Abstract
We analyzed the changes of glycemic control over 12 months and the factors influencing blood glucose in 162 Japanese patients with type 2 diabetes having inadequate glycemic control despite sulfonylurea‐based therapy who received add‐on sitagliptin. Hemoglobin A1c (HbA1c) decreased significantly after 4 weeks of treatment, and this improvement was maintained for 1 year, although HbA1c was slightly higher in week 52 than in week 24. Comparison of the patients showing a ≥0.4% increase of HbA1c between weeks 24 and 52 (n = 57) with the others (n = 105) showed a significant difference in the change of bodyweight, as well as the dose of glibenclamide (both P < 0.01). Although combined therapy with sitagliptin and a sulfonylurea seems to be effective for at least 1 year, blood glucose levels are more likely to increase again in patients who show greater weight gain after 24 weeks of treatment and those receiving a higher dose of glibenclamide.
Keywords: Blood glucose, Sitagliptin, Sulfonylurea
Introduction
The goal of treating type 2 diabetes mellitus is to prevent complications caused by chronic hyperglycemia, and it is important to maintain good glycemic control over a long period1. Japanese patients with type 2 diabetes mellitus are characterized by lower early phase insulin secretion after meal ingestion, hence treatment of Japanese patients is focused on activation of insulin secretion with sulfonylureas (SUs)6. Before the introduction of dipeptidyl peptidase‐4 (DPP‐4) inhibitors, sulfonylureas were most often used as monotherapy in Japan8. Sitagliptin is a new hypoglycemic agent9, which is often administered as add‐on therapy to patients showing inadequate glycemic control with SU treatment14. In the present study, we investigated the hypoglycemic effect of sitagliptin, and its durability in patients receiving combination therapy with a SU and sitagliptin.
Materials and Methods
The present study enrolled 162 patients (Table 1) with type 2 diabetes mellitus who had inadequate glycemic control despite oral SU‐based therapy (glimepiride n = 60, gliclazide n = 52 and glibenclamide n = 50) and received add‐on treatment with sitagliptin for 1 year. Other concomitant drugs included metformin (n = 99), pioglitazone (n = 47) and an α‐glucosidase inhibitor (n = 7).
Table 1. Clinical characteristics of the hemoglobin A1c elevated and hemoglobin A1c non‐elevated groups and P‐values.
| Total | HbA1c‐elevated group | HbA1c‐non‐elevated group | P‐value | |
|---|---|---|---|---|
| n | 162 | 57 | 105 | |
| Age (years) | 65.6 ± 10.0 | 63.9 ± 9.8 | 66.5 ± 10.0 | NS |
| Sex (male/female) | 98/64 | 30/27 | 68/37 | NS |
| Baseline BMI (kg/m2) | 24.4 ± 4.0 | 24.5 ± 3.9 | 24.3 ± 3.9 | NS |
| Duration of DM (years) | 13.2 ± 8.4 | 12.3 ± 7.7 | 13.8 ± 8.7 | NS |
| ΔBW0_24 week (kg) | 0.2 ± 1.2 | 0.4 ± 1.5 | 0.1 ± 0.7 | NS |
| ΔBW24_52 week (kg) | 0.3 ± 1.4 | 0.9 ± 1.8 | 0.0 ± 0.9 | <0.01 |
| Glimepiride (mg/day) | 1.7 ± 1.2 | 1.6 ± 1.0 | 1.8 ± 1.2 | NS |
| Gliclazide (mg/day) | 34.4 ± 19.4 | 33.1 ± 17.6 | 35.0 ± 20.1 | NS |
| Glibenclamide (mg/day) | 3.5 ± 2.2 | 4.6 ± 2.1 | 2.9 ± 1.9 | <0.01 |
| Metformin | 99 | 37 (64.9%) | 62 (59.0%) | NS |
| Pioglitazone | 47 | 17 (29.8%) | 30 (28.6%) | NS |
| α‐Glucosidase inhibitor | 7 | 3 (5.3%) | 4 (3.8%) | NS |
ΔBW0_24 week, difference of bodyweight between 0 and 24 weeks; ΔBW24_52 week, difference of bodyweight between 24 and 52 weeks; BMI, body mass index; DM, diabetes mellitus; HbA1c, hemoglobin A1c; NS, not significant.
Patients were divided into a hemoglobin A1c (HbA1c)‐elevated group consisting of those with an increase of HbA1c by 0.4% or more in week 52 compared with week 24, and a HbA1c‐non‐elevated group consisting of the remaining patients. Logistic regression analysis was carried out to investigate the factors involved in the increase of blood glucose during the later part of the treatment period in the patients taking glibenclamide, glimepiride or gliclazide. The factors assessed were the clinical characteristics of the patients (age, sex, body mass index, duration of diabetes), the change of bodyweight from week 24 to week 52, the dose of glibenclamide and the use of other medications.
The primary end‐point was the HbA1c level. The values of HbA1c in the present study were converted and expressed by National Glycohemoglobin Standardization Program values23.
All analyses were carried out using SPSS version 19 for Windows (SPSS, Chicago, IL, USA). Data on HbA1c and bodyweight were processed by one‐way analysis of variance (anova). The Mann–Whitney U‐test was used for comparison of the HbA1c‐elevated group with the non‐elevated group. Results are presented as the mean ± standard deviation, and P < 0.05 was considered significant.
The present retrospective observational study was carried out in accordance with the provisions of the Helsinki Declaration. Approval was obtained from the ethics committee of the Kansai Electric Power Hospital, and written informed consent was obtained from all patients.
Results
The mean HbA1c was 7.77 ± 0.73% at the start of treatment with sitagliptin, and it decreased significantly to 7.45 ± 0.71% after 4 weeks of treatment and to 7.25 ± 0.75% in week 52, suggesting that the improvement of glycemic control was maintained during 12 months of treatment (Figure 1a). However, the HbA1c level became slightly higher during the later part of the treatment period, and HbA1c was significantly higher in week 52 than it was between weeks 16 and 44 (anova, P < 0.01). The mean bodyweight showed no significant change during 12 months (Figure 1b).
Figure 1.
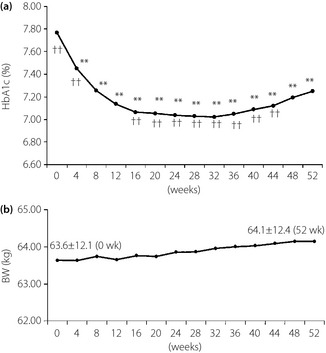
Profile of (a) hemoglobin A1c (HbA1c) and (b) bodyweight (BW) over 52 weeks. Analysis of variance vs week 0 **P < 0.01, vs week 52 ††P < 0.01.
To analyze the factors related to the slight increase of HbA1c in the later part of the treatment period, patients were divided into a HbA1c‐elevated group (n = 57) and a HbA1c non‐elevated group (n = 105; Figure 2). The characteristics of these two groups are compared in Table 1; there were significant differences regarding the change of bodyweight from week 24 to week 52 and the dose of glibenclamide. There was no significant difference in the change of bodyweight from week 0 to week 24. There was no significant difference in the dose of the other SUs or in the use of medications other than SUs between the two groups. When logistic regression analysis was carried out in the glibenclamide group to determine the factors related to poor glycemic control, it was found that the difference of bodyweight between 24 and 52 weeks (ΔBW24_52 week) and the dose of glibenclamide were significant (Table 2). Thus, greater weight gain from week 24 to week 52 and a higher dose of glibenclamide were associated with a larger increase of HbA1c, but the age, sex and baseline body mass index were not significant factors. Logistic regression analysis carried out in the glimepiride or gliclazide groups showed that ΔBW24_52 week was the only significant factor in both groups, whereas the dose of glimepiride or gliclazide was not significant.
Figure 2.
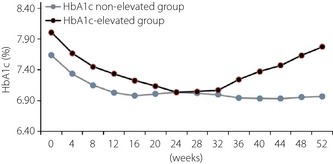
Profile of hemoglobin A1c (HbA1c) in a HbA1c‐elevated group and a HbA1c non‐elevated group over 52 weeks.
Table 2. Factors influencing the durability of combined sitagliptin and glibenclamide therapy according to logistic regression analysis.
| Independent variable | Partial regression coefficient | P‐value |
|---|---|---|
| ΔBW24_52 week | −0.829 | <0.05 |
| Dose of glibenclamide | −0.449 | <0.05 |
ΔBW24_52 week, difference of bodyweight between 24 and 52 weeks.
Discussion
In the present study, a significant decrease of blood glucose was achieved that persisted throughout the study period with no significant change of bodyweight. Comparison between the groups of patients showing poorer and better durability of therapeutic efficacy showed a significant increase in bodyweight from week 24 to week 52 in the group with the elevated HbA1c. We also found that patients in the poorer durability group received a higher dose of glibenclamide. Thus, weight gain from week 24 to week 52 and the dose of glibenclamide were factors significantly related to poorer glycemic control. As previous reports have suggested that sitagliptin has no effect on bodyweight, the cause of the weight gain in the present study might be due to inadequate diet and/or exercise therapy11. Indeed, Tajiri et al.24 reported that blood glucose levels were likely to increase over time in patients on sitagliptin therapy with low lifestyle scores, which is consistent with our present results. Seasonal changes of glycemic control are often observed during the treatment of diabetes, and weight gain might have been related to such seasonal changes. Therefore, when sitagliptin is administered as add‐on therapy to patients who have developed secondary failure of SU‐based treatment, it is important to ensure that diet and exercise therapy are adequate to prevent weight gain in order to maintain glycemic control over a long period16.
It is curious that only a higher dose of glibenclamide was associated with poor glycemic control independently of weight gain. In contrast, the doses of glimepiride and gliclazide were similar in both groups, and these drugs were used at relatively low doses compared with the glibenclamide‐treated group. Logistic regression analysis showed that the dose of glimepiride or gliclazide was not a significant factor. The incidence of so‐called secondary failure has been reported to differ among SU drugs25. Both Harrower25 and Satoh et al.26 reported that the incidence of secondary failure was higher for glibenclamide than gliclazide. The SU drugs show differences of various properties27, and it is not yet clear which properties of SU drugs have an influence on the incidence of secondary failure25. The results of the present study are likely to have been influenced by differences in the rate of secondary failure among SU drugs.
DPP‐4 inhibitors reduce blood glucose levels by increasing insulin secretion by an incretin effect. Accordingly, it is likely that endogenous insulin secretion was lower in the patients treated with higher doses of glibenclamide than in those receiving lower doses of this drug. Further studies are required to resolve these issues.
In summary, we analyzed the profile of glycemic control in patients receiving combination therapy with SUs and sitagliptin. The present results show that avoiding both weight gain and high‐dose glibenclamide therapy can contribute to the maintenance of better glycemic control.
However, it is unclear whether other factors also contributed to the decrease in efficacy of DPP‐4 inhibitor therapy or the secondary failure of DPP‐4 inhibitor treatment in the present study, hence further investigations are required.
Acknowledgment
The authors declare no conflicts of interest with regard to this report.
J Diabetes Invest 2014; 5: 445–448
References
- 1.Holman RR, Paul SK, Bethel MA, et al. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589 [DOI] [PubMed] [Google Scholar]
- 2.Ray KK, Seshasai SR, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta‐analysis of randomised controlled trials. Lancet 2009; 373: 1765–1772 [DOI] [PubMed] [Google Scholar]
- 3.Emerging Risk Factors Collaboration . Diabetes mellitus, fasting glucose, and risk of cause‐specific death. N Engl J Med 2011; 364: 829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UKPDS Group . Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 854–865 [PubMed] [Google Scholar]
- 5.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355: 2427–2443 [DOI] [PubMed] [Google Scholar]
- 6.Kuroe A, Fukushima M, Usami M, et al. Impaired beta‐cell function and insulin sensitivity in Japanese subjects with normal glucose tolerance. Diabetes Res Clin Pract 2003; 59: 71–77 [DOI] [PubMed] [Google Scholar]
- 7.Fukushima M, Usami M, Ikeda M, et al. Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross‐sectional study of Japanese type 2 diabetes. Metabolism 2004; 53: 831–835 [DOI] [PubMed] [Google Scholar]
- 8.Arai K, Matoba K, Hirao K, et al. Present status of sulfonylurea treatment for type 2 diabetes in Japan: second report of a cross‐sectional survey of 15,652 patients. Endocr J 2010; 57: 499–507 [DOI] [PubMed] [Google Scholar]
- 9.Seino Y, Fukushima M, Yabe D. GIP and GLP‐1, the two incretin hormones: similarities and differences. J Diabetes Invest 2010; 1: 8–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada Y, Hayami T, Nakamura K, et al. Human gastric inhibitory polypeptide receptor: cloning of the gene (GIPR) and cDNA. Genomics 1995; 29: 773–776 [DOI] [PubMed] [Google Scholar]
- 11.Iwamoto Y, Taniguchi T, Nonaka K, et al. Dose‐ranging efficacy of sitagliptin, a dipeptidyl peptidase‐4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Endocr J 2010; 57: 383–394 [DOI] [PubMed] [Google Scholar]
- 12.Iwamoto Y, Tajima N, Kadowaki T, et al. Efficacy and safety of sitagliptin monotherapy compared with voglibose in Japanese patients with type 2 diabetes: a randomized, double‐blind trial. Diabetes Obes Metab 2010; 12: 613–622 [DOI] [PubMed] [Google Scholar]
- 13.Odawara M, Kadowaki T, Tajima N, et al. Long‐term safety, tolerability, and efficacy of the dipeptidyl peptidase‐4 inhibitor sitagliptin in Japanese patients with type 2 diabetes. Diabetol Int 2011; 2: 94–105 [Google Scholar]
- 14.Aaboe K, Knop FK, Vilsboll T, et al. KATP channel closure ameliorates the impaired insulinotropic effect of glucose‐dependent insulinotropic polypeptide in patients with type 2 diabetes. J Clin Endocrinol Metab 2009; 94: 603–608 [DOI] [PubMed] [Google Scholar]
- 15.Hermansen K, Kipnes M, Luo E, et al. Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 2007; 9: 733–745 [DOI] [PubMed] [Google Scholar]
- 16.Tajima N, Kadowaki T, Odawara M, et al. Addition of sitagliptin to ongoing glimepiride therapy in Japanese patients with type 2 diabetes over 52 weeks leads to improved glycemic control. Diabetol Int 2011; 2: 32–44 [Google Scholar]
- 17.Pratley RE, Kipnes MS, Alogliptin Study 007 Group . Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor alogliptin in patients with type 2 diabetes inadequately controlled by glyburide monotherapy. Diabetes Obes Metab 2009; 11: 167–176 [DOI] [PubMed] [Google Scholar]
- 18.Garber AJ, Foley JE, Banerji MA, et al. Effects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulphonylurea. Diabetes Obes Metab 2008; 10: 1047–1056 [DOI] [PubMed] [Google Scholar]
- 19.Kubota A, Matsuba I, Saito T, et al. Secretory units of islets in transplantation index is a useful clinical marker to evaluate the efficacy of sitagliptin in treatment of type 2 diabetes mellitus. J Diabetes Invest 2011; 2: 377–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maeda H, Kubota A, ASSET‐K Study group . The safety, efficacy and predictors for HbA1c reduction of sitagliptin in the treatment of Japanese type 2 diabetes. Diabetes Res Clin Pract 2012; 95: e20–e22 [DOI] [PubMed] [Google Scholar]
- 21.Kubota A, Maeda H, Kanamori A, et al. Efficacy and safety of sitagliptin monotherapy and combination therapy in Japanese type 2 diabetes patients. J Diabetes Invest 2012; 3: 503–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubota A, Maeda H, Kanamori A, et al. Pleiotropic effects of sitagliptin in the treatment of type 2 diabetes mellitus patients. J Clin Med Res 2012; 4: 309–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seino Y, Nanjo K, Tajima N, et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tajiri Y, Tsuruta M, Ohki T, et al. Long‐term efficacy of sitagliptin for the treatment of type 2 diabetic patients in Japan. Endocr J 2012; 59: 197–204 [DOI] [PubMed] [Google Scholar]
- 25.Harrower AD. Comparison of efficacy, secondary failure rate, and complications of sulfonylureas. J Diabetes Complications 1994; 8: 201–203 [DOI] [PubMed] [Google Scholar]
- 26.Satoh J, Takahashi K, Takizawa Y, et al. Secondary sulfonylurea failure: comparison of period until insulin treatment between diabetic patients treated with gliclazide and glibenclamide. Diabetes Res Clin Pract 2005; 70: 291–297 [DOI] [PubMed] [Google Scholar]
- 27.Gregorio F, Ambrosi F, Cristallini S, et al. Therapeutical concentrations of tolbutamide, glibenclamide, gliclazide and gliquidone at different glucose levels: in vitro effects on pancreatic α‐ and β‐cell function. Diabetes Res Clin Pract 1992; 18: 197–206 [DOI] [PubMed] [Google Scholar]
- 28.Noda Y, Mori A, Packer L. Gliclazide scavenges hydroxyl, superoxide and nitric oxide radicals: an ESR study. Res Commun Mol Pathol Pharmacol 1997; 96: 115–124 [PubMed] [Google Scholar]
- 29.Seino S, Takahashi H, Takahashi T, et al. Treating diabetes today: a matter of selectivity of sulphonylureas. Diabetes Obes Metab 2012; 14(Suppl. 1): 9–13 [DOI] [PubMed] [Google Scholar]
- 30.Seino S. Cell signalling in insulin secretion: the molecular targets of ATP, cAMP and sulfonylurea. Diabetologia 2012; 55: 2096–2108 [DOI] [PubMed] [Google Scholar]


