of average multifunctionality and between processes with different units and absolute magnitudes. The statistical significance and coefficients of the complexity by fertilization treatments are given in Table 1 and the full process data in Fig. S1. Only standard litter decomposition responds in a qualitative manner (i.e., there is a positive complexity effect and no nitrogen or interaction effect) that is consistent with multifunctionality, but the absolute magnitude of the decomposition response to the community manipulation is much greater than for multifunctionality. The discontinuity between the individual process and multifunctionality responses raises questions about the practical and mechanistic interpretation of multifunctionality indices.
Net primary production strongly decreased with loss of functional complexity and increased with nitrogen fertilization (Fig. 2 and Fig. S1). Increased NPP with fertilization is to be anticipated, but the positive effect of larger-bodied fauna on NPP was surprising. Macro- and mesofauna do commonly accelerate processes such as litter decomposition and nutrient cycling (17, 29, 30). However, these effects do not seem to propagate to higher-order processes such as NPP and ecosystem respiration in speciose plant communities, leading to the expectation that these processes are robust to the loss of larger-bodied fauna (6, 16, 31). Our experiment differs, however, from previous work in the complexity of the systems combined with the long timescale of the experiment. For example, our experimental microcosms took 7 mo to construct, as soil horizons were reconstructed, hundreds of individuals of 10 co-occurring plant species were planted, and soil microflora and fauna were systematically added over many events. The community treatments were then allowed 225 d to develop before the fertilizer treatment was applied and data were collected over a further 280 d (Materials and Methods). There was therefore likely enough time and ecological complexity within our experimental grasslands for feedbacks between belowground and plant community composition to develop and translate to alterations in an important ecosystem process (14). These aboveground–belowground interactions influenced all of the ecosystem processes and modified their response to fertilization; we discuss these interactions in turn below.
The marginally significant complexity by fertilization interaction effect on NPP arose because NPP responded strongly only to fertilization in the high-complexity treatment (Fig. 2 and Fig. S1), suggesting stronger plant nitrogen limitation in the absence of larger-bodied fauna. In support of this inference, legume biomass was >30% of NPP in the low-complexity treatment and <1% in the high-complexity treatment (Table S1). In addition, plant-available soil nitrogen was lower on average under high complexity (although this difference was not statistically significant) and was significantly increased by fertilization (Table S1). Furthermore, greater nitrogen limitation is consistent with the fact that the grass biomass in the high-complexity treatment had significantly lower foliar nitrogen contents than in the low-complexity treatment and responded most strongly (although not significantly) to fertilization (Table S1). Our results suggest that, from a management perspective, loss of soil functional complexity might not translate to decreased grassland performance because losses in aboveground yields trade off with increased forage quality (i.e., changes in the plant community through higher grass nitrogen content and higher legume abundance).
The low abundance of legumes and lower nitrogen content of grasses in the high-complexity treatment highlights the ability of belowground community composition to influence aboveground community composition and stoichiometry (7). Although it can be difficult to interpret such single “snapshot” metrics, our process data also support the importance of belowground–aboveground interactions in shaping system functioning. Specifically, the presence of larger-bodied fauna increased, as expected (29, 32, 33), decomposition rates of a standard litter substrate (Table 1, Fig. 2, and Fig. S1). However, the decomposition of the aboveground litter returned to the same experimental replicate was unaffected by the community treatments (Table 1, Fig. 2, and Fig. S1). Presumably, the lower foliage litter quality in the high-functional-complexity treatment, due to lower legume abundances and low grass nitrogen contents, retarded decomposition. Such findings highlight the importance of decomposer community composition as an arbiter of how changes in plant communities affect ecosystem processes (14).
Respiration rates were measured at the level of the whole community and so include both autotrophic and heterotrophic ecosystem respiration. Given the greater plant biomass in the high-complexity treatment, both aboveground (i.e., NPP) and belowground (i.e., roots) (Table S1), it is surprising that this greater biomass did not translate to greater respiration rates. In fact, respiration was ∼25% lower in the high-complexity treatment (Fig. 2 and Fig. S1). We therefore investigated whether the dominant source of heterotrophic respiration (i.e., soil microbes) was suppressed by the presence of larger-bodied fauna. Soil microbial biomass, however, was unaffected by the complexity and fertilization treatments being 3.6 ± 0.29 mg microbial C g soil−1 (mean ± SE) across all replicates. We did not measure other properties of the microbial community, such as its structure or physiology, which might have helped explain the respiration responses (34). However, a plausible explanation for the higher respiration in the low-complexity treatment and the stronger fertilization effect on respiration in the high-complexity treatment (explaining the complexity × fertilization interaction; Table 1), is that the responses are plant-mediated. Specifically, N2 fixation by legumes is energetically demanding (35), and nitrogen contents of foliage correlate with the abundance of the photosynthetic enzyme, rubisco, which incurs a high respiratory cost for maintenance (36, 37). Therefore, we suggest that the low abundance of legumes and the lower nitrogen contents in the grasses in the high-complexity treatment reduced autotrophic respiration, which was then stimulated by nitrogen fertilization because it increased NPP and/or grass nitrogen content, despite reducing root biomass (Table S1 and Fig. 2).
Increases in aboveground NPP under the high-complexity and nitrogen fertilization treatments did not translate to greater net carbon capture (i.e., NEP) because carbon gains through higher plant productivity were generally offset by increased respiratory losses (Fig. 2 and Fig. S1). Indeed, the respiration response to the treatments was always opposite to the NEP response (compare the sign of the coefficients in Table 1). These opposite responses are best represented by the effects of nitrogen fertilization, which increased aboveground NPP but also stimulated respiration, resulting in a significant and negative effect on NEP (Table S1, Fig. 2, and Fig. S1). Such dynamics represent how interconnected individual biogeochemical processes are in an ecosystem context, resulting in both positive and negative relationships that could aggregate to a common diversity–multifunctionality relationship with different underlying causes.
Implications for Management.
The concept of ecosystem multifunctionality is being used to guide management of systems to provide multiple services (38–40), with biodiversity recommended as an ecosystem property that can be managed to increase or sustain multifunctionality (15). Our data show that three different multifunctionality metrics—all applied recently to investigate the consequences of diversity loss from various communities, including plants, soil biota, and salt marsh consumers (16, 27, 28)—show a consistent negative response to loss of soil functional complexity. These consistent responses seem to suggest that multifunctionality indices could help to provide a quantitative basis for improving ecosystem service provision in relation to managing communities. The mismatch between our community and fertilization effects on multifunctionality and the individual processes, however, cautions against using the framework as a predictive tool for achieving desired levels of functioning for multiple, specified ecosystem services. For the framework to be effective as a predictive tool requires that desired individual processes respond to community change in a positive, correlated fashion. In contrast, our data reveal that manipulations of belowground communities can elicit responses of individual processes that are widely divergent and that these divergent responses seem to arise through belowground effects on aboveground communities that feed back to affect ecosystem functioning.
Materials and Methods
Experimental Setup and Design.
Ten terrestrial microcosms were established across 7 mo and then maintained in the Ecotron-controlled environment facility (41) for 505 d. The work presented here represents research conducted between experiment day 225 and 505 (280 d total), and research earlier in the experiment showed the potential for the functional complexity manipulations to affect plant community structure and carbon cycle dynamics (6, 29, 42). Each microcosm was 1 m2 and housed within a 2- × 2- × 2-m walk-in chamber. Full details of the construction and conditions of the microcosms are provided in Bradford et al. (6). Briefly, the microcosms were created from upland, acid grassland at Sourhope Experimental Farm (UK National Grid Reference NT855196) in Scotland. Seeds of the 10 most dominant plants at Sourhope were collected, greenhouse-germinated, and introduced into the microcosms as seedlings (384 individuals per community). They included six grasses, two forbs, one Juncus sp., and one legume. All species established and persisted. The soil profile was reconstructed and included five horizons: FH (plant litter-humified organic matter mix; 3.5 cm deep), H (humified organic matter; 2.0 cm), Ah “upper” (surface mineral soil with humified organic matter accumulation; 6.9 cm), Ah “lower” (surface mineral with less humified organic matter accumulation; 11.4 cm), and AB (surface-subsurface mineral soil; 6.5 cm). Photoperiod was 18 h, including a gradual dawn and dusk of 2 h. Temperature and relative humidity followed sine curves between 21.1 °C during the day and 9.5 °C at night and 83% after rainfall (3.5 mm day−1) and a minimum of 63%, respectively.
Functional complexity was manipulated through introductions of soil organisms after the soil was first treated with CH3Br to remove meso- and macrofauna. Microorganisms and fauna were then introduced selectively to create belowground communities that differed in organism body size. We adhered to the general size classification for terrestrial decomposer food webs (32), establishing a “biodiversity loss” treatment containing microbiota only (<100-μm-diameter body size; primarily bacteria, fungi, Protozoa, and Nematoda) and compared performance of these grassland ecosystems to those with soil communities containing microbiota, as well as mesofauna (100-μm to 2-mm diameter; primarily Collembola, Acari, and Enchytraeidae) and macrofauna (>2-mm diameter; primarily earthworms, slugs, insect larvae, and staphylinid beetles). All taxa survived and established at field densities (6).
Treatments were randomly assigned within five blocks, creating five replicates of each community treatment. For the work presented here, a nitrogen-fertilization treatment was crossed with the community manipulations. Specifically, at experiment day 225, each microcosm was divided in half and nitrogen was added on day 281 (see below) to one side in the form of NH4NO3 at a rate equivalent to 240 kg⋅N ha−1⋅y−1. This addition rate mimics that typically applied as fertilizer to upland temperate grassland, and our experimental additions were made across 2 d to minimize leaching. Due to a mechanical error with the precipitation simulators, one side of each microcosm received more precipitation than the other side over the course of the experiment. This error created a drier side and a wetter side. In our two community manipulations, three of the replicates had nitrogen applied to the drier side and two replicates had it applied to the wetter side, meaning that we could include soil moisture (dry vs. wet) in our statistical models to account for differences in soil moisture from uneven precipitation.
Measurement of Individual Ecosystem Functions.
All ecosystem processes were measured five times (once before and four times after fertilization). Each process was measured within the 56-d cutting cycle. Specifically, plant biomass was cut to 9 cm every 56 d to simulate periodic, unselective grazing. After the first 56-d period (biomass cut on experiment day 281), nitrogen was applied, and then for the next four cuts (experiment days 337, 393, 449, and 505) total oven-dried (80 °C) biomass was used to estimate NPP, and subsamples were sorted to functional group [legumes, other forbs and grasses, with the Juncus (Juncaceae) species grouped with the latter] to estimate community composition and for percentage nitrogen determinations of the plant tissue. We report the percentage of the cut biomass that legumes made up (Table S1). The remainder was primarily grasses because the cut biomass of nonleguminous forbs was negligible (≤0.2 ± 0.3% mean ± SE of NPP).
NEP, a measure of ecosystem carbon exchange, was measured as the sum of day and night CO2 fluxes. Measures were taken using a community cover box (0.5 m2) connected to an infrared gas analyzer (6). Day rates were determined from difference in CO2 concentration between cover-box input and output air. Night rates were determined from the linear buildup of CO2 in recirculating air over time, which was also used as the estimate of ecosystem respiration rates. Decomposition rates of the standard litter type and the community-returned biomass (cut above 9 cm) were measured using the litterbag approach (29). Standard litterbags contained greenhouse-grown Agrostis capillaris, providing grass litter of uniform quality, whereas litter returned from the previous biomass cut varied in quality owing to the effects of the community and fertilization treatments. Use of these two litter types enabled quantification of the direct effect of functional complexity on litter decomposition and also the combined impacts of the direct effects and any indirect effect mediated through changes in plant community litter quality. Litterbags were removed after 28 d, and decomposition rate was estimated as the percentage of mass loss compared with the initial, dry litter mass.
At the end of the experiment (day 505), we measured a suite of additional supporting variables. Root density was estimated from the dry mass of roots that were physically extracted from a known volume of soil for both the organic (FH + H) and the surface mineral (Ah upper) horizons. Along with plant-available nitrogen, estimated via 2-M KCl extractions (43) for the FH + H + Ah upper soil, there were treatment effects on these variables (Table S1). Other supporting variables were not affected by the community or fertilization treatments, including the KCl-extractable dissolved organic N and C (mean ± SE: 72 ± 2.7, 491 ± 27.5 μg N and C g soil−1, respectively); the pH of the FH + H and Ah upper horizons (4.69 + 0.05 in water for both); the chloroform-fumigation extractable microbial biomass C (43); and the total percentage C and N for the Ah upper soil: 5.98 ± 0.17% and 0.56 ± 0.02%, respectively.
Data Analysis.
We constructed three metrics of multifunctionality: average, single-threshold, and multiple-threshold multifunctionality (28) for the five biogeochemical processes that we evaluated (NPP, NEP, respiration, standard, and returned litter decomposition). The threshold metrics differ only in the number of thresholds examined, and the strengths and weaknesses of all multifunctionality approaches are discussed in Byrnes et al. (28). We used the three approaches that could be assessed using our experimental design [the species turnover approach sensu Hector and Bagchi (11) cannot be assessed with our design] to permit comparison with previous work and because each metric provides unique information (28).
Average multifunctionality determines the average level of a suite of functions by standardizing each function to a common scale and taking their mean (15, 44–47). Inclusion of strongly positively correlated (i.e., r > 0.5) individual processes is not recommended (46) in the calculation of multifunctionality indices, but our strongest correlation was 0.37 between the two litter-decomposition processes. We standardized the processes using a z-score transformation, which has advantages over other standardization procedures for the linear model-based statistics that we use (15). Specifically, a standardized function is subtracted by its mean and divided by its SD. The second index, the single-threshold approach, determines if multiple functions are simultaneously maintained above certain levels, determined as whether they exceed a specified threshold percentage of maximum functioning (12, 47, 48). This approach calculates the maximum value of each process across all observations and counts the number of functions—at each observational unit—that exceed a threshold established by the researcher. To define maximum functioning, we took the mean of the five highest values for each function across all treatment types. We defined single-thresholds of 20 and 80%, representing stronger and weaker community effects on functioning, respectively. The multiple-thresholds approach does not require the investigator to choose a threshold value and instead investigates a continuous gradient of thresholds (28). Specifically, we calculated in 1% increments the values for 5–99% of maximum functioning, in addition to the number of functions per plot exceeding each threshold level. Threshold-based analysis was performed using the multifunc package in R (28). Further information on these metrics, as well as the linear mixed models used to assess relationships between the treatments and multifunctionality or single functions, are included in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dan Flynn, Jesse Lasky, and Shahid Naeem for comments on an early version of the manuscript; Maria Uriarte for suggestions regarding statistical methods; and George Tordoff, John Newington, and Kerry Hutcheson for technical assistance. This research was funded by the Natural Environment Research Council (United Kingdom) under their Soil Biodiversity Thematic Programme.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.S. is a Guest Editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1413707111/-/DCSupplemental.
References
- 1.Cardinale BJ, et al. The functional role of producer diversity in ecosystems. Am J Bot. 2011;98(3):572–592. doi: 10.3732/ajb.1000364. [DOI] [PubMed] [Google Scholar]
- 2.Hooper DU, et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol Monogr. 2005;75(1):3–35. [Google Scholar]
- 3.Naeem S, Duffy JE, Zavaleta E. The functions of biological diversity in an age of extinction. Science. 2012;336(6087):1401–1406. doi: 10.1126/science.1215855. [DOI] [PubMed] [Google Scholar]
- 4.Falkowski PG, Fenchel T, Delong EF. The microbial engines that drive Earth’s biogeochemical cycles. Science. 2008;320(5879):1034–1039. doi: 10.1126/science.1153213. [DOI] [PubMed] [Google Scholar]
- 5.Wall DH, et al., editors. Soil Ecology and Ecosystem Services. Oxford: Oxford Univ Press; 2012. [Google Scholar]
- 6.Bradford MA, et al. Impacts of soil faunal community composition on model grassland ecosystems. Science. 2002;298(5593):615–618. doi: 10.1126/science.1075805. [DOI] [PubMed] [Google Scholar]
- 7.De Deyn GB, et al. Soil invertebrate fauna enhances grassland succession and diversity. Nature. 2003;422(6933):711–713. doi: 10.1038/nature01548. [DOI] [PubMed] [Google Scholar]
- 8.Setälä H, Marshall VG, Trofymow JA. Influence of body size of soil fauna on litter decomposition and 15N uptake by poplar in a pot trial. Soil Biol Biochem. 1996;28(12):1661–1675. [Google Scholar]
- 9.Heemsbergen DA, et al. Biodiversity effects on soil processes explained by interspecific functional dissimilarity. Science. 2004;306(5698):1019–1020. doi: 10.1126/science.1101865. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen UN, Ayres E, Wall DH, Bardgett RD. Soil biodiversity and carbon cycling: A review and synthesis of studies examining diversity-function relationships. Eur J Soil Sci. 2011;62:105–116. [Google Scholar]
- 11.Hector A, Bagchi R. Biodiversity and ecosystem multifunctionality. Nature. 2007;448(7150):188–190. doi: 10.1038/nature05947. [DOI] [PubMed] [Google Scholar]
- 12.Gamfeldt L, Hillebrand H, Jonsson PR. Multiple functions increase the importance of biodiversity for overall ecosystem functioning. Ecology. 2008;89(5):1223–1231. doi: 10.1890/06-2091.1. [DOI] [PubMed] [Google Scholar]
- 13.Bowker MA, Maestre FT, Mau RL. Diversity and patch-size distributions of biological soil crusts regulate dryland ecosystem multifunctionality. Ecosystems. 2013;16:923–933. [Google Scholar]
- 14.Eisenhauer N, Reich PB, Isbell F. Decomposer diversity and identity influence plant diversity effects on ecosystem functioning. Ecology. 2012;93(10):2227–2240. doi: 10.1890/11-2266.1. [DOI] [PubMed] [Google Scholar]
- 15.Maestre FT, et al. Plant species richness and ecosystem multifunctionality in global drylands. Science. 2012;335(6065):214–218. doi: 10.1126/science.1215442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagg C, Bender SF, Widmer F, van der Heijden MGA. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc Natl Acad Sci USA. 2014;111(14):5266–5270. doi: 10.1073/pnas.1320054111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handa IT, et al. Consequences of biodiversity loss for litter decomposition across biomes. Nature. 2014;509(7499):218–221. doi: 10.1038/nature13247. [DOI] [PubMed] [Google Scholar]
- 18.Wardle DA. Communities and Ecosystems. Princeton: Princeton Univ Press; 2002. [Google Scholar]
- 19.de Vries FT, et al. Soil food web properties explain ecosystem services across European land use systems. Proc Natl Acad Sci USA. 2013;110(35):14296–14301. doi: 10.1073/pnas.1305198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters RH. The Ecological Implications of Body Size. Cambridge, UK: Cambridge Univ Press; 1983. [Google Scholar]
- 21.Brussaard L, et al. Biodiversity and ecosystem functioning in soil. Ambio. 1997;26(8):563–570. doi: 10.1007/s13280-021-01507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavelle P. Faunal activities and soil processes: Adaptive strategies that determine ecosystem function. Adv Ecol Res. 1997;27:93–132. [Google Scholar]
- 23.Naeem S, Wright JP. Disentangling biodiversity effects on ecosystem functioning: Deriving solutions to a seemingly insurmountable problem. Ecol Lett. 2003;6(6):567–579. [Google Scholar]
- 24.Isbell F, et al. High plant diversity is needed to maintain ecosystem services. Nature. 2011;477(7363):199–202. doi: 10.1038/nature10282. [DOI] [PubMed] [Google Scholar]
- 25.Hillebrand H, Matthiessen B. Biodiversity in a complex world: Consolidation and progress in functional biodiversity research. Ecol Lett. 2009;12(12):1405–1419. doi: 10.1111/j.1461-0248.2009.01388.x. [DOI] [PubMed] [Google Scholar]
- 26.Gelman A, Shor B, Bafumi J, Park D. Rich state, poor state, red state, blue state: What’s the matter with Connecticut? Quart J Poli Sci. 2007;2:345–367. [Google Scholar]
- 27.Hensel MJS, Silliman BR. Consumer diversity across kingdoms supports multiple functions in a coastal ecosystem. Proc Natl Acad Sci USA. 2013;110(51):20621–20626. doi: 10.1073/pnas.1312317110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byrnes JEK, et al. Investigating the relationship between biodiversity and ecosystem multifunctionality: Challenges and solutions. Methods Ecol Evol. 2014;5(2):111–124. [Google Scholar]
- 29.Bradford MA, Tordoff GM, Eggers T, Jones TH, Newington JE. Microbiota, fauna, and mesh size interactions in litter decomposition. Oikos. 2002;99(2):317–323. [Google Scholar]
- 30.García-Palacios P, Maestre FT, Kattge J, Wall DH. Climate and litter quality differently modulate the effects of soil fauna on litter decomposition across biomes. Ecol Lett. 2013;16(8):1045–1053. doi: 10.1111/ele.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunt HW, Wall DH. Modelling the effects of loss of soil biodiversity on ecosystem function. Glob Change Biol. 2002;8(1):33–50. [Google Scholar]
- 32.Swift MJ, Heal OW, Anderson JM. Decomposition in Terrestrial Ecosystems. Studies in Ecology. Vol 5 Oxford: Blackwell Scientific; 1979. [Google Scholar]
- 33.Wall DH, et al. Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Glob Change Biol. 2008;14(11):2661–2677. [Google Scholar]
- 34.Allison SD, Wallenstein MD, Bradford MA. Soil-carbon response to warming dependent on microbial physiology. Nat Geosci. 2010;3:336–340. [Google Scholar]
- 35.Vance CP, Heichel GH. Carbon in N2 fixation: Limitation or exquisite adaptation. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:373–392. [Google Scholar]
- 36.Shipley B, Lechowicz MJ, Wright I, Reich PB. Fundamental trade-offs generating the worldwide leaf economics spectrum. Ecology. 2006;87(3):535–541. doi: 10.1890/05-1051. [DOI] [PubMed] [Google Scholar]
- 37.Tjoelker MG, Oleksyn J, Lorenc-Plucinska G, Reich PB. Acclimation of respiratory temperature responses in northern and southern populations of Pinus banksiana. New Phytol. 2009;181(1):218–229. doi: 10.1111/j.1469-8137.2008.02624.x. [DOI] [PubMed] [Google Scholar]
- 38.Milder JC, Hart AK, Dobie P, Minai J, Zaleski C. Integrated landscape initiatives for African agriculture, development, and conservation: A region-wide assessment. World Dev. 2014;54:68–80. [Google Scholar]
- 39.Lovell ST, Taylor JR. Supplying urban ecosystem services through multifunctional green infrastructure in the United States. Landscape Ecol. 2013;28(8):1447–1463. [Google Scholar]
- 40.Schindler S, et al. Multifunctionality of floodplain landscapes: Relating management options to ecosystem services. Landscape Ecol. 2014;29(2):229–244. [Google Scholar]
- 41.Lawton JH. The Ecotron facility at Silwood Park: The value of “big bottle” experiments. Ecology. 1996;77(3):665–669. [Google Scholar]
- 42.Bradford MA, et al. Carbon dynamics in a model grassland with functionally different soil communities. Funct Ecol. 2007;21(4):690–697. [Google Scholar]
- 43.Bradford MA, Fierer N, Reynolds JF. Soil carbon stocks in experimental mesocosms are dependent on the rate of labile carbon, nitrogen and phosphorus inputs to soils. Funct Ecol. 2008;22(6):964–974. [Google Scholar]
- 44.Hooper DU, Vitousek PM. Effects of plant composition and diversity on nutrient cycling. Ecol Monogr. 1998;68(1):121–149. [Google Scholar]
- 45.Maestre FT, Castillo-Monroy AP, Bowker MA, Ochoa-Hueso R. Species richness effects on ecosystem multifunctionality depend on evenness, composition and spatial pattern. J Ecol. 2012;100(2):317–330. [Google Scholar]
- 46.Mouillot D, Villéger S, Scherer-Lorenzen M, Mason NWH. Functional structure of biological communities predicts ecosystem multifunctionality. PLoS ONE. 2011;6(3):e17476. doi: 10.1371/journal.pone.0017476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pasari JR, Levi T, Zavaleta ES, Tilman D. Several scales of biodiversity affect ecosystem multifunctionality. Proc Natl Acad Sci USA. 2013;110(25):10219–10222. doi: 10.1073/pnas.1220333110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zavaleta ES, Pasari JR, Hulvey KB, Tilman GD. Sustaining multiple ecosystem functions in grassland communities requires higher biodiversity. Proc Natl Acad Sci USA. 2010;107(4):1443–1446. doi: 10.1073/pnas.0906829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.