Abstract
Aims/Introduction
In Japan, liraglutide was recently approved for patients with type 2 diabetes. To our knowledge, there are no markers predicting successful switching from insulin therapy to liraglutide monotherapy in Japanese patients with type 2 diabetes and renal impairment. We therefore assessed clinical characteristics predicting successful switching.
Materials and Methods
We analyzed 21 patients with type 2 diabetes and estimated glomerular filtration rates <60 mL/min/1.73 m2 receiving long‐term insulin in Shiga University of Medical Science Hospital, Otsu, Shiga, Japan. Their β‐cell function was assessed by measuring urinary C‐peptide and C‐peptide immunoreactivity (CPR) index, along with glucagon loading and oral glucose tolerance tests. Blood glucose concentration and blood pressure were measured daily before and after switching from insulin to liraglutide, and glycated hemoglobin (HbA1c; National Glycohemoglobin Standardization Program) was assessed 12 weeks after switching to liraglutide.
Results
Baseline HbA1c was significantly lower in successfully switched than in unsuccessfully switched patients. CPR index, urinary C‐peptide concentration and 6‐min post‐glucagon increment in CPR (ΔCPR) did not differ significantly in the two groups. ΔCPR 120 min after 75 g oral glucose was significantly higher in successfully than unsuccessfully switched patients. Mean blood glucose concentrations before breakfast, after breakfast, before lunch and after dinner were significantly lower in successfully switched patients. HbA1c did not change significantly in either group.
Conclusions
Measurement of oral glucose‐stimulated ΔCPR120 min is recommended when considering switching Japanese type 2 diabetes patients with renal impairment from insulin to liraglutide monotherapy.
Keywords: Liraglutide, Oral glucose tolerance test, Renal impairment
Introduction
Type 2 diabetes mellitus is a progressive, multifactorial, debilitating disease marked by a gradual decrease in pancreatic β‐cell function and concomitant deterioration in insulin secretion against a background of increased insulin resistance. Many patients with type 2 diabetes have renal impairment, a late complication of inadequate glycemic control1. Microalbuminuria, the earliest indicator of nephropathy attributable to diabetes, affects 25% of patients with type 2 diabetes within 10 years of diagnosis2. Diabetic nephropathy develops in 5–10% of patients with both type 2 diabetes and microalbuminuria each year3. Therefore, there is a need for therapies that can achieve glycemic control in type 2 diabetes, and that are also safe and effective in patients with renal dysfunction. The glucose‐lowering actions of glucagon‐like peptide‐1 (GLP‐1), an incretin hormone, are glucose‐dependent, which limits the risk of hypoglycemia8. GLP‐1 induces insulin secretion and reduces glucagon secretion, resulting in potent reduction of blood glucose concentrations.
Because the pharmacokinetics of exenatide (exendin‐4), an incretin mimetic, are significantly affected by renal dysfunction, this agent is not recommended for patients with severe renal impairment or end‐stage renal disease (ESRD)10.
Liraglutide is a once‐daily human GLP‐1 analog under development for the treatment of hyperglycemia in patients with type 2 diabetes. Liraglutide has a high degree of sequence identity to human GLP‐1, but differs in having an Arg34Lys substitution and a glutamic acid and 16‐C free fatty acid addition to Lys2612. Its half‐life in humans after subcutaneous injection is approximately 13 h13, allowing once‐daily administration. The metabolism of liraglutide is similar to that of large peptides, in that it is fully degraded14. There is no evidence that the kidney is the main organ for its elimination.
Liraglutide monotherapy in patients with type 2 diabetes was found to significantly improve glycemic control and to reduce bodyweight with a small risk of hypoglycemia15. Liraglutide also has favorable effects on several indicators of β‐cell function17, and improves early markers of cardiovascular disease22. Regression analysis of log (area under the curve) for subjects with normal renal function and mild‐to‐severe renal impairment showed that decreasing creatinine clearance did not significantly affect the pharmacokinetics of liraglutide23.
Japanese authorities recently approved liraglutide for glycemic control in patients with type 2 diabetes. Most patients with type 2 diabetes and moderate‐to‐severe renal impairment receive insulin. Switching from insulin to liraglutide adversely affects glycemic control in patients with type 2 diabetes and poor β‐cell function. A recent study reported that postprandial serum C‐peptide is useful in selecting those patients with type 2 diabetes without renal impairment who can be safely switched from insulin to liraglutide24. To our knowledge, however, no guidelines to date have been formulated that predict which Japanese patients with type 2 diabetes and renal impairment can be safely and successfully switched from insulin to liraglutide monotherapy. We therefore assessed the clinical characteristics of patients predicting successful switching.
Materials and Methods
Patients, Liraglutide Dosage and Assessment of β‐Cell Function
A total of 21 patients (18 males and 3 females), ranging in age from 29 to 85 years (median age 61 years) with type 2 diabetes receiving long‐term (more than 1 year) complex insulin therapy in Shiga University of Medical Science Hospital, Otsu, Shiga, Japan were studied. Pregnant patients and those with liver cirrhosis were excluded. All patients had an estimated glomerular filtration rate (eGFR), as assessed by the Modification of Diet in Renal Disease study equation modified for Japanese patients25, less than 60 mL/min/1.73 m2. While still receiving insulin, and before being switched to liraglutide, β‐cell function in each patient was assessed and urinary C‐peptide in 24‐h urine was measured by a chemiluminescence immunoassay. Glucagon tests were carried out after overnight fasting. Venous blood samples for measurement of blood glucose and serum C‐peptide concentrations were obtained immediately before and 6 min after an intravenous bolus injection of 1 mg glucagon (Novo Industries, Copenhagen, Denmark). Increments of C‐peptide immunoreactivity (ΔCPR) after 6 min were calculated according to the formula:
Oral glucose tolerance tests were carried out in the same way after overnight fasting. Venous blood samples for measurement of blood glucose and serum C‐peptide concentrations were obtained immediately before and 30 and 120 min after a 75‐g glucose load, with immunoreactive insulin (IRI) measured before and 30 min after the 75‐g glucose load. ΔCPRs at 120 min were calculated according to the formula:
CPR indexes were calculated according to the formula:
Insulinogenic indexes (II) were calculated according to the formula:
Blood glucose concentrations were monitored daily in all patients before and after switching to liraglutide monotherapy. Patients were started on 0.3 mg liraglutide (Novo Nordisk, Bagsværd, Denmark) once daily for 3 days; if there was no nausea or vomiting, patients were increased to 0.6 mg/day for 3 days, and finally increased to 0.9 mg. Once adequate glycemic control was achieved, the dose of liraglutide was maintained.
Successful switching from insulin to liraglutide was defined as a mean blood glucose concentration <200 mg/dL 120 min after breakfast (AB), lunch (AL) and dinner (AD) on three consecutive days after reaching the final dose of liraglutide. Unsuccessfully switched patients resumed insulin therapy.
The present study was approved by the local institutional review board, and was carried out in accordance with the Declaration of Helsinki. All patients received full explanations of the study and the use of liraglutide, and provided informed consent.
Follow up of Patients Switched to Liraglutide
Blood glucose was measured daily before and after switching to liraglutide monotherapy. Glycated hemoglobin (HbA1c) was measured in 10 successfully‐switched patients at baseline and 4, 8, 12, and 24 weeks after starting liraglutide monotherapy using high‐performance liquid chromatography and an assay certified by the Japan Diabetes Society (JDS). HbA1c (%) was estimated as the National Glycohemoglobin Standardization Program (NGSP) equivalent value (%) and calculated according to the formula:
This equation converts the HbA1c (JDS) (%), measured using the previous standard Japanese method, to the HbA1c (NGSP) (%)26.
Blood pressure was measured daily before breakfast (BB) during hospitalization. Mean blood pressure over three consecutive days before and after switching to liraglutide in successfully switched patients, or before switching to liraglutide and after resuming insulin therapy in unsuccessfully switched patients, was calculated.
Statistical Analysis
Data were analyzed using SPSS version 17.0 (SPSS, Tokyo, Japan). The distribution of variables was analyzed by checking histograms and normal plots of the data, and normality was tested using Kolmogorov–Smirnov and Shapiro–Wilk tests. Student's t‐test was used to compare parameters at different time‐points, and the χ2‐test was used to compare proportions between variables. Pearson's or Spearman's rank correlation coefficients were calculated to determine correlations between variables. To evaluate the predictive factors of the model, Logistic regression was carried out. Values are expressed as means ± standard deviation, with P < 0.05 considered statistically significant.
Results
Patient Characteristics
Of the 21 patients, 16 were successfully switched from insulin to liraglutide, and five were unsuccessful. There were no significant differences between these two groups in age, sex, bodyweight, body mass index; total cholesterol, triglyceride and high‐density lipoprotein cholesterol concentrations; and eGFR. Interestingly, the doses of insulin also did not differ significantly in these two groups. HbA1c was significantly lower in successfully switched than in unsuccessfully switched patients (7.1 ± 1.6 vs 9.0 ± 1.6%, P = 0.03; Table 1).
Table 1. Baseline characteristics of patients.
| Successfully switched (n = 16) | Unsuccessfully switched (n = 5) | P‐value | |
|---|---|---|---|
| Age (years) | 59.7 ± 12.5 | 62.6 ± 13.4 | 0.66 |
| Sex (male/female) | 15/1 | 3/2 | 0.13 |
| Bodyweight (kg) | 72.5 ±16.4 | 60.0 ±15.3 | 0.15 |
| Body mass index | 26.4 ± 8.4 | 24.0 ± 3.9 | 0.55 |
| Insulin (units) | 25.9 ± 26.9 | 33.8 ± 11.0 | 0.54 |
| Duration (years) | 21.9 ± 10.6 | 20.2 ± 12.5 | 0.77 |
| T‐cho (mg/dL) | 200.8 ± 67.8 | 222.0 ± 35.9 | 0.52 |
| TG (mg/dL) | 197.4 ± 138.9 | 190.2 ± 124.5 | 0.92 |
| HDL (mg/dL) | 45.7 ± 21.0 | 51.4 ± 11.6 | 0.57 |
| eGFR (mL/min/1.73 m2) | 26.1 ± 14.6 | 19.8 ± 8.1 | 0.38 |
| HbA1c (%) | 7.1 ± 1.6 | 9.0 ± 1.6 | 0.03* |
Paired Student's t‐tests were used to compare values at different time‐points. *Values are expressed as means ± standard deviation, with P < 0.05 considered statistically significant. eGFR, estimated glomerular filtration rate; HDL, high density lipoprotein; LDL, low density lipoprotein; T‐cho, total cholesterol; TG, triglyceride.
β‐Cell Function
Comparisons of successfully and unsuccessfully switched patients showed no significant differences in CPR index (2.20 ± 1.48 vs 0.9 ± 0.65, P = 0.074), urinary C‐peptide (47.8 ± 36.4 vs 44.1 ± 40.2 μg/day, P = 0.85), ΔCPR 6 min after glucagon load (2.0 ± 1.25 vs 0.9 ± 0.72 ng/mL, P = 0.07) and II (0.1 ± 0.12 vs 0.04 ± 0.04, P = 0.21). ΔCPR 120 min after a 75‐g oral glucose load was significantly greater in successfully than unsuccessfully switched patients (5.2 ± 2.81 vs 1.2 ± 0.65 ng/mL, P = 0.006). The lowest 120 min ΔCPR in successfully switched patients was 2.4 ng/mL, and the highest in unsuccessfully switched patients was 1.6 ng/mL (Figure 1).
Figure 1.
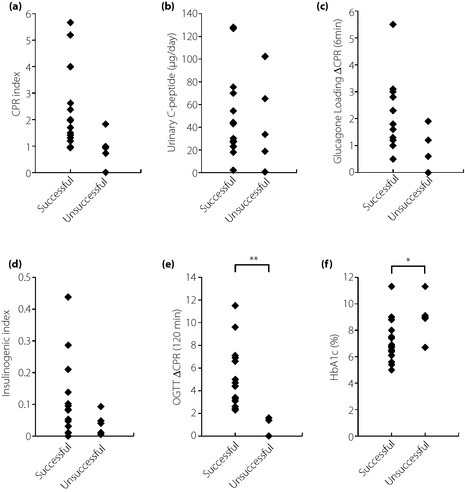
Baseline β‐cell function and glycated hemoglobin (HbA1c) in patients successfully and unsuccessfully switched from insulin to liraglutide. (a) C‐peptide immunoreactivity (CPR) index. (b) 24‐h urinary C‐peptide. (c) ΔCPR 6 min after a 1‐g glucagon load. (d). Insulinogenic index at 30 min. (e) ΔCPR 120 min after a 75‐g glucose load. (f) HbA1c (National Glycohemoglobin Standardization Program). *P < 0.05, **P < 0.01. OGTT, oral glucose tolerance test.
Follow up of Successful and Unsuccessful Switching to Liraglutide Monotherapy
In successfully switched patients, the mean blood glucose concentrations over three consecutive days after reaching the final dose of liraglutide were lower than before switching to liraglutide. In particular, mean blood glucose concentrations were significantly lower BB (123.9 ± 25.4 vs 109.6 ± 20.0 mg/dL, P = 0.010), AB (210.0 ± 53.3 vs 157.6 ± 26.7 mg/dL, P = 0.001), before lunch (BL; 178.9 ± 60.4 vs 119.5 ± 23.1 mg/dL, P = 0.001) and after dinner (AD; 200.4 ± 42.7 vs 159.9 ± 22.8 mg/dL, P = 0.002). In unsuccessfully switched patients, however, blood glucose concentrations BD increased significantly (188.4 ± 32.1 vs 301.6 ± 95.7 mg/dL, P = 0.038). Before switching to liraglutide, blood glucose concentrations in successfully and unsuccessfully switched patients did not differ significantly BB (123.9 ± 25.4 vs 119.0 ± 13.5 mg/dL, P = 0.69) AB (210.0 ± 53.3 vs 245 ± 32.8 mg/dL, P = 0.18), AL (181.7 ± 77.9 vs 240.4 ± 54.2 mg/dL, P = 0.14) and AD (200.4 ± 42.7 vs 231.4 ± 57.0 mg/dL, P = 0.20). However, blood glucose concentrations BL (178.9 ± 60.4 vs 259.6 ± 50.6 mg/dL, P = 0.01) and BD (121.1 ± 48.1 vs 188.4 ± 32.1 mg/dL, P = 0.01) differed significantly in the two groups (Figure 2).
Figure 2.
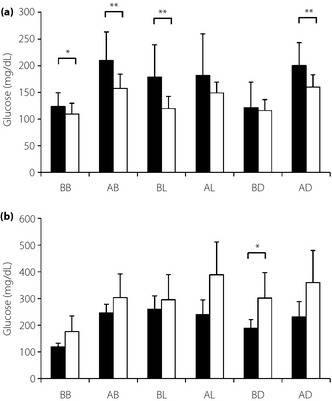
Daily blood glucose monitoring before (black bars) and after (white bars) switching from insulin to liraglutide monotherapy. (a) Successfully switched patients. (b) Unsuccessfully switched patients. Measurement times were 2 h after meals. *P < 0.05, **P < 0.01. AB, after breakfast; AD, after dinner; AL, after lunch; BB, before breakfast; BD, before dinner; BL, before lunch.
HbA1c did not differ significantly before and 24 weeks after successful switching in the 10 patients who were switched successfully (Figure 3).
Figure 3.
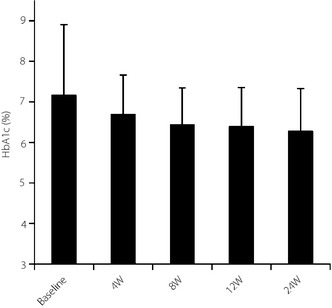
Glycated hemoglobin (HbA1c; National Glycohemoglobin Standardization Program) in successfully‐switched patients. W, weeks.
Successfully switched patients showed clinically significant reductions in systolic (142.0 ± 19.2 vs 123.6 ± 13.2 mmHg, P = 0.001) and diastolic (79.6 ± 13.4 vs 71.8 ± 9.8 mmHg, P = 0.036) blood pressure (BP) after switching. No significant changes in systolic or diastolic blood BP were observed in unsuccessfully switched patients. Before switching to liraglutide, systolic BP (142.0 ± 19.2 vs 143.2 ± 15.0 mmHg, P = 0.95) and diastolic BP (79.6 ± 13.4 vs 71.4 ± 16.7 mmHg, P = 0.28) did not differ significantly between successfully and unsuccessfully switched patients.
Discussion
The UK Prospective Diabetes Study showed that intensive glycemic control prevents microangiopathy in patients with type 2 diabetes28. However, the Action to Control Cardiovascular Risk in Diabetes Study Group (ACCORD) and the Advance Collaborative Group reported that intensive glycemic control does not reduce major macrovascular complications. Surprisingly, in the ACCORD trial, the mortality rate was significantly higher in the intensive than in the standard therapy group29. Therefore, treatments that less frequently induce hypoglycemia while improving glycemic control are more desirable, especially for type 2 diabetic patients with diabetic complications. The present study showed that liraglutide improves glycemic control without serious adverse effects, including hypoglycemia, in diabetic patients with renal impairment. Furthermore, switching to liraglutide from insulin worsened glycemic control in just five of 21 patients. These results suggest that liraglutide is an effective antidiabetic agent for type 2 diabetic patients with renal impairment.
The five patients who were unsuccessfully switched to liraglutide all had a long duration of diabetes mellitus and had received oral antidiabetic therapy immediately after diagnosis. Furthermore, all five were negative for anti‐glutamic acid decarboxylase and anti‐islet antigen 2 antibodies, indicating that none had type 1 diabetes.
Serum C‐peptide concentration has been reported to be a poor indicator of β‐cell function in type 2 diabetic patients with nephropathy, because the kidneys are the major sites of C‐peptide catabolism31. We found that CPR index and urinary C‐peptide were not useful predictors of successful switching from insulin to liraglutide in patients with renal impairment. In addition, CPR index and urinary C‐peptide have also been reported to be unrelated to β‐cell function in patients receiving complex insulin therapy. In such patients, glucagon or glucose load tests are more useful in evaluating insulin secretion. Although we observed no difference in glucagon test results between patients successfully and unsuccessfully switched to liraglutide, we found that oral glucose tolerance tests were useful in distinguishing between these two groups. ΔCPRs at 120 min were greater than 2.4 ng/mL in successfully switched patients, but lower than 1.6 ng/mL in unsuccessfully switched patients, suggesting that the cut‐off between the two is probably close to these concentrations. No significant correlations were observed between eGFR and ΔCPR at 120 min (data not shown). Interestingly, II did not differ significantly between successfully and unsuccessfully switched patients, suggesting that early insulin secretion is not important in switching to liraglutide. In contrast, although mean HbA1c differed significantly between these two groups, logistic regression analyses before and after adjustment for age and sex found that HbA1c concentration was not an independent predictor of successfully switched patients; the crude odds ratio was 0.59 (95% confidence interval 0.25–1.03, P = 0.06) and the age‐ and sex‐adjusted odds ratio was 0.38 (95% confidence interval 0.13–1.15, P = 0.09). Furthermore, CPR120 min was not correlated with HbA1c concentration (r = −0.115, P = 0.64), indicating that CPR120 min was a marker that independently predicted ‘successful switching’ independent of HbA1c concentration. Patients with impaired renal function generally require less insulin, mainly because their insulin clearance is prolonged32. We observed a very high rate of successful switching from insulin to liraglutide (76%, 16/21 patients). In patients without renal impairment, the rate of successful switching was reported to be 56%, although these patients had a shorter duration of diabetes than ours24. Thus, patients with renal impairment might require less autosecreted insulin to maintain good glycemic control than do patients without renal impairment, suggesting that liraglutide is appropriate for patients with renal impairment.
Clinical trials have shown that patients treated with 1.8 mg liraglutide experience decreases in systolic BP from baseline to 26 weeks of 2–5.6 mmHg, compared with decreases of 0.9–1.8 mmHg in patients receiving placebo33. The mechanism by which liraglutide reduces BP is unclear, but it might be through increased natriuresis38. It is difficult to assess the effect of natriuresis in patients with renal impairment, because almost all of these patients take diuretics. We found that patients who successfully switched from insulin to liraglutide experienced significant decreases in systolic and diastolic BP, suggesting that liraglutide monotherapy also reduces BP in patients with renal impairment.
In conclusion, the present findings showed that oral glucose‐stimulated serum C‐peptide should be measured when considering switching from insulin therapy to liraglutide monotherapy in Japanese type 2 diabetes patients with renal impairment. The distribution of CPR 120 min in the successfully switched group was completely separated from that in the unsuccessfully switched group, precluding receiver operating characteristic analysis. The highest CPR 120 min in the ‘unsuccessful’ group was 2.4 ng/mL, whereas the lowest CPR 120 min in the ‘successful’ group was 1.6 ng/mL, making the cut‐off somewhere between these two concentrations. Receiver operating characteristic curve analysis showed that a cut‐off value for CPR 120 min of 1.95 could optimally distinguish between successfully and unsuccessfully switched patients. Clinically, we recommended a cut‐off of 1.95 ng/mL. However, the present study was limited by the small number of patients. Larger studies are required to confirm the cut‐off value. In addition, the relationship between oral glucose‐stimulated C‐peptide and postprandial C‐peptide is unknown. Further studies are required to clarify the most useful predictor of successful switching.
Acknowledgments
The authors acknowledge the assistance of Mariko Soumura, Ayano Takagi and Shinya Ono at Shiga University of Medical Science. The authors declare no conflict of interest.
J Diabetes Invest 2014; 5: 435–441
References
- 1.Retnakaran R, Cull CA, Thorne KI, et al. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006; 55: 1832–1839 [DOI] [PubMed] [Google Scholar]
- 2.Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003; 63: 225–232 [DOI] [PubMed] [Google Scholar]
- 3.Parving HH, ØSterby R, Ritz E. Diabetic nephropathy. In: Brenner BM (ed). The Kidney. 6th edn. W.B.Saunders, Philadelphia, 2000; 1731–1773 [Google Scholar]
- 4.Ravid M, Savin H, Jutrin I, et al. Long‐term stabilizing effect of angiotensin‐converting enzyme inhibition on plasma creatinine and on proteinuria in normotensive type II diabetic patients. Ann Intern Med 1993; 118: 577–581 [DOI] [PubMed] [Google Scholar]
- 5.Nelson RG, Bennett PH, Beck GJ, et al. Development and progression of renal disease in Pima Indians with non‐insulin‐dependent diabetes mellitus. Diabetic Renal Disease Study Group. N Engl J Med 1996; 335: 1636–1642 [DOI] [PubMed] [Google Scholar]
- 6.Gaede P, Vedel P, Parving HH, et al. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet 1999; 353: 617–622 [DOI] [PubMed] [Google Scholar]
- 7.Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO‐HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet. 2000; 355: 253–259 [PubMed] [Google Scholar]
- 8.Chia CW, Egan JM. Incretin‐based therapies in type 2 diabetes mellitus. J Clin Endocrinol Metab 2008; 93: 3703–3716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drucker DJ, Nauck MA. The incretin system: glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696–1705 [DOI] [PubMed] [Google Scholar]
- 10.Linnebjerg H, Kothare PA, Park S, et al. Effect of renal impairment on the pharmacokinetics of exenatide. Br J Clin Pharmacol 2007; 64: 317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byetta(exenatide injection) Prescribing Information. Available from: http://pi.lilly.com/us/byetta-pi.pdf
- 12.Knudsen LB, Nielsen PF, Huusfeldt PO, et al. Potent derivatives of glucagon‐like peptide‐1 with pharmacokinetic properties suitable for once daily administration. J Med Chem 2000; 43: 1664–1669 [DOI] [PubMed] [Google Scholar]
- 13.Agerso H, Jensen LB, Elbrond B, et al. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long‐acting GLP‐1 derivative, in healthy men. Diabetologia 2002; 45: 195–202 [DOI] [PubMed] [Google Scholar]
- 14.Helleberg K, Malm‐Erjefält M, Bjørnsdottir I, et al. Metabolism and excretion of[Pal‐3H]‐liraglutide in human healthy subjects. Diabetes 2008; 57(Suppl.1): A581 [Google Scholar]
- 15.Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD‐3 Mono): a randomised, 52‐week, phase III, double‐blind, parallel‐treatment trial. Lancet 2009; 373: 473–481 [DOI] [PubMed] [Google Scholar]
- 16.Vilsboll T. Liraglutide: a once‐daily GLP‐1 analogue for the treatment of type 2 diabetes mellitus. Expert Opin Investig Drugs 2007; 16: 231–237 [DOI] [PubMed] [Google Scholar]
- 17.Juhl CB, Hollingdal M, Sturis J, et al. Bedtime administration of NN2211, a long‐acting GLP‐1 derivative, substantially reduces fasting and postprandial glycemia in type 2 diabetes. Diabetes 2002; 51: 424–429 [DOI] [PubMed] [Google Scholar]
- 18.Chang AM, Jakobsen G, Sturis J, et al. The GLP‐1 derivative NN2211 restores beta‐cell sensitivity to glucose in type 2 diabetic patients after a single dose. Diabetes 2003; 52: 1786–1791 [DOI] [PubMed] [Google Scholar]
- 19.Degn KB, Juhl CB, Sturis J, et al. One week's treatment with the long‐acting glucagon‐like peptide 1 derivative liraglutide (NN2211) markedly improves 24‐h glycemia and alpha‐ and beta‐cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes 2004; 53: 1187–1194 [DOI] [PubMed] [Google Scholar]
- 20.Madsbad S, Brock B, Perrild H, et al. Liraglutide significantly improves first‐phase insulin secretion and maximal beta‐cell secretory capacity. Diabetologia 2006; 49(Suppl. 1): A0004 [Google Scholar]
- 21.Vilsboll T, Brock B, Perrild H, et al. Liraglutide, a once‐daily human GLP‐1 analogue, improves pancreatic B‐cell function and arginine‐stimulated insulin secretion during hyperglycaemia in patients with Type 2 diabetes mellitus. Diabet Med 2008; 25: 152–156 [DOI] [PubMed] [Google Scholar]
- 22.Courreges JP, Vilsboll T, Zdravkovic M, et al. Beneficial effects of once‐daily liraglutide, a human glucagon‐like peptide‐1 analogue, on cardiovascular risk biomarkers in patients with Type 2 diabetes. Diabet Med 2008; 25: 1129–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobsen LV, Hindsberger C, Robson R, et al. Effect of renal impairment on the pharmacokinetics of the GLP‐1 analogue liraglutide. Br J Clin Pharmacol 2009; 68: 898–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwao T, Sakai K, Sata M. Postprandial serum C‐peptide is a useful parameter in the prediction of successful switching to liraglutide monotherapy from complex insulin therapy in Japanese patients with type 2 diabetes. J Diabetes Complications 2013; 27: 87–91 [DOI] [PubMed] [Google Scholar]
- 25.Imai E, Horio M, Nitta K, et al. Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease. Clin Exp Nephrol 2007; 11: 41–50 [DOI] [PubMed] [Google Scholar]
- 26.Seino Y, Nanjo K, Tajima N, et al. Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. J Diabetes Invest 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kashiwagi A, Kasuga M, Araki E, et al. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest 2012; 3: 39–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352: 837–853 [PubMed] [Google Scholar]
- 29.Hoogwerf BJ. Action to Control Cardiovascular Risk, Diabetes Study G. Does intensive therapy of type 2 diabetes help or harm? Seeking accord on ACCORD. Clevel Clin J Med 2008; 75: 729–737 [DOI] [PubMed] [Google Scholar]
- 30.Results of ADVANCE trial conducted in type 2 diabetic patients. Ter Arkh. 2008; 80: 88 (Russian). [PubMed] [Google Scholar]
- 31.Covic AM, Schelling JR, Constantiner M, et al. Serum C‐peptide concentrations poorly phenotype type 2 diabetic end‐stage renal disease patients. Kidney Int 2000; 58: 1742–1750 [DOI] [PubMed] [Google Scholar]
- 32.Weinrauch LA, Healy RW, Leland OS Jr, et al. Decreased insulin requirement in acute renal failure in diabetic nephropathy. Arch Intern Med 1978; 138: 399–402 [PubMed] [Google Scholar]
- 33.Marre M, Shaw J, Brandle M, et al. Liraglutide, a once‐daily human GLP‐1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD‐1 SU). Diabet Med 2009; 26: 268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)‐2 study. Diabetes Care 2009; 32: 84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human glucagon‐like peptide‐1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD‐4 Met+TZD). Diabetes Care 2009; 32: 1224–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell‐Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD‐5 met+SU): a randomised controlled trial. Diabetologia 2009; 52: 2046–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26‐week randomised, parallel‐group, multinational, open‐label trial (LEAD‐6). Lancet 2009; 374: 39–47 [DOI] [PubMed] [Google Scholar]
- 38.Gutzwiller JP, Tschopp S, Bock A, et al. Glucagon‐like peptide 1 induces natriuresis in healthy subjects and in insulin‐resistant obese men. J Clin Endocrinol Metab 2004; 89: 3055–3061 [DOI] [PubMed] [Google Scholar]


