Abstract
An efficient route towards biologically relevant pentose derivatives is described. The de novo synthetic strategy features an enantioselective α-oxidation reaction enabled by a chiral amine in conjunction with copper(II) catalysis. A subsequent Mukaiyama aldol coupling allows for the incorporation of a wide array of modular two-carbon fragments. Lactone intermediates accessed via this route provide a useful platform for elaboration, as demonstrated by the preparation of a variety of C-nucleosides and fluorinated pentoses. Finally, this work has facilitated expedient syntheses of pharmaceutically active compounds currently in clinical use.
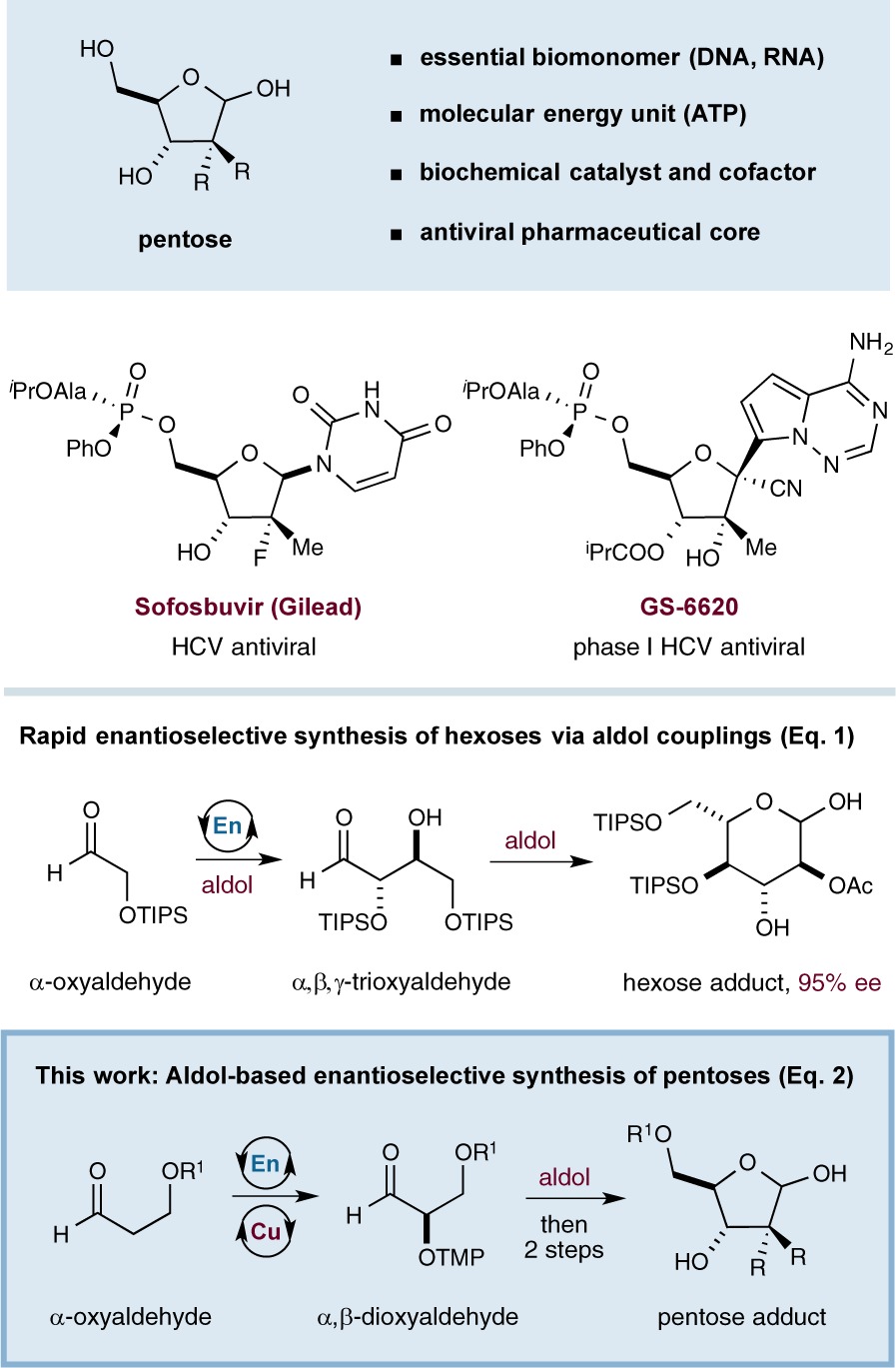
Carbohydrates represent compounds of both vast abundance and fundamental biological importance. Within this class, five-carbon saccharides (pentoses) are perhaps most readily recognized as the structural monomers composing the backbones of DNA and RNA, which enable the replication, transcription, and translation of genetic information. In addition, the ribose derivative adenosine triphosphate (ATP) represents the molecular unit of energy, while a variety of other elaborated pentoses serve as cofactors crucial to enzyme function.1 It is not surprising, therefore, that nucleoside frameworks are found at the core of many pharmaceutically active compounds and that significant research effort has been expended to gain synthetic access to non-natural pentose analogs.2 The most common strategy to build enantioenriched nucleosides is to employ natural sugars as starting materials;3 however, these protocols are typically protracted by the need to discriminate among four chemically similar hydroxyl groups, which further limits opportunities for the incorporation of unnatural moieties and stereochemical information. Clearly, an attractive alternative would involve a de novo synthetic sequence that rapidly and enantioselectively couples prefabricated fragments and is amenable to broad diversification of functional groups and nucleoside stereochemistry.4−6
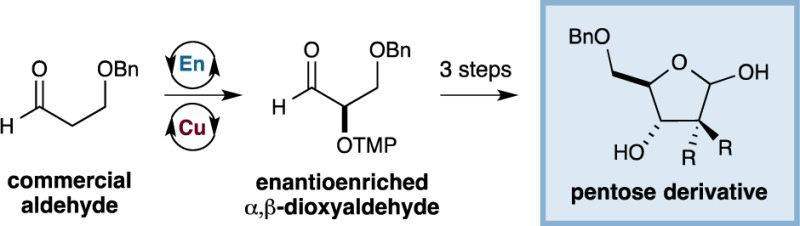
In 2004, our group described a two-step synthesis of orthogonally protected hexoses applying an enantioselective proline-catalyzed aldol coupling followed by a Lewis acid-mediated, diastereoselective Mukaiyama aldol reaction (eq 1). This approach allows for the rapid and asymmetric construction of gluco-, manno-, and allo-configured carbohydrates from simple starting materials.7 We questioned whether a similar strategy might provide access to their 5-carbon, nucleoside counterparts, beginning with the enantioselective catalytic production of an α,β-dioxygenated aldehyde (eq 2). By analogy to our hexose synthesis, we envisioned that this enantioenriched aldehyde could undergo aldol coupling to build the requisite nucleoside skeleton. Importantly, such a strategy would employ catalysis-derived starting materials in place of chiral pool precursors (e.g., isopropylidene-protected glyceraldehydes),8 which have been shown to be poorly or nonselective in similar de novo nucleoside syntheses.9 Moreover, our building blocks would be easily modified to provide a variety of differentially substituted products and would allow for preinstallation of protecting groups, thereby obviating the need for extraneous protection–deprotection sequences. Herein we describe the successful execution of these design ideals and outline a generic and enantioselective route to nucleoside architecture.
 |
Acknowledgments
Financial support was provided by NIHGMS (R01 GM103558-01) and gifts from Merck, AbbVie, and Amgen. We would like to thank Dr. Zoe R. Turner for X-ray crystallographic data.
Supporting Information Available
Experimental details and crystallographic data. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
† These authors contributed equally.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Nelson D. L.; Cox M. M.. Lehninger Principles of Biochemistry, 5th ed.; W. H. Freeman and Co.: New York, 2008; pp 271–299. [Google Scholar]
- Sofia M. J.; Bao D.; Chang W.; Du J.; Nagarathnam D.; Rachakanda S.; Reddy P. G.; Ross B. S.; Wang P.; Zhang H. R.; Bansal S.; Espiritu C.; Keilman M.; Lam A. M.; Micolochick Steuer H. M.; Niu C.; Otto M. J.; Furman P. A. J. Med. Chem. 2010, 53, 7202. [DOI] [PubMed] [Google Scholar]
- Clark J. L.; Hollecker L.; Mason J. C.; Stuyver L. J.; Tharnish P. M.; Lostia S.; McBrayer T. R.; Schinazi R. F.; Watanabe K. A.; Otto M. J.; Furman P. A.; Stec W. J.; Patterson S. E.; Pankiewicz K. W. J. Med. Chem. 2005, 48, 5504. [DOI] [PubMed] [Google Scholar]
- For a general review of the de novo synthesis of monosaccharides:Hudlicky T.; Entwistle D. A.; Pitzer K. K.; Thorpe A. J. Chem. Rev. 1996, 96, 1195. [DOI] [PubMed] [Google Scholar]
- For a review of the synthesis of carbohydrates from acyclic precursors:Ager D. J.; East M. B. Tetrahedron 1993, 49, 5683. [Google Scholar]
- a For a review of the asymmetric de novo synthesis of monosaccharides:Vogel P.; Robina I.. De Novo Synthesis of Monosaccharides. In Glycoscience; Fraser-Reid B., Tatsuka K., Thiem J., Eds.; Springer-Verlag: Berlin, 2008, pp 857–956. [Google Scholar]; b Trost B. M.; Nübling C. Carbohydr. Res. 1990, 202, 1. [DOI] [PubMed] [Google Scholar]
- Northrup A. B.; MacMillan D. W. C. Science 2004, 305, 1752. [DOI] [PubMed] [Google Scholar]
- a Bär E.; Fischer H. O. L. J. Am. Chem. Soc. 1939, 61, 761. [Google Scholar]; b Debost J. L.; Gelas J.; Horton D. J. Org. Chem. 1983, 48, 1381. [Google Scholar]
- a Hertel L. W.; Kroin J. S.; Misner J. W.; Tustin J. M. J. Org. Chem. 1988, 53, 2406. [Google Scholar]; b Zhang P. S.; Idling H.; Cedilote M.; Brunner S.; Williamson T.; Cleary T. P. Tetrahedron: Asymmetry 2009, 20, 305. [Google Scholar]
- Simonovich S. P.; Van Humbeck J. F.; MacMillan D. W. C. Chem. Sci. 2012, 3, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Humbeck J. F.; Simonovich S. P.; Knowles R. R.; MacMillan D. W. C. J. Am. Chem. Soc. 2010, 132, 10012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M.; Matsuo J. Angew. Chem., Int. Ed. 2013, 52, 9109. [DOI] [PubMed] [Google Scholar]
- Reaction of enol silanes was successful in two cases, but silyl ketene acetals provided a more robust scope; details in SI.
- Reactions with prochiral silyl ketene acetals generally provide with low to moderate selectivity:; a Kita Y.; Tamura O.; Itoh F.; Yasuda H.; Kishino H.; Ke Y. Y.; Tamura Y. J. Org. Chem. 1988, 53, 554. [Google Scholar]; b Terada M.; Gu J.-H.; Deka D. C.; Mikami K.; Nakai T. Chem. Lett. 1992, 21, 29. [Google Scholar]
- a For a review of the synthesis and biological applications of C-nucleosides: Stambasky J.; Hocek M.; Kocovsky P. Chem. Rev. 2009, 109, 6729. [DOI] [PubMed] [Google Scholar]; b For a general review of the synthesis of C-glycosides: Du Y.; Linhardt R. J. Tetrahedron 1998, 54, 9913. [Google Scholar]
- In some cases, the product mixture also contained the open-chain aryl ketone, which was competent in the reduction step.
- Reduction of pentose-derived lactols in the presence of a Lewis acid and triethylsilane to provide predominantly β-C-nucleosides is well precedented:; Metobo S. E.; Xu J.; Saunders O. L.; Butler T.; Aktoudianakis E.; Cho A.; Kim C. U. Tetrahedron Lett. 2012, 53, 484. [Google Scholar]
- Review of the synthesis of fluorinated nucleosides:Liu P.; Sharon A.; Chu C. K. J. Fluorine Chem. 2008, 129, 743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Use of the bulky TIPS group was found to be essential in order to avoid β-elimination during enolization.
- a Chou T. S.; Heath P. C.; Patterson L. E.; Poteet L. M.; Lakin R. E.; Hunt A. H. Synthesis 1992, 565. [Google Scholar]; b Chang Y. K.; Lee J.; Park G.-S.; Lee M.; Park C. H.; Kim H. K.; Lee G.; Lee B.-Y.; Baek J. Y.; Kim K. S. Tetrahedron 2010, 66, 5687. [Google Scholar]
- Wang P.; Chun B.-K.; Rachakonda S.; Du J.; Zhan N.; Shi J.; Stec W.; Cleary D.; Ross B. S.; Sofia M. J. J. Org. Chem. 2009, 74, 6819. [DOI] [PubMed] [Google Scholar]
- Absolute stereochemistry of 37 was confirmed by X-ray crystallography (data in SI).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


