Abstract
Aims/Introduction
The purpose of the study was to determine the feasibility and effect of a reward‐based, task‐setting strategy for low‐income outpatients with type 2 diabetes.
Materials and Methods
Indigent diabetes outpatients without glucometers were eligible to participate in this trial. A total of 132 cases were randomly recruited. Participants in group B used glucometers for self‐monitoring at no cost. Group A participants could keep the glucometers only if the glycosylated hemoglobin level declined compared with the baseline visit; for those not achieving a reduction in the glycosylated hemoglobin level, the glucometers would have to be returned. Group C served as the control group without self‐monitoring setout. Diabetes education was provided to all groups. Metabolic indices and self‐management were evaluated after 6 months of follow up.
Results
Group A had a significant decline in the glycosylated hemoglobin level (−0.97%) and medical costs (−159 yuan) compared with the baseline visit, whereas groups B and C had a decrease in the glycosylated hemoglobin levels alone (−0.62 and −0.57%, respectively). The body mass index did not change significantly in any group. There was a statistical difference in the glycosylated hemoglobin level of group A compared with groups B and C. Self‐management in group A improved the outcome relative to groups B and C.
Conclusions
This preliminary evidence suggests that the program is feasible, acceptable for improving patient self‐management, and cost‐effective in reducing the glycosylated hemoglobin level and medical costs.
Keywords: Low‐income outpatients, Reward‐based, Task‐setting
Introduction
Economic status is associated with the prevalence and glycemic control of patients with type 2 diabetes1. Indigent patients with diabetes are more prone than other groups to have worse outcomes and poor measures of control5. Thus, researchers should pay more attention to this population. In China, the prevalence of diabetes reached 9.7%, and there were over 92 million adults with diabetes in 20107. The cost of medical care is a critical barrier to treatment for patients of low socioeconomic status or no health insurance coverage in China8. Improving the outcomes of low socioeconomic status patients would therefore be beneficial to decrease the prevalence of diabetes and comorbidities, and to alleviate the economic burden.
Diabetes education plays an important role in glycemic control, preventing acute complications and reducing the risk of long‐term complications9. Various active methods (e.g., empowerment, motivational interviewing and goal‐setting) have been provided to patients with diabetes to improve their ability for self‐management. However, the American Diabetes Association has shown that there is no one ‘best’ diabetes education model that suits all patients10. Consequently, education of diabetes patients should be ‘targeted’ rather than universal, suggesting a different approach and content for different conditions. Currently, there are no uniquely effective diabetes education models for indigent patients with diabetes. The intrinsic characteristic of a practical dilemma, augmenting the risk for undesirable outcomes among low‐income patients, suggests that this target population has a specific need for interventions to inspire intrinsic factors to effectively engage in diabetes self‐management, leading to improved outcomes, including knowledge, understanding, skills and quality of life.
As low socioeconomic status is a significant barrier for glycemic control in indigent patients with type 2 diabetes, Bruni et al.11 reported that incentive‐based payment schemes had an active impact on general practitioner behavior; however, the empirical literature on financial incentives in healthcare has shown mixed testimony to support the effectiveness12. Johnson et al.14 suggested that providing glucometers for patients gratuitously was of limited clinical effectiveness for glycemic control in patients with type 2 diabetes. Furthermore, these studies were carried out on the general population instead of the target indigent group. In response to these premises, alternative strategies have been considered, as incentive schemes that do not condition remuneration on the achievement of presetting individual targets have drawbacks that cannot be overlooked. The lack of an association between health outcomes and financial rewards strongly attenuates the influence on patient behavior. The strategy that patients entailed to complete the task on healthcare for rewards rather than without compensation should be available, as it would motivate patients' intrinsic intentions to foster cooperation, insist on self‐management and change behaviors. Consequently, in the present work, a trial among indigent outpatients with type 2 diabetes was used to determine the impact of combining a reward (a glucometer, blood glucose test strips and lancets) with the task in diabetes control compared with subjects with a gratis reward without the task based on diabetes education. There is little evidence to provide guidance related to whether or not such a special program is feasible to improve self‐management, given the economic barriers that are present in this population.
Materials and Methods
Educational Program
The present trial used a variety of teaching methods, including group education, one‐on‐one counseling, and written materials (pamphlets) to disseminate diabetes knowledge and skills training. Diabetes pamphlets were dispensed at baseline. The trained leader delivered a lecture applicable to each group in the first and second month (1 h per course). The group education showed that diabetes education is a key part of the therapy, advocating appropriate activities and exercises, encouraging optimal health, psychological well‐being and relaxation, and appropriate diet, and conveying no smoking and glucose monitoring. A one‐on‐one control program for outpatients was provided in the third and fourth months that focused on reminding patients about behavioral change (i.e., self‐management of blood glucose, diet, exercise, emotions and medication adherence), open‐ended questioning, reflective listening, summarization, barrier solving in education and adjusting the dosage or switching to a different medication, if necessary. Other educators were in charge of recruiting patients through inclusion/exclusion criteria, and collecting and coordinating the data of participants. They informed all of the patients to participate in the group education course and one‐on‐one counseling by telephone. If patients had questions during the call, their efforts were also to create a concise diabetes education intervention that offered a counseling approach of diabetes knowledge and skills training; for example, helping outpatients identify barriers and promoting problem solving. The researchers were dedicated to assisting all participants in identifying realistic targets for behavioral changes, and offered encouragement and emotional support in self‐management plans.
Participants
Participants were recruited from the diabetes outpatients. The following inclusion criteria were used: aged between 30 and 70 years; diagnosed with type 2 diabetes >5 years; poorly controlled diabetes (glycosylated hemoglobin [HbA1c] ≥7.0% at the time of screening); income <2,000 yuan (approximately 300 USD) per month; no glucometers for glucose monitoring; and cared for by the same specialist for >3 months. The exclusion criteria were as follows: presence of serious diabetes complications; pregnancy; cancer; alcohol abuse; severe physiological or psychiatric disorders that would interfere with participation in the intervention; regular moderate physical activity; and previous group education.
Research Design
Participants were randomly assigned to one of three groups (group A, B or C) using a computer‐generated random numbers table. Then, the researchers concealed the sequence of random allocation. The specialists were blinded to participants with respect to types of medication, dosage and educational curriculum. One graduate student was responsible for each of the three groups. Diabetes educational manuals and guidance were created based on concepts developed by Channon et al.15
According to the study protocol, each participant in groups A and B received a glucometer. During the study, 30 diabetes test strips and lancets were offered for self‐monitoring per month. Group B participants were provided this equipment at no cost. Group A participants kept the glucometers and an additional 90 diabetes test strips and lancets, valued at approximately 300 USD, if the HbA1c level declined compared with the baseline visit; for those participants who did not achieve a reduction in the HbA1c level, the glucometers would have to be returned, and no additional diabetes test strips and lancets were provided as rewards. Patients had to return the equipment if they dropped out of the trial. Group A participants were expected to accomplish the task with adequate initiatives for the rewards. The researchers issued the glucometers, and 30 diabetes test strips and lancets at baseline. The remaining diabetes test strips and lancets were granted when patients attended the group education and one‐on‐one counseling, and were issued in the fifth and sixth months. Group C served as the control group and did not receive the equipment.
Outcome Measures
The researchers carried out an interview to record the participants' basic characteristics when consent was obtained. The laboratory measurements consisted of body mass index (BMI), cholesterol, triglycerides, low‐density lipoprotein cholesterol (LDL‐c) and last available HbA1c (mean = 45 days before recruitment), and were collected through clinical information systems by the graduate students. The researchers evaluated the ability of self‐management of patients through the Diabetes Self‐Care Scale (DSCS) at baseline. The DSCS was developed by Hurley and Shea16. Lee et al.17 evaluated the properties of the DSCS, and showed that respondent separation reliability was acceptable (0.80) and item separation reliability was high (0.99). Wang et al.18 showed that Cronbach's α was as high as 0.82, test–retest reliability reached 0.95 and the Kaiser–Meyer–Olkin value was 0.68. The scale consisted of six dimensions, including self‐management on exercise (four items), diet (six items), medication adherence (three items), self‐monitoring (four items), foot care (five items) and response to hyper‐ or hypoglycemics (four items). Each item used a five‐point scale to measure how often the participant developed self‐management. The frequency categories were: never, rarely, sometimes, often and always, and ranged from 1 to 5. The total score of self‐management ranged from 26 to 130, indicating the ability of the patients to maintain self‐management and to engage in diabetes self‐care activities. To analyze the results, the test score was switched to a standard score, according to the following formula:
According to the standard score, a value of ≥80 points was identified as good, 60–79 was considered general and <60 was poor. The researchers encouraged participants to describe any problem solving regarding the action plan and additional behavioral changes. The rate of attendance in programs was recorded by the researchers. Participants were expected to return their records monthly to the researchers, who reviewed the records on receipt and offered standardized feedback. The researchers would coordinate final data collections at the final visit.
Statistical Analysis
The demographic and diabetes‐related characteristics of the sample were assessed by descriptive statistics. One‐way anova was used for continuous variables, and a χ2‐test was used for categorical variables to compare baseline characteristics across the three groups. The degree of engagement in each intervention was reported as the percentage of participants attending the group education curriculum and one‐on‐one counseling sessions. The researchers measured 6‐month changes for each outcome in each group. The mean values and 6‐month changes in self‐management scale scores were presented. An intention‐to‐treat basis was utilized to analyze the test. The unadjusted values at 6 months and the calculated differences for each intervention group were shown.
The applicable conditions of one‐way anova met the homogeneity of variance arrhythmia monitored by the Levene statistic. The correction value of Brown–Forsythe and Welch was chosen to test the equality of means using spss 18.0 statistical software (SPSS Inc., Chicago, IL, USA). Post‐hoc tests were used to explain the difference between two groups. The statistical outcomes showed by Dunnett's T3 were used when unequal variances existed. To examine intervention effects, changes across the three groups were compared by two‐tailed tests with an α of 0.05.
Results
Figure 1 summarizes the flow of participants throughout each stage of the trial.
Figure 1.
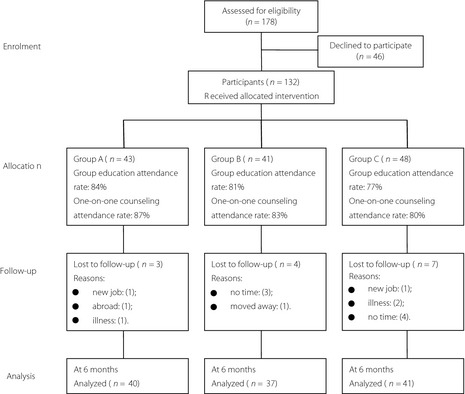
The flow of participants through each stage of the trial.
Participant Characteristics
Participants had a mean age of 58 years, greater than one‐half had health insurance, the minority was non‐smokers and the majority had not received a high‐school education. The females accounted for 56.1% of the study population. The mean number of outpatient visits (prior year) and medical costs were 14 and 1026 yuan, respectively. The mean HbA1c and BMI at enrolment were 8.27% and 25.05 kg/m2, respectively. The triglycerides, cholesterol and LDL‐c values were 1.72, 4.56 and 2.68 mmol/L, respectively. No statistical difference existed among the three groups regarding patient characteristics at baseline (Table 1).
Table 1. Characteristics of patients at baseline.
| Characteristics | All participants | Group A | Group B | Group C | P * |
|---|---|---|---|---|---|
| n | 132 | 43 | 41 | 48 | |
| Gender (female) | 56.1 | 55.8 | 53.7 | 58.3 | 0.91 |
| Mean age (years) | 57.56 ± 6.77 | 57.07 ± 6.84 | 56.83 ± 6.33 | 58.63 ± 7.06 | 0.39 |
| Duration of diabetes (years)‡ | 5.38 ± 1.99 | 5.40 ± 1.85 | 5.59 ± 1.94 | 5.18 ± 2.15 | 0.64 |
| Smoking† | 13.6 | 14.0 | 12.2 | 14.6 | 0.95 |
| <high school education† | 83.3 | 83.7 | 85.4 | 81.3 | 0.87 |
| Insurance† | 80.3 | 79.1 | 80.5 | 81.2 | 0.97 |
| Income (yuan)‡ | 1538 ± 255 | 1518 ± 266 | 1526 ± 261 | 1566 ± 241 | 0.62 |
| Medical costs (yuan/month)‡ | 1026 ± 127 | 1035 ± 111 | 1023 ± 141 | 1019 ± 129 | 0.84 |
| HbA1c (%)‡ | 8.27 ± 0.67 | 8.30 ± 0.70 | 8.21 ± 0.62 | 8.20 ± 0.69 | 0.80 |
| BMI (kg/m2)‡ | 25.05 ± 0.97 | 25.11 ± 1.05 | 24.96 ± 0.98 | 25.06 ± 0.92 | 0.78 |
| Triglycerides (mmol/L)‡ | 1.72 ± 0.82 | 1.74 ± 0.76 | 1.70 ± 0.85 | 1.71 ± 0.85 | 0.97 |
| Cholesterol (mmol/L)‡ | 4.56 ± 0.82 | 4.53 ± 0.82 | 4.57 ± 0.87 | 4.59 ± 0.80 | 0.94 |
| LDL‐c (mmol/L)‡ | 2.68 ± 0.34 | 2.70 ± 0.42 | 2.68 ± 0.25 | 2.66 ± 0.37 | 0.84 |
*P for overall difference between groups based on one‐way anova for continuous variables, χ2 tests for categorical variables. †Proportion %. ‡Mean ± Standard deviation
Engagement with Interventions
Group A participants had higher attendance at group education (84%) and one‐on‐one counseling (87%) than the other participants, whereas the mean number of attendances did not differ between the three groups. Of all the participants, 14 failed to complete follow‐up interventions before the end of the study. The main reason for loss to follow up was time constraints. Three participants were lost to follow up because of serious illness (i.e., acute heart failure, acute myocardial infarction and stroke). Three participants were lost to follow‐up in group A, leaving 40 participants completing the research; 37 and 41 participants remained in groups B and C at the end‐point, respectively. The drop‐out rate at the 6‐month visit, however, did not differ between the groups. The values for metabolic outcomes, mean medical costs and BMI were available for 89% of the participants.
Clinical Events of the End‐Point
The average time each patient in groups A and B self‐monitored was 135 and 127 days, respectively. The frequency of blood glucose monitoring in group C was calculated to be 24 days per patient during the 6‐month follow up. A greater percentage of group A participants engaged the four programs (88.4%), compared with 80.5% and 77.1% in groups B and C, respectively. Furthermore, there was no significant difference amongst groups A, B and C in the amount of feedback provided. In a comparison of the time involved in calling the patients for participation in the program, the mean time expended by groups A, B, and C was 7.4, 2.5 and 2.2 min, respectively. Participants in group A expressed more interest and desire to learn knowledge and skills of diabetes than the other groups. The activity of glycemic control was more significant in group A. The mean blood glucose levels were high during Chinese traditional festivals and feasts as a result of poor dietary control in all three groups. Seven participants did not achieve a lower HbA1c level in group A at the end‐point. Thus, the glucometers were returned, and no additional diabetes test strips and lancets were provided, according to the protocol. Severe hypoglycemia, defined as requiring assistance from another person, did not occur.
Effects on Metabolic Outcomes
Each group had a significant reduction in the HbA1c level compared with their corresponding baseline scores after 6 months. Finally, 33 patients (77%) had a reduction in HbA1c compared with the baseline in group A. There were 19, 22, and 26 patients with HbA1c levels >7% at the end point in groups A, B and C, respectively. Notably, group A had a decline in the HbA1c level of 0.97% compared with the baseline value, and similar improvements were observed in groups B and C (−0.62 and −0.57%, respectively), with a significant decline compared with the baseline data (P < 0.00). The HbA1c level was significantly different in group A compared with groups B and C (P < 0.05) based on mean percentage differences in the HbA1c level, as the baseline values differed between groups, whereas there was no statistical significance between groups B and C (7.60 ± 0.51% vs 7.73 ± 0.57%, P > 0.05). There were no significant changes in BMI for any group, although the levels had dropped compared with the baseline values. Table 2 shows no significant changes in lipids across the three groups.
Table 2. Comparison of 6‐month follow‐up outcomes†.
| Metabolic indicators | Results | 95% Confidence interval | P | |
|---|---|---|---|---|
| Relative to Group C | Group A vs Group B | |||
| HbA1c (%) | ||||
| Group A | 7.29 ± 0.58 | −0.45 (−0.69 to −0.20) | 0.01 | |
| Group B | 7.60 ± 0.51 | −0.13 (−0.38 to −0.12) | 0.29 | |
| Group C | 7.73 ± 0.57 | −0.31 (−0.56 to −0.06) | 0.02 | |
| Medical cost (yuan) | ||||
| Group A | 881 ± 95 | −119 (−163 to −14) | 0.01 | |
| Group B | 991 ± 103 | −8 (−37 to 53) | 0.72 | |
| Group C | 1000 ± 105 | −111 (−156 to −64) | 0.01 | |
| BMI (kg/m2) | ||||
| Group A | 25.05 ± 0.92 | 0.14 (−0.24 to 0.52) | 0.47 | |
| Group B | 24.96 ± 0.82 | 0.05 (−0.34 to 0.44) | 0.79 | |
| Group C | 24.91 ± 0.86 | 0.88 (−0.30 to 0.48) | 0.66 | |
| Triglycerides (mmol/L) | ||||
| Group A | 1.68 ± 0.55 | −0.11 (−0.42 to 0.19) | 0.47 | |
| Group B | 1.66 ± 0.77 | −0.14 (−0.45 to 0.18) | 0.39 | |
| Group C | 1.79 ± 0.74 | 0.03 (−0.29 to 0.34) | 0.87 | |
| Cholesterol (mmol/L) | ||||
| Group A | 4.53 ± 0.52 | −0.10 (−0.37 to 0.17) | 0.48 | |
| Group B | 4.60 ± 0.63 | −0.04 (−0.31 to 0.24) | 0.78 | |
| Group C | 4.64 ± 0.68 | −0.06 (−0.33 to 0.22) | 0.68 | |
| LDL‐C (mmol/L) | ||||
| Group A | 2.64 ± 0.40 | −0.08 (−0.23 to 0.08) | 0.28 | |
| Group B | 2.73 ± 0.32 | 0.01 (−0.15 to 0.16) | 0.92 | |
| Group C | 2.72 ± 0.31 | −0.09 (−0.25 to 0.06) | 0.25 | |
†95% Confidence Interval and P for overall difference between groups based on one‐way ANOVA for continuous variables, chi‐square for categorical variables, and further Post Hoc Tests for revealing the difference between two groups. Number of participants in groups A, B and C was 40, 37 and 41, respectively.
Effects on Medical Costs
Medical costs included the fees of insulin, oral hypoglycemic drugs and diabetes laboratory measurements. The expense of laboratory measurements in hospitals decreased in groups A and B because of the use of glucometers at no cost. Medical costs decreased in groups B and C compared with the baseline visit, but did not reach a significant difference (P > 0.05). Importantly, there was a significant reduction in group A (−159 yuan) compared with groups B and C (P < 0.01); the patients in group A saved a year of medical expenses equal to the value of a glucometer, diabetes test strips and lancets.
Effects on Self‐Management
Table 3 shows the results of self‐management. The total score of self‐management among groups A, B and C showed no statistical difference at baseline. Self‐management of patients identified as good was 9.3, 9.7, and 12.5% in groups A, B and C, respectively. Most patients had general self‐management capacity (69.8, 70.7 and 66.7%, respectively). In these six dimensions, medication adherence had the highest score (88.7, 90.7 and 90.0, respectively). The minimum score was in the self‐monitoring dimension regarded as poor (48.5, 50.1 and 50.1, respectively).
Table 3. Score of three groups on self‐management.
| Dimension | Time | Group A (n = 40) | Group B (n = 37) | Group C (n = 41) | P |
|---|---|---|---|---|---|
| Total score of self‐management | Baseline | 84.0 ± 9.3 | 85.0 ± 7.5 | 85.2 ± 5.2 | 0.72 |
| Follow‐up | 99.3 ± 4.1 | 96.6 ± 5.3 | 92.4 ± 4.2 | 0.00* | |
| Dietary | Baseline | 18.5 ± 3.1 | 18.4 ± 3.2 | 19.1 ± 2.6 | 0.49 |
| Follow‐up | 21.5 ± 2.9 | 20.9 ± 3.3 | 19.8 ± 2.2 | 0.03** | |
| Exercise | Baseline | 13.9 ± 2.3 | 14.3 ± 2.1 | 14.0 ± 1.8 | 0.72 |
| Follow‐up | 17.1 ± 1.4 | 15.6 ± 1.9 | 15.6 ± 1.6 | 0.00*** | |
| Medicine adherence | Baseline | 13.3 ± 1.0 | 13.6 ± 1.3 | 13.5 ± 1.1 | 0.56 |
| Follow‐up | 14.0 ± 0.9 | 14.1 ± 0.8 | 14.1 ± 0.9 | 0.89 | |
| Self‐monitoring | Baseline | 9.7 ± 2.9 | 10.2 ± 2.4 | 10.2 ± 2.6 | 0.61 |
| Follow‐up | 14.8 ± 2.2 | 14.3 ± 2.1 | 10.7 ± 2.5 | 0.00**** | |
| Foot care | Baseline | 15.5 ± 1.8 | 15.9 ± 2.1 | 15.2 ± 1.6 | 0.21 |
| Follow‐up | 16.3 ± 2.3 | 16.5 ± 2.3 | 16.5 ± 2.4 | 0.86 | |
| Care on hyper‐ or hypo‐glycemics | Baseline | 13.1 ± 2.1 | 12.7 ± 2.2 | 13.2 ± 1.6 | 0.46 |
| Follow‐up | 15.5 ± 1.5 | 15.3 ± 1.7 | 15.6 ± 1.9 | 0.67 |
*groups A and B vs C, P < 0.05; group B vs group A, P < 0.05. **Levene statistic shows F = 3.80, P < 0.05. Brown‐Forsythe correction value, P < 0.05; Welch correction value correction value, P < 0.05. Dunnett's T3 shows group A vs group C, P < 0.05. ***group A vs groups B and C, P < 0.01. ****groups A and B vs group C, P < 0.01.
At the end of the study, the total score of self‐management had an improved outcome in the three groups. The percentage of good self‐management was 16.3, 14.6, and 16.7% in groups A, B and C, respectively. In addition, there was a significant difference in group A compared with groups B and C (P < 0.05); group B was statistically different compared with group C (P < 0.01). The comparison between groups A and C was statistically different in the dietary dimension (P < 0.05). In contrast, participants in group A reported improvements in the exercise dimension relative to groups B and C (P < 0.01). Participants in groups A and B had similar improvements in the self‐monitoring dimension relative to group C participants (P < 0.01), with no significant differences existing between participants in groups A and B. No significant increase occurred in the foot care and care on hyper‐ or hypoglycemics dimensions between the three groups. Patients in groups A and B had significant changes in the self‐monitoring dimension identified as general, whereas group C participants with no improvement were considered as poor.
Discussion
Patients living in poverty have difficulty planning their daily life (including meals and physical activities) and making use of costly medications. Therefore, those patients might benefit from adjusted strategies of intervention, such as reward‐based, task‐setting and repeated specific, rather than general guidelines, for diabetes self‐management. The current trial confirmed the feasibility of an incentive and reward‐based scheme in reducing medical costs, indicating broad acceptance and improvement in blood glucose control in low‐income patients with uncontrolled diabetes, as the content was tailored to meet the participants' common needs and adapted as necessary for socioeconomic status.
A significant decrease in the HbA1c level, medical costs and improved self‐management in group A compared with the other groups showed that the model of reward‐based, task‐setting appeared effective for improving patient outcomes and decreasing the burden of diabetes treatment compared with the other groups. This strategy may be implemented by affecting the patients' psychological changes. Group A was rewarded a glucometer, diabetes test strips and lancets for achieving primary goals (i.e., a reduction in HbA1c value). To obtain the rewards, the participants had to change behaviors with regular self‐management. Thereby, the participants were encouraged to carry out self‐management through diabetes education. The psychological changes had a positive impact, including an increase in self‐awareness and the enhancement of mental effort directed to accomplishment of tasks19. Diabetes educational programs incorporating behavioral and psychosocial strategies could produce improved outcomes. In addition, providing socioeconomic support, and lower glycemic task‐setting might evoke participant inherent motivation, facilitating the conduct of ongoing self‐management. Psychological factors related to diabetes self‐management are emotional factors, such as depression, attitude, beliefs, self‐motivation and cognitive ability22. Those with greater beliefs were more committed to their target and were more content with their performance. Furthermore, a positive attitude linked specifically to diabetes was associated with specific self‐management behaviors26. The financial incentive as a reward resulted in a positive attitude (e.g., higher motivation and better self‐regulation) than more temporally distant, long‐term goals25, and produced higher goal commitment and fostered enthusiasm amongst the participants. Diabetes is a patient‐managed disease. Patients, not providers, implement the majority of daily actions (e.g., choices in diet and physical activity, and self‐monitoring) regarding diabetes care. The reward‐based, task‐setting strategy is rooted in the extrinsic inspiration eliciting self‐initiative opinion, sharing a common characteristic of patient‐oriented self‐management, and promoting collaborative goals in the form of behavioral ‘action plans’, wherein participants assess capability, and adjust monitoring progress and the strategy as required. The innovative method reduced patient ambivalence, improved the perceptions of the importance of self‐management, helped patients recover from disappointment, encouraged self‐incentives and maintenance of behavioral modification, and promoted good therapeutic compliance. External motivation, rewards with tasks, combined the intrinsic drive, psychological changes, induced positive outcomes in glycemic control and self‐management, which was recognized as the determinant of indigent patients' decision to change behaviors.
Previous studies showed that reward strategies without requirements given for behavior changes had no treatment effects, but goal‐setting was an effective strategy27. Group A had a significant difference in the total score of self‐management and HbA1c compared with group B. This result shows that the strategy of rewards with task‐setting had a more significant impact than merely delivering financial incentives. Group A participants had a significant improvement in the exercise and dietary dimensions, showing that they were driven to reduce the blood glucose levels. Group A did not show better self‐management in dietary change compared with group B, which might relate to the period that the trial was implemented (autumn and winter). Those days included many Chinese traditional festivals and feasts, filled with delicious sweets, greasy foods and wine, and the freedom to enjoy food plays an important role in one's quality of life in the Chinese culture19, and served as barriers preventing the participants from engaging in diabetes self‐management of dietary control. This phenomenon corresponded to the feedback reflecting elevated blood glucose levels. Herein, the education would emphasize a healthy diet during Chinese traditional festivals, and encouraged participants to adhere to the treatment regimen to prevent self‐indulgence in diversified feasts in the future. As in the present trial, previous studies showed that additional glucometer use was of limited clinical effect on improving glycemic control in diabetes patients22. However, the use of glucose monitoring by groups A and B enhanced an enthusiasm for self‐management, and made diabetes patients more effectively manage their condition, detect hyperglycemia or hypoglycemia, and receive information for medication adjustment, dietary content and physical exercises.
Participants had preferable changes after the intervention. However, changed behaviors easily regress to previous habits, and good educational outcomes are hard to sustain29. Thus, a lifelong management intervention had to encourage patients' efforts in achieving and maintaining self‐management goals in a ‘real‐world’ setting, whereas ongoing diabetes self‐management support is necessary to effectively manage diabetes and sustain changed behaviors10. The results of the present study can help target public health measures in deprived regions. As the government plays a prominent role in diabetes management and care30, the medical insurance policy should offer long‐term reward‐based blood glucose test strips and lancets to patients according to the reduction in HbA1c levels to encourage behavioral changes and maintenance. Thus, the ongoing management intervention following a reward‐based medical insurance approach is patient‐driven, and flexible to individual needs, priorities and ‘real‐life’ conditions.
Coupling socioeconomic support with reward‐based task‐setting in China might be an effective strategy for imparting the skills necessary for diabetes self‐management. When patients accept reward‐based goals and commit themselves to attaining the goals, the benefits are as strong as goal achievement. This preliminary evidence suggests that the program was feasible, acceptable to improve patients' self‐management, and cost‐effective in reducing HbA1c levels and medical costs.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81170773 to Zhongming Wu, and 81070645 to Demin Yu) and from Tianjin Natural Science Foundation (12JCYBJC17700 to Jingna Lin). The authors declare that they have no conflict of interest.
J Diabetes Invest 2014; 5: 410–417
References
- 1.Gnavi R, Karaghiosoff L, Costa G, et al. Socio‐economic differences in the prevalence of diabetes in Italy: the population‐based Turin study. Nutr Metab Cardiovasc Dis 2008; 18: 678–682 [DOI] [PubMed] [Google Scholar]
- 2.Maier W, Holle R, Hunger M, et al. The impact of regional deprivation and individual socio‐economic status on the prevalence of type 2 diabetes in Germany. A pooled analysis of five population‐based studies. Diabet Med. 2013; 30: e78–86 [DOI] [PubMed] [Google Scholar]
- 3.Rabi DM, Edwards AL, Southern DA, et al. Association of socio‐economic status with diabetes prevalence and utilization of diabetes care services. BMC Health Serv Res 2006; 6: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fano V, Pezzotti P, Gnavi R, et al. The role of socio‐economic factors on prevalence and health outcomes of persons with diabetes in Rome, Italy. Eur J Public Health 2013. doi: 10.1093/eurpub/cks168 [DOI] [PubMed] [Google Scholar]
- 5.Leone T, Coast E, Narayanan S, et al. Diabetes and depression comorbidity and socio‐economic status in low and middle income countries (LMICs): a mapping of the evidence. Global Health 2012; 8: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peek ME, Cargill A, Huang ES. Diabetes health disparities: a systematic review of health care interventions. Med Care Res Rev 2007; 64: S101–S156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang WY, Lu JM, Weng JP, et al. Prevalence of diabetes among men and women in China. N Engl J Med 2010; 362: 1090–1101 [DOI] [PubMed] [Google Scholar]
- 8.Lou Q, Wu L, Dai X, et al. Diabetes education in mainland China—A systematic review of the literature. Patient Educ Couns 2011; 85: 336–347 [DOI] [PubMed] [Google Scholar]
- 9.Ellis SE, Speroff T, Dittus RS, et al. Diabetes patient education: a meta‐analysis and meta‐regression. Patient Educ Couns 2004; 52: 97–105 [DOI] [PubMed] [Google Scholar]
- 10.Funnell MM, Brown TL, Childs BP, et al. National standards for diabetes self‐Management education 2012. Diabetes Care 2012; 35: S101–S108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruni ML, Nobilio L, Ugolini C. Economic incentives in general practice: the impact of pay‐for‐participation and pay‐for‐compliance programs on diabetes care. Health Policy 2009; 90: 140–148 [DOI] [PubMed] [Google Scholar]
- 12.Frølich A, Talavera JA, Broadhead P, et al. A behavioural modelo f clinician responses to incentives to improve quality. Health Policy 2007; 80: 179–193 [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal MB, Frank RG. What is the empirical basis for paying for quality in health care? Med Care Res Rev 2006; 63: 135–157 [DOI] [PubMed] [Google Scholar]
- 14.Johnson JA, Majumdar SR, Bowker SL, et al. Self‐monitoring in type 2 diabetes: a randomized trial of reimbursement policy. Diabet Med 2006; 23: 1247–1251 [DOI] [PubMed] [Google Scholar]
- 15.Channon SJ, Huws‐Thomas MV, Rollnick S, et al. A multicenter randomized controlled trial of motivational interviewing in teenagers with diabetes. Diabetes Care 2007; 30: 1390–1395 [DOI] [PubMed] [Google Scholar]
- 16.Hurley AC, Shea CA. Self‐efficacy: strategy for enhancing diabetes self‐care. Diabetes Educ 1992; 18: 146–150 [DOI] [PubMed] [Google Scholar]
- 17.Lee NP, Fisher WP. Evaluation of the Diabetes Self‐care Scale. J Appl Meas 2005; 6: 366–381 [PubMed] [Google Scholar]
- 18.Wang JX, Wang RX, Ling QJ. Self‐care behaviors and related factors in outpatients newly diagnosed with non‐insulin‐dependent diabetes mellitus. J Nurs 1998; 45: 60–75 (in Chinese). [Google Scholar]
- 19.Liao C, Masters RSW. Self‐focused attention and performance failure under psychological stress. J Sport Exerc Psychol 2002; 24: 289–305 [DOI] [PubMed] [Google Scholar]
- 20.Williams AM, Vickers J, Rodrigues S. The effects of anxiety on visual search, movement, kinematics, and performance in table tennis: a test of Eysenck and Calvo's processing efficiency theory. J Sport Exerc Psychol 2002; 24: 438–455 [Google Scholar]
- 21.Wilson M, Smith NC, Chattigton M, et al. The role of effort in moderating the anxiety‐performance relationship: testing the prediction of processing efficiency theory in simulated rally driving. J Sports Sci 2006; 24: 1223–1233 [DOI] [PubMed] [Google Scholar]
- 22.Nam S, Chesla C, Stotts NA, et al. Barriers to diabetes management: patient and provider factors. Diabetes Res Clin Pract 2011; 93: 1–9 [DOI] [PubMed] [Google Scholar]
- 23.Surwit RS, van Tilburg MA, Zucker N, et al. Stress management improves long‐term glycemic control in type 2 diabetes. Diabetes Care 2002; 25: 30–34 [DOI] [PubMed] [Google Scholar]
- 24.McMahon GT, Gomes HE, Hickson Hohne S, et al. Web‐based care management in patients with poorly controlled diabetes. Diabetes Care 2005; 28: 1624–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Primožič S, Tavčar R, Avbelj M, et al. Specific cognitive abilities are associated with diabetes self‐management behavior among patients with type 2 diabetes. Diabetes Res Clin Pract 2012; 95: 48–54 [DOI] [PubMed] [Google Scholar]
- 26.Fisher L, Glasgow RE, Strcker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care 2010; 33: 1034–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spahn JM, Reeves RS, Keim KS, et al. State of the evidence regarding behavior change theories and strategies in nutrition counseling to facilitate health and food behavior change. J Am Diet Assoc 2010; 110: 879–891 [DOI] [PubMed] [Google Scholar]
- 28.Sheu WHH. Addressing self‐monitoring of blood glucose: advocating paired glycemic testing for peoplewith type 2 diabetes. J Diabetes Invest 2012; 3: 337–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norris SL, Lau J, Smith SJ, et al. Self‐management education for adults with type 2 diabetes – A meta‐analysis of the effect on glycemic control. Diabetes Care 2002; 25: 1159–1171 [DOI] [PubMed] [Google Scholar]
- 30.Jiang YD, Shiu RS, Chuang LM, et al. Is the development of a diabetes care system important for quality care? An analysis in Taiwan J Diabetes Invest 2011; 2: 79–81 [DOI] [PMC free article] [PubMed] [Google Scholar]


