Abstract
Aims/Introduction
To investigate the efficacy and safety of vildagliptin, a potent dipeptidyl peptidase‐4 inhibitor, as add‐on to nateglinide, compared with switching to vildagliptin in Japanese type 2 diabetes patients poorly controlled with nateglinide.
Materials and Methods
A total of 40 patients inadequately controlled with nateglinide were randomized to the switching group (n = 20, switching from nateglinide to vildagliptin) or combination group (n = 20, nateglinide plus vildagliptin). A meal tolerance test was carried out at weeks 0 and 24.
Results
The mean changes in glycated hemoglobin from baseline to week 24 were −1.2 ± 0.3% and −0.3 ± 0.5% in patients of the combination and switching groups, respectively, and the difference between the groups was statistically significant (P < 0.001). The mean changes in area under the curve of glucose from 0 to 180 min (AUC0–180 min) from baseline to week 24 was −361 ± 271.3 mmol·min/L in patients of the combination group compared with 141 ± 241.9 mmol·min/L in those of the switching group (P < 0.001). The incidence of hypoglycemic events was low (three in the combination group), and none of the patients developed severe hypoglycemia. Although the addition of vildagliptin to nateglinide did not significantly increase insulin secretion relative to glucose elevation (ISG) after meal load (ISG0–180 min: AUC0–180 min insulin / AUC0–180 min glucose) in comparison with that in baseline, the mean ISG0–30 min 24 weeks after addition of vildagliptin to nateglinide was significantly higher than that at baseline. In contrast, switching from nateglinide to vildagliptin reduced the mean ISG0–180 min, relative to baseline.
Conclusions
The combination therapy of vildagliptin and nateglinide is effective and safe in Japanese type 2 diabetes, and the improved glycemic control is as a result of augmentation of nateglinide‐induced early phase insulin secretion. This trial was registered with UMIN (no. ID000004010).
Keywords: Dipeptidyl peptidase‐IV inhibitors, Glinides, Insulin secretion
Introduction
Early type 2 diabetes is currently considered an important target for drug therapy, because large prospective and randomized studies proved that early and aggressive intervention to ensure proper glycemic control prevents both cardiovascular events and microvascular complications1. The earliest determinant of progression to type 2 diabetes is loss of early insulin secretion, a defect that results in postprandial hyperglycemia, particularly in the Asian population1. In this regard, glinides, a class of rapidly acting insulin secretagogues, selectively enhances early meal‐induced insulin secretion, and thus improves mealtime glucose control5. Consistent with the physiological nature of the categories of these drugs, the overall insulin exposure is relatively lower than that produced by sulfonylurea compounds in type 2 diabetes5. However, glinides are not necessarily effective in all diabetic patients. These drugs are sometimes less effective in patients with advanced diabetes, especially those with persistent fasting hyperglycemia and those who are treated with these drugs for a long period of time. In such patients, many physicians select sulfonylureas as the next line antidiabetic agents, but these drugs do not effectively improve the postmeal spike in glucose levels9. In this regard, the combination of nateglinide and dipeptidyl peptidase‐IV (DPP‐IV) inhibitors, which exert their glucoregulatory actions through the prevention of incretin degradation, thus causing potentiation of glucagon‐like peptide‐1 (GLP‐1) and glucose‐dependent insulinotropic polypeptide (GIP) actions10, could potently lower both postprandial and fasting hyperglycemia. It has been reported that administration of DPP‐IV inhibitors strongly enhanced the potential of insulin secretion from insulin secretagogues, including glinides in animals14 and sulfonylurea in healthy male subjects15. The present study was designed to prove the therapeutic efficacy of the combination strategy of nateglinide and vildagliptin, a stable, selective and orally effective DPP‐IV inhibitor16, in comparison with that of switching strategy from nateglinide to vildagliptin.
Methods
Patients
We screened type 2 diabetic patients who regularly attended Juntendo University Hospital between November 2010 and October 2011. Among them, we selected those with the following criteria: (i) on nateglinide treatment (90 mg t.i.d.) for more than 1 year; (ii) older than 18 years‐of‐age; (iii) glycated hemoglobin (HbA1c) of >6.9%, but <8.4%; (iv) stable glycemic control with HbA1c variation <1.0% during the preceding 6 months; and (v) negative history of DPP‐IV inhibitor, sulfonylurea or alpha‐glucosidase inhibitor therapy. Patients were also excluded if they had concomitant chronic diseases, such as anemia (hemoglobin ≤11.0 g/dL), kidney (plasma creatinine >1.50 mg/dL), liver (aspartase aminotransferase >80 IU/L or alanine aminotransferase >80 IU/L), or serious cardiovascular, pancreas or digestive organ disease; recent acute illness, such as serious infectious disease or injury; progressive diabetic complications, such as proliferative diabetic retinopathy, serious diabetic neuropathy; or other conditions, such as current steroid therapy, or were suspected or confirmed to be pregnant. Heavy alcohol drinkers or cancer patients were also excluded. The study protocol was carried out in accordance with the ethical principles stated in the Declaration of Helsinki17, and approved by the ethics review committee of Juntendo University Hospital. All patients provided written informed consent and confirmed their willingness to participate in this study. The equation between the Japan Diabetes Society and the National Glycohemoglobin Standardization Program values of HbA1c has been defined as previously reported18.
Randomized Clinical Trial
Among 65 inadequately controlled nateglinide‐treated type 2 diabetic patients, 40 were invited to participate in an open labeled randomized controlled trial carried out at Juntendo University Hospital, Japan. The primary end‐points of the present study were: (i) comparison of the effects of the combination of nateglinide + vildagliptin with those of switching from nateglinide to vildagliptin after 24 weeks on glycemic control (HbA1c and area under the curve [AUC] of serum glucose under standard meal loading test); and (ii) comparison of the safety of the two regimens. The secondary end‐points included comparison of the effects of both therapeutic regimens on secretion of insulin and glucagon, serum lipid profile under standard meal load, and effects on blood pressure (BP) and body mass index (BMI).
After the 4‐week screening period, eligible patients were randomized either to the combination therapy group (oral vildagliptin 50 mg b.i.d. and nateglinide 90 mg t.i.d. just before each meal [n = 20]) or to the switching group (vildagliptin monotherapy without nateglinide [n = 20]) based on a computer‐generated assignment. Patients were provided with recommendations for diet therapy during the screening period and after randomization. Restriction was imposed on patients taking any oral diabetic agents, insulin and other oral medications, such as BP‐lowering and lipid‐lowering drugs for both groups, except for avoidance of the adverse effects, such as hypoglycemia, by the attending physicians' decision during the study.
At and after the screening visit, baseline laboratory data, including plasma adiponectin, BP and BMI, were determined for each participant. A standard meal loading test was also carried out at weeks 0 and 24. Blood samples were obtained for the measurement of serum lipids (total cholesterol, high‐density lipoprotein cholesterol and triglycerides) and HbA1c by standard laboratory techniques. BP was measured with a mercury sphygmomanometer. The baseline clinical characteristics of the participants are shown in Table 1. Each patient was reviewed at least every 2 months, and their general health, compliance with medications, laboratory data, blood pressure, and diet and exercise status were checked at each visit. At 24 weeks after the intervention, baseline laboratory data, BP, BMI and standard meal loading test were determined again for each participant. The mean laboratory values for the observation period were calculated from the data obtained at each visit. Safety was also assessed by general physical examination, assessment of vital signs, clinical hematology and chemistry, urinalysis, and reporting of adverse events, in particular, hypoglycemic episodes.
Table 1. Demographic and baseline characteristics (randomized population).
| Vildagliptin 100 mg/day + nateglinide 270 mg/day (n = 17) | Vildagliptin 100 mg/day (n = 19) | |
|---|---|---|
| Age (years)† | 66.3 ± 10.0 | 63.0 ± 13.2 |
| <65‡ | 8 (47.1) | 9 (47.4) |
| ≥65‡ | 9 (52.9) | 10 (52.6) |
| Sex‡ | ||
| Males | 10 (58.8) | 15 (78.9) |
| Females | 7 (41.2) | 4 (21.1) |
| Body mass index (kg/m2†) | 25.0 ± 4.1 | 24.7 ± 2.6 |
| BMI <25 kg/m2‡ | 10 (58.8) | 10 (52.6) |
| BMI ≥25 kg/m2‡ | 7 (41.2) | 9 (47.4) |
| HbA1c (%)† | 7.6 ± 0.6 | 7.5 ± 0.6 |
| HbA1c ≤7‡ | 5 (29.4) | 4 (21.1) |
| HbA1c >7 to ≤8‡ | 8 (47.1) | 12 (63.2) |
| HbA1c >8‡ | 4 (23.5) | 3 (15.8) |
| Fasting plasma glucose (mmol/L)† | 8.44 ± 1.56 | 8.45 ± 1.48 |
| Fasting plasma insulin (pmol/L)† | 7.1 ± 4.0 | 7.4 ± 6.0 |
| Disease duration (years)† | 11.8 ± 5.6 | 11.6 ± 9.0 |
| Systolic blood pressure (mmHg)† | 140.8 ± 17.1 | 134.5 ± 13.5 |
| Diastolic blood pressure (mmHg)† | 82.1 ± 11.0 | 77.7 ± 11.2 |
| Medications (n)‡ | ||
| Metformin | 6 (35.2) | 4 (21.1) |
| Pioglitazone | 4 (23.5) | 1 (5.3) |
| Calcium‐channel blockers | 4 (23.5) | 5 (26.3) |
| ARB, ACEi | 6 (35.2) | 7 (36.8) |
| Diuretics | 1 (5.9) | 1 (5.3) |
| β‐Blockers | 0 (0) | 3 (15.8) |
| α‐Blockers | 0 (0) | 2 (10.5) |
| Statins | 9 (52.9) | 9 (47.4) |
| Fibrates | 0 (0) | 1 (5.3) |
| Ezetimibe | 1 (5.9) | 1 (5.3) |
| Anti‐platelet agents | 4 (23.5) | 4 (21.1) |
†Values are mean ± standard deviation.‡Values numbers of patients or percentages. ACE, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II type 2 receptor antagonist; BMI, body mass index; HbA1c, glycated hemoglobin.
Standard Meal Loading Test
The participants attended the Diabetes Unit at 09.00 hours after a 12‐h fast (from 21.00 hours on the day before each test), and were given 90 mg of nateglinide at baseline for both groups, and 50 mg vildagliptin with or without 90 mg of nateliginide after intervention just before an oral standard‐meal load (coordinated by the Japan Diabetes Society20). The total energy content of the standard meal was 460 kcal, with 56.5 g of carbohydrate, 18.0 g of fat and 18.0 g of protein; with a total of 51.4 energy% (E%) from carbohydrate, 33.3 E% from fat and 15.3 E% from protein. The meal had to be eaten within 15 min after receiving the drug. The participants were at rest and sitting throughout testing.
An intravenous line was inserted into one forearm vein before drug administration and kept patent using 0.9% NaCl for repeated blood sampling. Blood was drawn at 0, 15, 30, 60, 120 and 180 min after the meal. Time zero corresponds to immediately before drug administration. We measured glucose, insulin, glucagon and lipids (triglycerides [TG], low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol and total cholesterol) at 0, 15, 30, 60, 120 and 180 min; interleukin (IL)‐6, total adiponectin, active GLP‐1, and high‐sensitivity C reactive protein (hsCRP) at 0, 30 and 120 min.
Statistical Analysis
Data were analyzed with the use of pasw statistics (version 18; IBM, Tokyo, Japan). All data are expressed as mean ± standard deviation unless otherwise indicated. To compare between‐group differences during the course of the study, non‐paired t‐test was carried out. To compare the differences between baseline and 24 weeks, paired t‐test was carried out. The AUC was calculated by the trapezoidal method. A P‐value <0.05 was considered statistically significant. Responder rates in each treatment group (percentage of patients achieving end‐point HbA1c <7.0%) were compared by the χ2‐test. A P‐value of <0.05 denoted the presence of significant statistical difference.
Results
Patients
Figure 1 shows a flow chart of the trial profile of the present study. A total of 65 patients were recruited, and 25 of them were excluded based on the aforementioned criteria. The remaining patients were randomly assigned to either the combination therapy group (combination group, n = 20) or the switching to vildagliptin group (switching group, n = 20). A total of 17 of 20 (85%) patients of the combination group, and 19 of 20 (95%) patients of the switching group completed the study. The reason for discontinuation in the combination group was absolutely mild hypoglycemia (three cases), and that in the switching group was liver dysfunction, which improved after discontinuation of vildagliptin.
Figure 1.
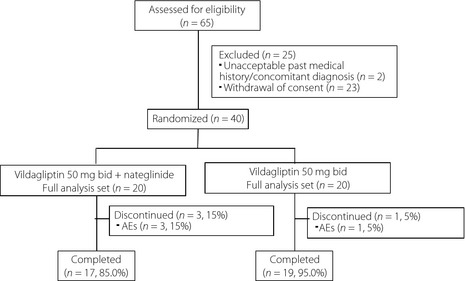
Flow chart of the patient recruitment process.
The baseline characteristics of the randomized patients are presented in Table 1. The two groups were well balanced at baseline, with a mean age, BMI and HbA1c of 66 ± 10 years, 25.0 ± 4.1 kg/m2, and 7.6 ± 0.6% for the combination group, 63 ± 13 years, 24.7 ± 2.6 kg/m2, and 7.5 ± 0.6% for the switching group, respectively. Patients were predominantly males (>60%), with a mean disease duration of 11.8 ± 5.6 years for the combination group and 11.6 ± 9.0 years for the switching group.
Efficacy (changes in HbA1c)
Figure 2a shows the serial changes in mean HbA1c during 24 weeks of treatment with vildagliptin‐alone (switching group) and vildagliptin with nateglinide (combination group). At baseline, HbA1c was similar in the two groups (combination group 7.6 ± 0.6%, switching group 7.5 ± 0.6%). The pattern of decrease in HbA1c was different in the two groups: the mean HbA1c level decreased rapidly in the combination group from the start of the treatment until week 16, but decreased more gradually thereafter until the end of the treatment period. In contrast, the mean HbA1c level was slightly, but significantly, lower after 24 weeks of treatment, relative to the baseline in the switching group. The mean change in HbA1c (from baseline to end‐point, week 24) was −1.2 ± 0.3% in the combination group and −0.3 ± 0.5% in switching group, and the difference was significant (P < 0.001; Figure 2b). The target HbA1c (<7.0% at end‐point) was achieved by 82.3% of the patients in the combination group compared with 47.4% in switching group (P < 0.05).
Figure 2.
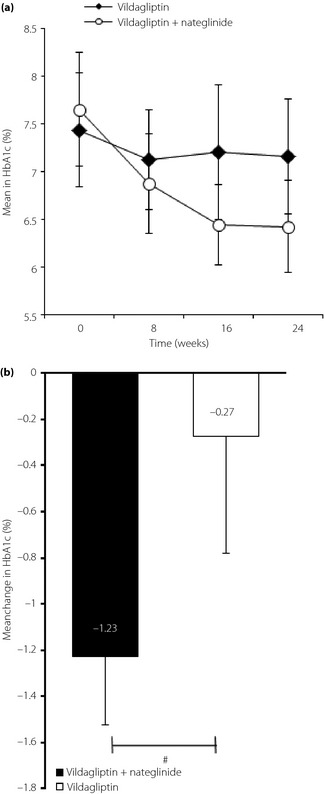
(a) Serial changes in glycated hemoglobin (HbA1c) during the 24 weeks with vildagliptin or vildagliptin + nateglinide. (b) Mean changes in HbA1c at the end‐point in the two treatment groups. #P < 0.001, the combination group versus the switching group.
Standard Meal Test
Figure 3a–c shows the serial changes in mean serum glucose concentrations before and after standard meal load (Figure 3a) and mean change in fasting serum glucose level from baseline to 24 weeks (Figure 3b). The addition of vildagliptin to nateglinide significantly reduced glucose levels at 15, 30, 60, 120 and 180 min compared with switching from nateglinide to vildagliptin (8.4 and 10.0 at 15 min, P < 0.01; 9.0 and 11.5 at 30 min, P < 0.001; 9.8 and 13.4 at 60 min, P < 0.001; 8.4 and 11.2 at 120 min, P < 0.001; 6.4 and 8.4 mmol/L at 180 min, P < 0.01, respectively; Figure 3a). There was a significant improvement in fasting glucose level in each group, relative to the baseline, but no significant difference between the groups (end‐point: switching group 7.9 ± 1.6 mmol/L, combination group 7.4 ± 1.4 mmol/L, baseline 8.5 ± 1.5 mmol/L and 8.4 ± 1.6 mmol/L, respectively). However, a significant increase in the mean AUC of glucose from 0 to 180 min (AUC0–180 min glucose) was observed after switching from nateglinide to vildagliptin (baseline 1852 ± 303 mmol·min/L, end‐point 1,992 ± 360 mmol·min/L; P < 0.05), whereas the mean AUC0–180 min glucose decreased significantly after the addition of vildagliptin to nateglinide (baseline 1,881 ± 327 mmol·min/L, end‐point 1,520 ± 256 mmol·min/L; P < 0.001). The mean change in AUC0–180 min glucose from baseline to week 24 after the addition of vildagliptin to nateglinide was significantly lower than that observed in the switching group (−361 ± 271 mmol·min/L and +141 ± 242 mmol·min/L, respectively, P < 0.001; Figure 3c). These results show that vildagliptin strongly improved both fasting and postprandial hyperglycemia when combined with nateglinide.
Figure 3.
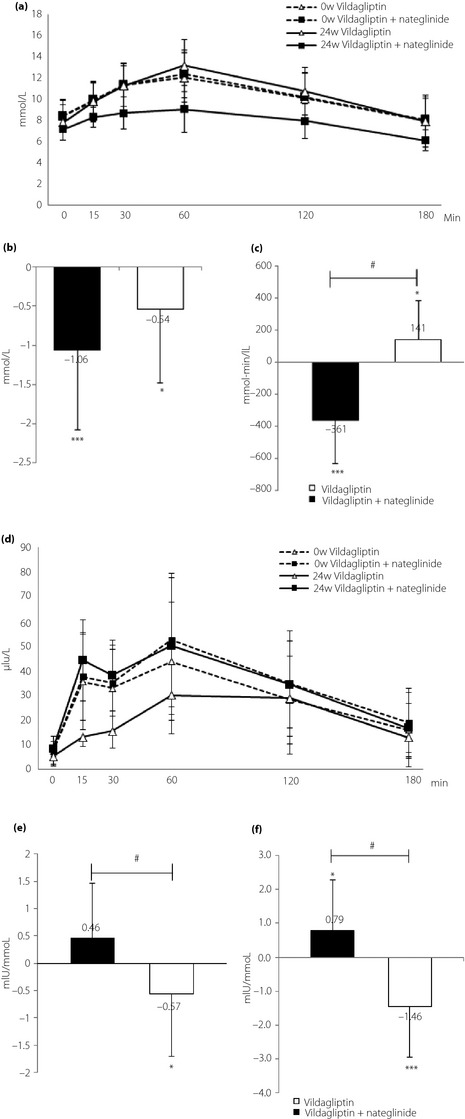
(a) Serial changes in plasma glucose level at baseline with nateglinide and after 24 weeks with vildagliptin or vildagliptin + nateglinide after standard meal load. (b) Mean changes in fasting plasma glucose. ***P < 0.001, baseline versus 24 weeks in the combination group. *P < 0.05, baseline versus 24 weeks in the switching group. (c) Mean changes in areas under the curve of glucose from baseline to end‐point after standard meal load. #P < 0.001, the combination group versus the switching group. ***P < 0.001, baseline versus 24 weeks in the combination group. *P < 0.05, baseline versus 24 weeks in the switching group. (d) Serial changes in plasma insulin level at baseline with nateglinide and after 24 weeks with vildagliptin or vildagliptin + nateglinide after standard meal load. (e) Mean change in insulin secretion relative to glucose elevation after meal load (ISG0?180 min; AUC0?180 min insulin/AUC0?180 min glucose) from baseline to endpoint. #P < 0.01, the combination group versus the switching group. *P < 0.05, baseline versus 24 weeks in the switching group. (f) Mean change in ISG0–30 min from baseline to end‐point. #P < 0.001, the combination group versus the switching group. *P < 0.05, baseline versus 24 weeks in the combination group. ***P < 0.001, baseline versus 24 weeks in the switching group.
Figure 3d and e shows serial changes in serum insulin (Figure 3d) and the mean change in insulin secretion relative to glucose elevation (ISG) after meal load (ISG0–180 min; AUC0–180 min insulin / AUC0–180 min glucose) from baseline to end‐point (Figure 3e). The addition of vildagliptin to nateglinide did not significantly increase serum insulin levels at any time‐point (Figure 3d) or in ISG0–180 min, compared with baseline (Figure 3e). In contrast, switching from nateglinide to vildagliptin significantly reduced serum insulin levels measured from 15 to 60 min, as well as mean in ISG0–180 min, compared with baseline. The mean change in ISG0–180 min from baseline to week 24 after switching from nateglinide was significantly lower than that observed after addition of vildagliptin to nateglinide (−0.57 ± 0.063 mIU/mmol and +0.46 ± 0.053 mIU/mmol, respectively; P < 0.01; Figure 3e). Although the combination improved glycemic control, it did not significantly change the mean in ISG0–180 min relative to the baseline (baseline 3.53 ± 1.91; end‐point 3.99 ± 1.94 mIU/mmol). However, the combination of vildagliptin and nateglinide significantly enhanced early phase insulin secretion, reflected by insulin secretion relative to glucose elevation after meal load (ISG0–30 min; AUC0–30 min insulin / AUC0–30 min glucose), compared with the baseline (baseline 3.04 ± 1.36, end‐point 3.83 ± 1.85 mIU/mmol; P < 0.05; Figure 3f). In the case of using delta insulin0–30 min / delta glucose0–30 min (termed as insulinogenic index) as early phase insulin secretion, the results were the same as those in the case of using in ISG0–30 min. The aforementioned changes resembled the original and specific features of nateglinide monotherapy on early phase insulin secretion.
Figure 4 shows the serial changes in glucagon (Figure 4a), and also the mean change in AUC0–180 min glucagon (Figure 4b). During the 24‐week treatment period, the AUC of glucagon tended to decrease from the baseline to end‐point in both groups, but the change was not significant. There was no significant difference between the two treatment groups with regard to the change in AUCs of glucagon (combination group –481 ± 3579 μIU·min/mL, switching group –1123 ± 2801 μIU·min/mL, P = 0.551).
Figure 4.
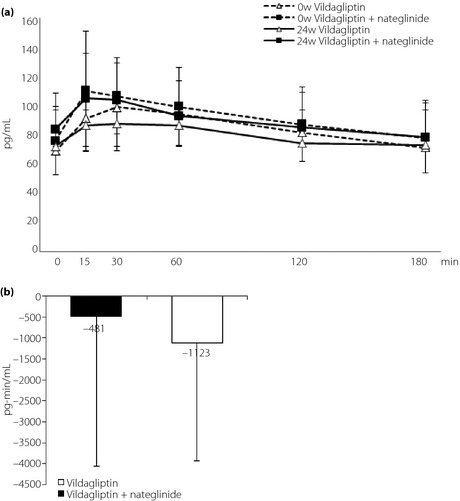
(a) Serial changes in glucagon at baseline with nateglinide and after 24 weeks with vildagliptin or vildagliptin + nateglinide after standard meal load. (b) Mean changes of area under the curve of glucose from 0 to 180 min glucagon after standard meal load from baseline to end‐point.
Figure 5 compares the increment of plasma active GLP‐1 concentrations after meal load (0–120 min) from baseline to end‐point in the combination and switching groups. The results showed no significant difference between the two groups. Similar changes were observed after 24‐week treatment in postprandial adiponectin, IL‐6 and hsCRP in the two groups (data not shown). A similar trend was noted for changes in lipid metabolism, except for significantly lowered fasting serum low‐density lipoprotein cholesterol in the combination group compared with the switching group (combination group −13.0 ± 19.1, switching group +1.7 ± 15.9 mg/dL; P < 0.05).
Figure 5.
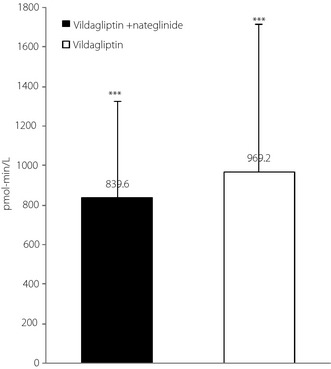
Increment of plasma active glucagon‐like peptide‐1 after standard meal load (0–120 min) measured at baseline with nateglinide and after 24 weeks with vildagliptin or vildagliptin + nateglinide. ***P < 0.001, baseline versus 24 weeks in both groups.
Tolerability
The overall incidence of adverse events was higher in the combination group than the switching group. The high incidence of adverse events in the combination group was as a result of the higher incidence of hypoglycemia. Mild nausea and liver dysfunction were observed in one patient of the switching group. There were no serious adverse events during the treatment period in patients of either group.
Hypoglycemic events were observed in just 15% (3/20) of patients in the combination group, whereas none developed severe hypoglycemia. All hypoglycemic events occurred several hours after administration of vildagliptin during exercise. All hypoglycemia‐related symptoms disappeared after reducing the dose of nateglinide.
There was no significant difference in changes in bodyweight between the two groups (combination group 1.0 ± 1.7 kg, switching group 0.4 ± 1.6 kg; P = 0.308). Furthermore, there were no major changes in hematological, biochemical and urinary parameters in the two groups, except for one patient from the switching group who developed a mild rise in liver transaminases.
Discussion
The present study is the first to assess the efficacy and safety of vildagliptin used in combination with nateglinide in Japanese type 2 diabetes patients who were inadequately controlled with nateginide.
Glinides are highly physiological, mealtime glucose regulators5, and effective and safe drugs for the treatment of early type 2 diabetics. However, their effects on insulin secretion are relatively weak compared with sulfonylureas, which produce potent and persistent stimulation of pancreatic insulin secretion. In the present study, we compared the effects of the combination of vildagliptin and nateglinde on glycemic control.
Vildagliptin, as an add‐on therapy to nateglinide (combination group), produced a significant and clinically meaningful reduction in HbA1c compared with switching to vildagliptin (switching group; 1.2 ± 0.3% vs 0.3 ± 0.5%, respectively; P < 0.001). HbA1c was reduced in each of the 20 patients of the combination group without exception. Among the patients of the combination group, 82.3% achieved HbA1c less than 7.0% at end‐point compared with 47.4% in the switching group. These findings suggest that to accomplish strict glycemic control, it is better to continue nateglinide than to withdraw it when vildagliptin is prescribed as second‐line treatment for patients with type 2 diabetes who were inadequately controlled with nateglinide.
Nateglinide is an insulin secretagogue known to specifically stimulate early phase insulin secretion from β‐cells7. In the present study, the insulin secretion relative to glucose elevation after meal load (ISG0–180 min: AUC0–180 min insulin / AUC0–180 min glucose) at 24 weeks after combination therapy of nateglinide and vildagliptin was significantly higher than that 24 weeks after switching from nateglinide to vildagliptin, although the difference relative to baseline was not significant. Interestingly, ISG0–30 min, which reflects the ability of early phase insulin secretion, was significantly higher at 24 weeks after combination therapy than at baseline. These findings show that vildagliptin enhanced the effect of nateglinide on insulin secretion, as observed during monotherapy. Unfortunately, only a few reports have compared the effects of DPP‐IV inhibitor on insulin secretion under stimulation with insulin secretagogues in diabetics. El‐Ouaghlidi et al.15 compared the effects of placebo, glibenclamide, vildagliptin or both on insulin secretion after 75‐g oral glucose loading in 16 healthy participants. Glibenclamide enhanced insulin and C‐peptide responses, and induced hypoglycemia. Vildagliptin alone did not enhance insulin secretory responses compared with placebo. Vildagliptin plus glibenclamide stimulated insulin and C‐peptide responses from 30 to 240 min after glucose loading, compared with glibenclamide alone, although these differences were not significant. Asakawa et al.21 examined the efficacy of alogliptin, another DPP‐IV inhibitor, on insulin secretion in diabetic rats with sulfonylurea‐induced secondary failure. In their study, alogliptin showed significant improvement in glucose excursion with significant increase in insulin secretion. To our knowledge, only a few reports have been published on the effect of the combination of glinide and incretin‐related drugs. Bell et al.22 reported the effects of exogenous GLP‐1 on the hypoglycemic effects of nateglinide after intravenous glucose administration in type 2 diabetes. Plasma glucose responses were lowest and mean AUC0–180 min insulin responses were highest with peak level at 15 min after intravenous glucose administration after the combination of nateglinide and GLP‐1. In that report, plasma DPP‐IV activity was lower and active GLP‐1 concentration was higher after the combination than after GLP‐1 or nateglinide alone, suggesting that nateglinide inhibits DPP‐IV activity, as reported previously23. The beneficial effects of the combination of nateglinide and vildagliptin might be indirectly mediated through DPP‐IV inhibition and increased bioactivity of incretins. However, measurement of active GLP‐1 concentration showed no significant differences between the combination and switching groups. These different results could be due to the single dose loading tests used in previous studies and the test after long medication with nateglinide in the present study. Miura et al.25 have recently reported the effects of the combination of nateglinide and vildagliptin in an animal model25. In their report, treatment with nateglinide alone significantly stimulated early‐phase insulin secretion and tended to improve postprandial glucose level in Zucker fatty rats. The combination of nateglinide with vildagliptin resulted in a more dramatic improvement in postprandial hyperglycemia, which resulted in decrease of AUC0–60 min insulin after meal load, compared with the control or nateglinide alone. Unfortunately ISG0–60 min was not calculated in that study, though it could be presumed to have increased significantly.
The present study showed that vildagliptin was well tolerated, and that the overall incidence of adverse effects was comparable between the two groups. Furthermore, discontinuation as a result of adverse effects was only necessary in 15% of patients of the combination group and 5% of the switching group. The overall incidence of hypoglycemia was low in the combination group (15%), and each event was mild and precipitated by exercise. No episodes of severe hypoglycemia were encountered in the present study, suggesting that vildagliptin can be added to nateglinide to achieve better glycemic control without increased risk of severe hypoglycemia. The rise in liver enzymes (more than threefold increase in γ‐glutamyltranspeptidase) in one patient of the switching group was transient, and liver dysfunction improved after cessation of vildagliptin therapy.
Bodyweight increased from baseline to end‐point in both groups, with greater changes observed in the combination group than the switching one, although this was statistically insignificant. These findings are consistent with those of another study involving the combination of vildagliptin and sulfonylurea, where bodyweight increased modestly in patients receiving vildagliptin 100 mg daily in combination with sulfonylurea26. In that study, the increase in bodyweight in the vildagliptin plus glimepiride group was 1.3 ± 0.3 kg (–0.4 ± 0.3 kg in the placebo group), which is similar to those in Japanese type 2 diabetes patients27. In general, vildagliptin is considered neutral with regard to bodyweight, even when combined with nateglinide, compared with the weight‐promoting effects of thiazolidinediones28.
Importantly, more than 80% of the patients treated with the combination of vildagliptin and nateglinide achieved target HbA1c (<7.0%). Overall, the combination treatment was associated with a low incidence of hypoglycemia and no severe hypoglycemia.
In summary, vildagliptin add‐on to nateglinide is efficacious and well‐tolerated in the management of Japanese patients with type 2 diabetes who are inadequately controlled with nateglinide. This effect is mediated at least in part through a mechanism that enhances the favorable insulinotropic feature of nateglinide.
The present study had certain limitations that need to be recognized. The study was carried out in a relatively small number of patients at a single institution. Further studies are required to evaluate the long‐term effects of vildagliptin with or without nateglinide on glycemic control and insulin secretion in a larger number of patients, and after more intensive education.
Acknowledgments
HW has received honoraria for scientific lectures from MSD, Eli Lilly, Takeda, Novartis, Dainippon Sumitomo, Sanofi and Daiichi Sankyo, and also research funds from MSD, Eli Lilly, Takeda, Kowa, Mochida, Sanwakagaku, Novo Nordisk, Kissei, Novartis, Boehringer Ingelheim, Astrageneca, Asteras, Tanabe Mitsubishi, Dainippon Sumitomo, Abbott, Sanofi Aventis, Pfizer, and Daiichi Sankyo. TH has received honoraria for scientific lectures from MSD, Eli Lilly, Takeda, Novartis, Dainippon Sumitomo, Novo Nordisk, Sanofi and Daiichi Sankyo, and also research funds from MSD, Eli Lilly, Takeda, Kowa, Mochida, Sanwakagaku, Novo Nordisk, Kissei, Novartis, Boehringer Ingelheim, Tanabe Mitsubishi, Dainippon Sumitomo, Telmo, Sanofi Aventis, Roche, Ono, and Daiichi Sankyo. YF has been received grant support from Takeda, MSD and Nippon Eli Lilly. YF and AK have also acted as spokespersons for Novartis Pharma, Nippon Eli Lilly, MSD and Sanofi Aventis. All other authors declare no conflict of interest.
J Diabetes Invest 2014; 5: 400–409
References
- 1.Weyer C, Bogardus C, Mott DM, et al. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999; 104: 787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saad MF, Knowler WC, Pettitt DJ, et al. Sequential changes in serum insulin concentration during development of non‐insulin‐dependent diabetes. Lancet 1989; 1: 1356–1359 [DOI] [PubMed] [Google Scholar]
- 3.Kosaka K, Hagura R, Kuzuya T, et al. Insulin secretory response of diabetics during the period of improvement of glucose tolerance to normal range. Diabetologia 1974; 10: 775–782 [DOI] [PubMed] [Google Scholar]
- 4.Kosaka K, Hagura R, Kuzuya T. Insulin responses in equivocal and definite diabetes, with special reference to subjects who had mild glucose intolerance but later developed definite diabetes. Diabetes 1977; 26: 944–952 [DOI] [PubMed] [Google Scholar]
- 5.Horton ES, Clinkingbeard C, Gatlin M, et al. Nateglinide alone and in combination with metformin improves glycemic control by reducing mealtime glucose levels in type 2 diabetes. Diabetes Care 2000; 23: 1660–1665 [DOI] [PubMed] [Google Scholar]
- 6.Hollander PA, Schwartz SL, Gatlin MR, et al. Importance of early insulin secretion: comparison of nateglinide and glyburide in previously diet‐treated patients with type 2 diabetes. Diabetes Care 2001; 24: 983–988 [DOI] [PubMed] [Google Scholar]
- 7.Hirose T, Mizuno R, Yoshimoto T. The effects of nateglinide following oral glucose load in impaired glucose tolerance subjects: rapid insulin stimulation by nateglinide in IGT subjects. Endocr J 2002; 49: 649–652 [DOI] [PubMed] [Google Scholar]
- 8.Uchino H, Niwa M, Shimizu T, et al. Impairment of early insulin response after glucose load, rather than insulin resistance, is responsible for postprandial hyperglycemia seen in obese type 2 diabetes: assessment using nateglinide, a new insulin secretagogue. Endocr J 2000; 47: 639–641 [DOI] [PubMed] [Google Scholar]
- 9.Esposito K, Giugliano D, Nappo F, et al. Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation 2004; 110: 214–219 [DOI] [PubMed] [Google Scholar]
- 10.Mentlein R. Dipeptidyl‐peptidase IV (CD26)–role in the inactivation of regulatory peptides. Regul Pept 1999; 85: 9–24 [DOI] [PubMed] [Google Scholar]
- 11.Holst JJ, Deacon CF. Inhibition of the activity of dipeptidyl‐peptidase IV as a treatment for type 2 diabetes. Diabetes 1998; 47: 1663–1670 [DOI] [PubMed] [Google Scholar]
- 12.Deacon CF. Therapeutic strategies based on glucagon‐like peptide 1. Diabetes 2004; 53: 2181–2189 [DOI] [PubMed] [Google Scholar]
- 13.Green BD, Flatt PR, Bailey CJ. Inhibition of dipeptidylpeptidase IV activity as a therapy of type 2 diabetes. Expert Opin Emerg Drugs 2006; 11: 525–539 [DOI] [PubMed] [Google Scholar]
- 14.Yamazaki K, Yasuda N, Inoue T, et al. Effects of the combination of a dipeptidyl peptidase IV inhibitor and an insulin secretagogue on glucose and insulin levels in mice and rats. J Pharmacol Exp Ther 2007; 320: 738–746 [DOI] [PubMed] [Google Scholar]
- 15.El‐Ouaghlidi A, Rehring E, Holst JJ, et al. The dipeptidyl peptidase 4 inhibitor vildagliptin does not accentuate glibenclamide‐induced hypoglycemia but reduces glucose‐induced glucagon‐like peptide 1 and gastric inhibitory polypeptide secretion. J Clin Endocrinol Metab 2007; 92: 4165–4171 [DOI] [PubMed] [Google Scholar]
- 16.Banerjee M, Younis N, Soran H. Vildagliptin in clinical practice: a review of literature. Expert Opin Pharmacother 2009; 10: 2745–2757 [DOI] [PubMed] [Google Scholar]
- 17.Rickham PP. Human Experimentation. Code of Ethics of the World Medical Association. Declaration of Helsinki. Br Med J 1964; 2: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Committee on the standardization of diabetes mellitus‐related laboratory tesiting of the Japan Diabetes Society . International clinical harmonaization of glycated hemoglobin in Japan; From Japan Diabetes Society to National Glycohemoglobin Standardization program values. Diabetol Int 2012; 3: 8–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kashiwagi A, Kasuga M, Araki E, et al. International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest 2012; 3: 39–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshino G, Tominaga M, Hirano T, et al. The test meal A: a pilot model for the international standard of test meal for assessment of both postprandial hyperglycemia and hyperlipidemia. J Japan Diab Soc 2006; 49: 361–371 (Japanese). [Google Scholar]
- 21.Asakawa T, Moritoh Y, Kataoka O, et al. A novel dipeptidyl peptidase‐4 inhibitor, alogliptin (SYR‐322), is effective in diabetic rats with sulfonylurea‐induced secondary failure. Life Sci 2009; 85: 122–126 [DOI] [PubMed] [Google Scholar]
- 22.Bell PM, Cuthbertson J, Patterson S, et al. Additive hypoglycaemic effect of nateglinide and exogenous glucagon‐like peptide‐1 in type 2 diabetes. Diabetes Res Clin Pract 2011; 91: e68–e70 [DOI] [PubMed] [Google Scholar]
- 23.Duffy NA, Green BD, Irwin N, et al. Effects of antidiabetic drugs on dipeptidyl peptidase IV activity: nateglinide is an inhibitor of DPP IV and augments the antidiabetic activity of glucagon‐like peptide‐1. Eur J Pharmacol 2007; 568: 278–286 [DOI] [PubMed] [Google Scholar]
- 24.McKillop AM, Duffy NA, Lindsay JR, et al. Insulinotropic actions of nateglinide in type 2 diabetic patients and effects on dipeptidyl peptidase‐IV activity and glucose‐dependent insulinotropic polypeptide degradation. Eur J Endocrinol 2009; 161: 877–885 [DOI] [PubMed] [Google Scholar]
- 25.Miura K, Kitahara Y, Yamagishi S. Combination therapy with nateglinide and vildagliptin improves postprandial metabolic derangements in Zucker fatty rats. Horm Metab Res 2010; 42: 731–735 [DOI] [PubMed] [Google Scholar]
- 26.Garber AJ, Foley JE, Banerji MA, et al. Effects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulphonylurea. Diabetes Obes Metab 2008; 10: 1047–1056 [DOI] [PubMed] [Google Scholar]
- 27.Kikuchi M, Haneda M, Koya D, et al. Efficacy and tolerability of vildagliptin as an add‐on to glimepiride in Japanese patients with Type 2 diabetes mellitus. Diabetes Res Clin Pract 2010; 89: 216–223 [DOI] [PubMed] [Google Scholar]
- 28.Bolli G, Dotta F, Colin L, et al. Comparison of vildagliptin and pioglitazone in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Obes Metab 2009; 11: 589–595 [DOI] [PubMed] [Google Scholar]
- 29.McEwan P, Evans M, Bergenheim K. A population model evaluating the costs and benefits associated with different oral treatment strategies in people with type 2 diabetes. Diabetes Obes Metab 2010; 12: 623–630 [DOI] [PubMed] [Google Scholar]


