Abstract
Aims/Introduction
Diabetes mellitus is a major risk factor in the development of cardiovascular diseases (CVDs). The presence of advanced glycation end‐products (AGEs) promotes CVDs by upregulating endothelial cell (EC) inflammatory and thrombotic responses, in a similar manner as disturbed shear stress. However, the combined effect of disturbed shear stress and AGEs on EC function has yet to be determined. Our goal was to evaluate these effects on EC responses.
Materials and Methods
ECs were incubated with AGEs for 5 days. ECs were then subjected to physiological or pathological shear stress. Cell metabolic activity, surface expression of intercellular adhesion molecule‐1, thrombomodulin, connexin‐43 and caveolin‐1, and cytoskeleton organization were quantified.
Results
The results show that irreversibly glycated albumin and pathological shear stress increased EC metabolic activity, and upregulated and downregulated the EC surface expression of intercellular adhesion molecule‐1 and thrombomodulin, respectively. Expression of connexin‐43, caveolin‐1 and cytoskeletal organization was independent of shear stress; however, the presence of irreversibly glycated AGEs markedly increased connexin‐43, and decreased caveolin‐1 expression and actin cytoskeletal connectivity.
Conclusions
Our data suggest that irreversibly glycated albumin and disturbed shear stress could promote CVD pathogenesis by enhancing EC inflammatory and thrombotic responses, and through the deterioration of the cytoskeletal organization.
Keywords: Advanced glycation end‐products, Cardiovascular disease, Shear stress
Introduction
It has been established that diabetes mellitus is a major risk factor for stroke and cardiovascular disorders, such as coronary artery disease and peripheral arterial disease1. The diabetic vasculature differs from the normal vasculature, resembling the vasculature of cardiovascular diseases (CVDs). Recent studies have reported that a diabetic vasculature induces a two‐ to threefold increase in the likelihood for atherosclerotic complications2. During diabetes, chronic hyperglycemia and insulin resistance result in the formation of advanced glycation end‐products (AGEs), which play a pivotal role in the development of vascular damage. Accumulation of AGEs within the vessel wall has been shown to affect vessel homeostasis, increase vessel wall stiffness, and alter the structure and functions of cellular proteins1.
In a diabetic vasculature, AGEs are formed by the early glycation (1–2 weeks) and oxidation of plasma proteins resulting in the formation of a reversible Schiff base. Further glycation for longer times (6–8 weeks) cause proteins to undergo Amadori rearrangements, which lead to the formation of irreversible AGEs3. AGEs are heterogeneous compounds that alter cellular effects through the receptor for advanced glycation end‐products (RAGE). AGE–RAGE interactions induce the generation of reactive oxygen species and the activation of nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB)4. This leads to the enhanced expression of adhesion molecules (inter‐cellular adhesion molecule [ICAM‐1], vascular cell adhesion molecule [VCAM‐1]) and pro‐inflammatory cytokines (interleukin‐1, interleukin‐6). AGEs also reduce vascular antithrombotic properties and the bioavailability of endothelium derived nitric oxide, which might lead to endothelial dysfunction5. Although it has been reported that higher AGE concentrations have a deleterious effect on the endothelium6, there has been no consensus on the role of glycation extent on these effects; for example, whether or not the reversibility of glycation contributes to pathological cellular changes. Our aim was to determine how varying levels of glycation alter endothelial cell (EC) function during exposure to physiological and pathological shear stress.
The formation of atherosclerotic lesions during CVDs are governed by the vessel geometry, and the magnitude, duration and pattern of flow‐induced shear stress7. The nature of the shear stress plays a pivotal role in controlling EC functions, such as inflammation, thrombosis, cytoskeletal dynamics, gap junction formation (connexin‐43 and caveolin‐1) and angiogenic potential (caveolin‐1)8. Laminar unidirectional shear stress (1–1.5 Pa) is atheroprotective9, whereas disturbed wall shear stress (elevated magnitude, low mean oscillatory or recirculation) promotes an atherogenic phenotype10. It has been reported that elevated, low mean oscillatory and recirculation shear upregulate pro‐inflammatory and pro‐thrombotic markers11, along with disrupting cytoskeletal organization. Interestingly, the presence of AGEs exerts similar effects through NF‐κB activation4. Additionally, a disruption in the connexin‐43 network has been shown to be associated with EC dysfunction under diabetic conditions12. There have been numerous studies that report the individual effects of AGEs or disturbed shear stress on EC responses; however, there has been no study that elucidates how the presence of AGEs might synergistically alter cellular responses under physiological dynamic shear stresses.
In a recent study, we observed an additive effect of constant shear stress (pathologically low and high) and irreversibly glycated AGEs on CVD progression13. In the present study, our aim was to determine how glycation extent would affect endothelial function under exposure to physiological and disturbed wall shear stress to promote CVDs. We hypothesized that in the presence of AGEs and disturbed wall shear stress, there will be an upregulation of inflammatory and thrombotic markers, reorganization of cytoskeleton and an enhanced gap junction activity. These effects will also be a function of the glycation extent (reversible vs irreversible); with irreversibly glycated albumin the responses would be promoted more than their counterparts.
Materials and Methods
Synthesis of Advanced Glycation End Products
Bovine serum albumin was glycated as previously reported14. Briefly, 50 mg/mL albumin (≥98% pure; Sigma–Aldrich, St. Louis, MO, USA; all materials purchased from Sigma–Aldrich unless otherwise noted) was incubated with 250 mmol/L D‐(+)‐glucose, 5 mmol/L phenylmethyl‐sulfonyl‐fluoride (Pierce; Rockford, IL, USA), 2 mg/mL aprotinin, 0.5 mg/mL leupeptin, 100 μg/mL penicillin and 100 U/mL streptomycin in phosphate‐buffered saline (PBS; pH 7.4) at 37°C for a total of 8 weeks (termed AGE). Non‐glycated albumin samples were incubated as aforementioned without glucose (termed BSA (bovine serum albumin)). After 2 weeks (reversibly glycated), 6 weeks (irreversibly glycated) and 8 weeks (irreversibly glycated), samples were dialyzed against PBS. Albumin concentration and the glycation extent of the dialyzed samples were quantified14 and confirmed to be endotoxin free as described previously15. The extent of glycation was confirmed to be the same as our previous reports14. A previous study of ours carried out a dose–response curve for AGE concentration and platelet activation, and we concluded that lower concentrations of AGE can induce a detectable rise in platelet outcomes, but a concentration of 2 mg/mL was a more physiologically relevant concentration of AGE under these conditions15.
Endothelial Cell Culture
Human umbilical vein endothelial cells (HUVECs) were purchased from ScienCell Research Laboratories (Carlsbad, CA, USA) as passage one, and used for experiments from passage two through seven. HUVECs were maintained in EC growth media supplemented with 5% fetal bovine serum, 1X growth supplement, 10 U/mL penicillin and 10 μg/mL streptomycin (all from ScienCell) at 37°C and 5% CO2. Confluent cells were passaged by trypsin digestion, and then glycated or non‐glycated albumin samples were added at a final concentration of 2 mg/mL14. For each experiment, there was a paired no albumin additive control. Cells in the presence of additives were incubated for 3 days (‘Early’ duration) or 5 days (‘Late’ duration). For statistical purposes, the seeding density of cells during each experiment was maintained at approximately 1,000 cells/cm². Note that all Late experiments were paired with Early experiments.
Application of Dynamic Shear Stress
Three physiologically relevant shear stress waveforms were applied to HUVECs by a hemodynamic cone and plate shearing device. The details of the shearing device have been reported previously16. A computational fluid dynamic method was used to develop physiologically relevant waveforms in an adult left coronary artery as described and verified by us16. Briefly, unidirectional pulsatile shear (normal shear stress) varied from 0.03 to 1.0 Pa (time average 0.37 Pa) every 0.9 s. Stenosis shear waveform varied from 0.3 to 6.5 Pa (time average 2.2 Pa). Finally, the recirculation shear waveform varied between 0.062 and 0.4 Pa (time average 0.091 Pa). After the incubation with AGEs and BSAs, ECs were subjected to either a normal, stenosis or recirculation shear waveform for the duration of 1 h at 37°C. The additives (AGEs and BSAs) were paired during individual shear stress waveforms, and the data was normalized to the no added albumin control.
Endothelial Cell Metabolic Activity Assay
A standard MTT (3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide) assay (TOX1 Kit, Sigma‐Aldrich, St. Louis, Missouri, USA) was carried out to quantify EC metabolic activity, by incubating cells with MTT reagent for 3 h at 37°C. During incubation, formazan crystals form as a result of the activity of mitochondrial dehydrogenases, and formed crystals were solubilized using 10% Triton‐X and 0.1 N HCl in anhydrous isopropanol. The absorbance of the solution was quantified at 630 nm (DTX 880, Beckman Coulter, Indianapolis, Indiana, USA). All MTT data were normalized to the no added albumin control of the Early culture duration and the cell concentration for each shear stress waveform. The MTT assay provides a measure of the viability and activity of the cells.
Enzyme‐linked Immunosorbent Assay
Enzyme‐linked immunosorbent assay was used to quantify the surface expression of intercellular adhesion molecule (ICAM‐1), tissue factor (TF) and thrombomodulin (TM). Post‐shearing, the supernatant was aspirated off, and the cells were washed with PBS (pH 7.4) and fixed with 0.5% glutaraldehyde for 15 min at 37°C. Glutaraldehyde was neutralized, and non‐specific binding was blocked with 100 mmol/L glycine – 0.1% BSA for 30 min at 37°C followed by a Tris‐buffered saline wash (1X tris buffered saline; pH 7.4). ECs were then treated with a primary antibody against ICAM‐1 (1 μg/mL in PBS; Ancell Corporation, San Diego, CA, USA), TF (1 μg/mL in PBS; Abcam, Cambridge, MA, USA) or TM (1 μg/mL in PBS; Abcam) for 1 h at 37°C. The primary antibody binding was detected by treating ECs with an alkaline phosphatase conjugated secondary antibody (1 μg/mL in PBS) for 1 h at 37°C followed by a Tris‐buffered saline wash. The cells were then incubated with p‐Nitrophenyl phosphate solution for 20 min, and the absorbance at 405 nm was quantified. Early and Late culture durations were paired for individual shear stress waveforms, and the data was normalized to the no added albumin control after subtraction of the negative control for each independent experiment (cell density was constant per paired experiment).
Immunofluorescence Microscopy
The expression of connexin‐43 and caveolin‐1 was quantified with immunofluorescence microscopy. Post‐shear, ECs were washed with PBS and fixed with 1.5% glutaraldehyde for 15 min at 37°C. Glutaraldehyde was neutralized and non‐specific binding was blocked as aforementioned. Cells were washed with PBS‐0.1% BSA and incubated with 0.6 μg/mL anti‐human connexin‐43 (Abcam) and 2 μg/mL anti‐human caveolin‐1 (Invitrogen, Carlsbad, CA, USA) for 60 min at 37°C. The cells were again washed and incubated for 60 min with appropriate secondary antibodies (5 μg/mL; Invitrogen). A Coolsnap Fast Cooled (ES2) digital camera interfacing with NIS Elements Software using a 20X (Nikon, Tokyo, Japan, Plan Fluor ELWD, N.A. 0.45) objective was used to image ECs. The connexin‐43 and caveolin‐1 intensity was quantified using a customized method previously described13. All data was paired by the glycation extent (AGEs and BSAs) and applied shear stress waveform, and normalized to the no added albumin control for each independent experiment, and by the cell number.
Immunofluorescence microscopy was also used to quantify the changes in actin cytoskeleton and the intercellular connectivity. After the application of shear stress, the cells were fixed, the fixative neutralized and non‐specific binding blocked as aforementioned. To visualize the plasma membrane, the cells were incubated with wheat germ agglutinin (WGA; Invitrogen) at a concentration of 5 μg/mL for 10 min, in the dark at room temperature. The cell membrane was permeabilized with 0.2% Triton‐X for 5 min. F‐actin was visualized with 5 U/mL phallodin (Invitrogen) for 20 min in the dark at room temperature. DNA was visualized with 300 nmol/L DAPI (4',6‐Diamidino‐2‐phenyindole) for 5 min in the dark at room temperature. Actin fiber structure and connectivity was quantified using a customized method previously reported13. Each image was normalized by the number of cells per image.
Statistical Analysis
Statistical analysis was carried out using SAS (V 9.0, SAS Institute, Cary, North Carolina, USA), and significance was tested at α = 0.05. In most cases, a three‐way analysis of variance (ANOVA) was used to analyze the differences of means within the normalized data. The data was grouped by glycation extent, culture duration and shear stress waveform. Significant differences were found based on the least squares means values from the ANOVA results. The data for each independent experiment was normalized to the no added albumin control. Normalized data from a minimum of four independent experiments were used.
Results
Endothelial Cell Metabolic Activity
The metabolic activity of HUVECs in response to varying levels of glycated albumin and physiological shear stress waveforms was quantified (Figure 1). In the presence of glycated albumin, there was an overall enhancement of metabolic activity (approximately 40% taking into account all shear waveforms) for Late culture durations compared with Early culture durations. After exposure to recirculation or stenosis shear waveforms, the metabolic activity of ECs was somewhat impaired as compared with the normal waveform (Figure 1b). The presence of glycated albumin enhanced metabolic activity as compared with non‐glycated albumin and no added albumin samples, and under most cases this was a function of glycation extent. The presence of irreversibly glycated albumin (8‐week AGE) exerted the most effect (20–50% increase) on metabolic activity for all waveforms and for both durations, and this was additive to the normal effects of shear stress waveform.
Figure 1.
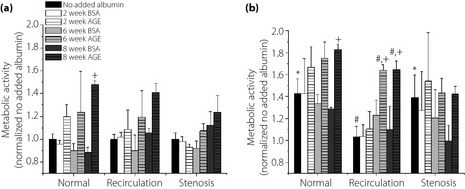
Human umbilical vein endothelial cells (HUVEC) metabolic activity under dynamic shear stress condition in cells in the presence of additives incubated for (a) 3 days (Early) and (b) 5 days (Late) culture duration as a function of varying levels of glycated albumin and the nature of the shear stress waveform. All data are reported as mean ± standard error of the mean of four independent experiments. +Significantly different from no added albumin and paired BSA (paired by shear stress and culture duration, three way ANOVA; P < 0.05). *Significantly different from Early (paired by shear stress and glycation extent, three way ANOVA; P < 0.05). #Significantly different from normal shear stress (paired by glycation extent and culture duration, three way ANOVA; P < 0.05). AGE, advanced glycation end‐products.
Expression of Inflammatory and Thrombotic Mediators
The expression of ICAM‐1, tissue factor and thrombomodulin were measured to determine the effect of glycation extent and shear stress on EC inflammatory and thrombogenic potential (Figure 2). The presence of irreversibly glycated albumin (8‐week AGE) induced the highest ICAM‐1 expression, and this was most significant after exposure to a stenosis shear waveform (approximately 100% increase compared with no added albumin and non‐glycated albumin; 60–100% increase compared with 2‐week AGE) for both Early and Late culture durations. AGEs also induced a higher expression of ICAM‐1 under recirculation shear waveform. Our data suggest that the presence of albumin glycated to a greater extent, and exposure to pathological shear stress significantly increased the expression of ICAM‐1 in an additive manner.
Figure 2.
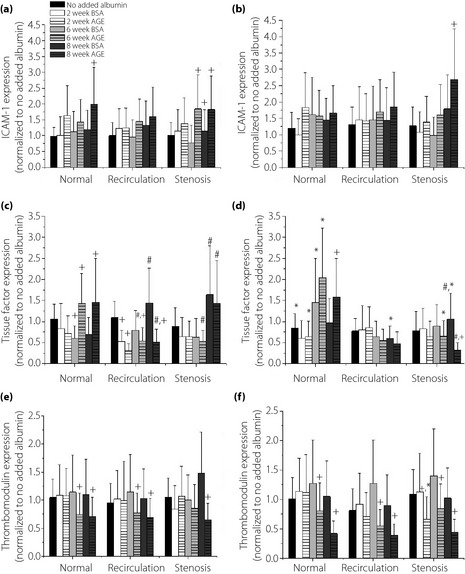
Human umbilical vein endothelial cells (HUVEC) surface expression of (a,b) intercellular adhesion molecule (ICAM)‐1, (c,d) tissue factor, and (e,f) thrombomodulin for cells in the presence of additives incubated for (a,c,e) 3 days (Early) and (b,d,f) 5 days (Late) culture durations, as a function of varying levels of glycated albumin, and the nature of the shear stress waveform. All data are reported as mean ± standard error of the mean of four independent experiments. +Significantly different from no added albumin and paired BSA (paired by shear stress and culture duration, three‐way ANOVA; P < 0.05). *Significantly different from Early (paired by shear stress and glycation extent, three‐way ANOVA; P < 0.05). #Significantly different from normal shear stress (paired by glycation extent and culture duration, three way ANOVA; P < 0.05). AGE, advanced glycation end‐products.
Tissue factor (Figure 2c,d) and thrombomodulin (Figure 2e,f) expression was measured as a marker for thrombogenic potential. The presence of AGEs induced a significant increase (40–60%) in TF expression compared with no added albumin and non‐glycated albumin under normal shear stress. However, under recirculation and stenosis shear stress, there was a significant decrease (30–40%) in TF expression for cells exposed to glycated albumin as compared with the paired non‐glycated albumin. There was no visible difference in TF expression between the Early and Late culture durations. Additionally, there was a significant decrease in TM expression when EC were incubated with irreversibly glycated albumin (6‐ and 8‐week AGE) for both Early (approximately 30%) and Late (approximately 50%) durations compared with no added albumin and non‐glycated albumin. The presence of AGEs and a longer culture duration exerted more effect on the surface expression of TM than shear stress waveform, and thus the presence of AGEs dominated the thrombogenic response.
Expression of Connexin‐43 and Caveolin‐1
The expression of connexin‐43 and caveolin‐1 was quantified as aforementioned (Figure 3). There was a significant increase (10–30%) in connexin‐43 expression in the presence of irreversibly glycated albumin (6‐ and 8‐week AGE) under all waveforms compared with no added albumin and non‐glycated samples (Figure 3a). The expression of caveolin‐1 significantly decreased (approximately 20%) in the presence of higher glycation extents for all shear stress waveforms (Figure 3b). The effects of AGE vs BSA were independent of shear stress waveform. The quantified data might be qualitatively compared with sample immunofluorescence images (Figure 4a–d), by comparing the intensity of the images per condition (the exposure time was kept constant throughout each experiment). As previously published, the expression for connexin‐43 and caveolin‐1 was independent of the culture duration13; therefore, this comparison was not made here. There was somewhat of an additive effect between shear stress and presence of glycated albumin14, but shear stress exposure regulated the expression of connexin‐43 and caveolin‐1 more strongly than AGE.
Figure 3.
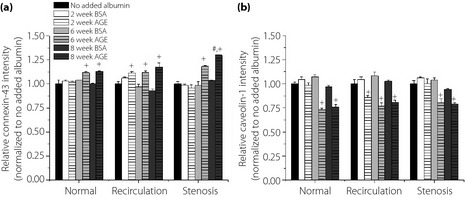
Human umbilical vein endothelial cells (HUVEC) surface expression for (a) connexin‐43 and (b) caveolin‐1 under dynamic shear stress condition, as a function of varying levels of glycated albumin and the nature of the shear stress waveform. All data are reported as mean ± standard error of the mean of four independent experiments. +Significantly different from no added albumin and paired BSA (paired by shear stress and culture duration, three‐way ANOVA; P < 0.05). #Significantly different from normal shear stress (paired by glycation extent and culture duration, three‐way ANOVA; P < 0.05). AGE, advanced glycation end‐products.
Figure 4.
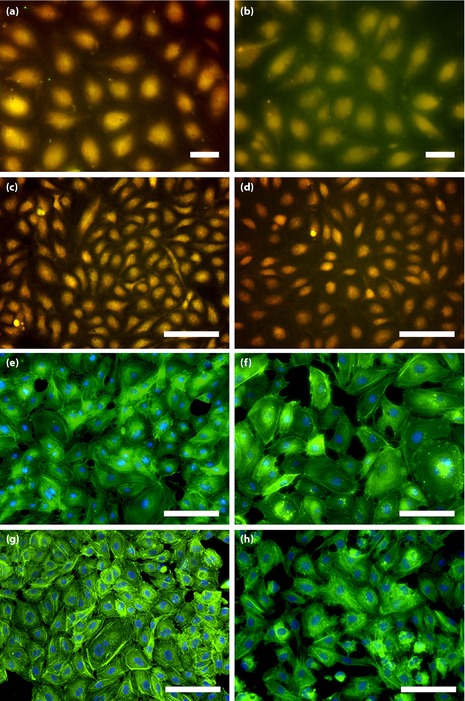
Representative images of human umbilical vein endothelial cells (HUVEC) surface expression of (a–d) connexin‐43/caveolin‐1 and (e–h) actin cytoskeleton structure and connectivity by immunofluorescence microscopy (connexin‐43, green; caveolin‐1, red; co‐localization, yellow, actin filament, green; nucleus, blue). This figure compares (a,c,e,g) no added albumin samples with (b,d,f,h) 8‐week irreversibly glycated albumin samples under (a,b,e,f) recirculation and (c,d,g,h) stenosis shear waveforms. (a–d) A higher expression of connexin‐43 and lower expression of caveolin‐1 is shown after incubation with (b,d) irreversibly glycated albumin samples compared with (a,c) paired no added albumin samples. (e–h) Higher actin structure and less connectivity in glycated samples compared to no added albumin samples is shown. Bars,100 μm.
Cytoskeletal Structure and Organization
The effect of the glycation extent and physiological shear stress waveforms on actin structure and organization was measured to determine the adaptability of ECs to shear. Under recirculation and stenosis shear stress, there was an increase in the percent of filamentous actin when the cells were exposed to irreversibly glycated albumin (Late culture durations; Figure 5a,b). The quantitative data can be visualized qualitatively by the immunofluorescence images in Figure 4. Interestingly, normal shear stress exerted the opposite effect in the presence of glycated albumin by diminishing actin fibrous structure. The actin connectivity was measured considering the intercellular network formation of the actin filaments. The results show that there is a higher connectivity of fibers under no added albumin and non‐glycated albumin samples compared with glycated samples. The connectivity was significantly reduced (15–30%) under all shear stress conditions (Figure 5c,d). Again, there were some additive effects of the combined incubation14, but it appears that connectivity is more susceptible to the shear application.
Figure 5.
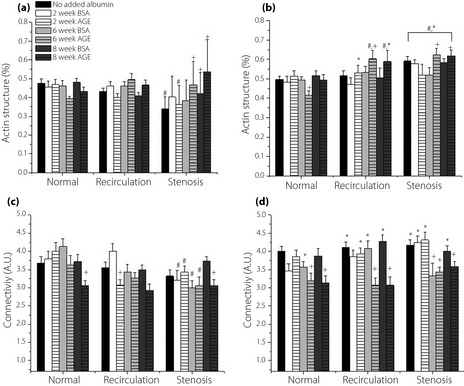
Human umbilical vein endothelial cells (HUVEC) (a,b) cytoskeleton structure and (c,d) actin connectivity under dynamic shear stress condition in cells in the presence of additives incubated for (a,c) 3 days (Early) and (b,d) 5 days (Late) culture duration, as a function of varying levels of glycated albumin and the nature of the shear stress waveform. All data are reported as mean ± standard error of the mean of four independent experiments. +Significantly different from no added albumin and paired BSA (paired by shear stress and culture duration, three‐way ANOVA; P < 0.05). *Significantly different from Early (paired by shear stress and glycation extent, three‐way ANOVA; P < 0.05). #Significantly different from normal shear stress (paired by glycation extent and culture duration, three‐way ANOVA; P < 0.05). AGE, advanced glycation end‐products.
Discussion
The metabolic activity of HUVECs when exposed to various glycated albumin samples and shear waveforms was measured (Figure 1). The present results show an overall increase in metabolic activity for Late culture durations in the presence of irreversibly glycated albumin for all shear stress waveforms. However, pathological waveforms reduced metabolic activity as compared with normal waveforms (there was still an enhancement in metabolic activity for cells exposed to glycated albumin). These results are in agreement with our previous work13. It is well documented that the presence of AGEs in a diabetic vasculature exerts a toxic effect, and impairs metabolic activity by diminishing mitochondrial activity and production of metabolites15. However, it has been reported that laminar or pulsatile17 and oscillatory shear stress19 (all within our studied range) stimulate metabolism. Our data suggest that the presence of shear stress overpowers the inhibitory effect of glycated albumin, and that the presence of glycated albumin somewhat adds to the shear effects. As the glycation extent increases, the most significant effect on metabolic activity is observed, suggesting that more severe diabetic conditions, combined with disturbed wall stresses, can increase EC metabolic activity.
The EC expression of ICAM‐1 was measured to determine the alteration of EC inflammatory potential under these conditions (Figure 2a,b). Our data suggest an additive increase in the expression of ICAM‐1 after exposure to irreversibly glycated albumin and pathological shear stress, which is in agreement with our previous findings14. Previously, it has been reported that AGEs can mediate an enhancement of ICAM‐1 expression both in vivo and in vitro4. Furthermore, ICAM‐1 expression has been shown to be sensitive to shear stress in vitro9, and in vivo ICAM‐1 expression is most pronounced at locations of disturbed wall shear stress20. Our findings are in agreement with these studies, and suggest that the presence of irreversibly glycated albumin during disturbed shear stress enhances EC inflammatory potential, which is a hallmark of CVDs.
Endothelial cell thrombogenic responses were also observed (Figure 2c–f). After exposure to irreversibly glycated albumin and pathological shear stress, there was a significant decrease in the expression of the antithrombogenic TM, which agrees with our previous findings. However, exposure to pathological shear stresses in the presence of irreversibly glycated albumin also downregulated the expression of the pro‐thrombogenic TF as compared with the paired non‐glycated albumin. Glycated albumin14 and disturbed shear stress21 has been reported to reduce TM expression, which would enhance the hemostatic response. Additionally, it has been reported that glycated albumin14 or disturbed wall shear stress16 enhance the expression and activity of the procoagulant TF22 during short durations. Our data agree with previous TM findings, but not with previous TF findings. However, some report that disturbed wall shear stress can decrease TF activity by activating tissue factor pathway inhibitor (TPFI)23, which binds to and inhibits TF. Although we did not quantify TFPI activity, it might account for the decreased TF expression, or it is possible that soluble TF concentration increased. Interestingly, our results show that normal shear upregulates TF expression in the presence of irreversibly glycated albumin13, whereas disturbed shear stress inhibits the effect of pro‐thrombogenic TF activity; possibly suggesting a mechanism for TPFI activity. Overall, our data suggests that irreversibly glycated albumin and pathological shear stress somewhat increase the EC thrombotic potential towards CVD progression, but there was no clear additive or synergistic interactions.
The expression of the gap junction protein connexin‐43 and the pro‐angiogenic marker caveolin‐1 was measured. Here, irreversibly glycated albumin and disturbed shear stress waveforms additively upregulated connexin‐43 expression, agreeing with our previous report13. To date there has not been a consensus on the effect of glycated albumin on connexin‐43 expression, and findings appear to be dependent on the particular conditions of the study24. However, the effects of disturbed shear stress are clearer. Elevated expression of connexin‐43 has been observed both in vitro26 and in vivo26, in regions of disturbed blood flow26. These findings are in agreement with our current data. The surface expression of caveolin‐1, a regulator for transcytosis, membrane permeability and angiogenesis29, decreased in the presence of irreversibly glycated albumin for all shear stress waveforms, agreeing with our previous report13. According to other reports, AGEs enhance the expression of caveolin‐130; whereas laminar or disturbed wall shear stress downregulate caveolin‐131. The downregulation of caveolin‐1, which is a negative regulator of the angiogenic factor nitric oxide (NO), could enhance NO‐dependent membrane permeability, tumor angiogenesis and facilitate disease progression under diabetic conditions29. Thus, combining our findings, our data suggests that the combination of disturbed flow conditions and glycated albumin can additively enhance the expression of connxin‐43 and caveolin‐1, which is indicative of CVD progression.
The adaptability of actin, the major structural component of the EC cytoskeleton, was observed. Proper structure and function of actin is crucial, as it regulates adhesion, migration, morphology and permeability33. The results showed a higher percentage of fibrillar actin and a reduced connectivity when cells were exposed to irreversibly glycated albumin and pathological shear stress. Glycated albumin can induce the polymerization of peripheral actin fibers followed by a redistribution of the fibers towards the nucleus34. Similar observations were documented in the presence of elevated shear stress, in vitro and in vivo35. Higher filamentous actin structure has also been reported in regions of elevated and oscillatory shear stress33. The results of these studies concur with the findings of our current study. It is important to note that a higher cell density during Late durations might have facilitated ECs retaining the fibrous actin structure. In contrast to the actin structure, we observed less connectivity under the pathological conditions, agreeing with our previous findings. So far, there has been no other study that reports the distribution of the cytoskeleton in this manner; however, there have been a few studies that report alteration in cellular network formation under physiological shear loading36. Overall, our data suggest that the presence of irreversibly glycated albumin and pathological shear stress waveforms alter cytoskeletal dynamics and mature network formation potential.
The goal of the present study was to determine in what manner the combined effect of AGEs and disturbed shear stress would alter EC metabolic and gap junction activity, inflammatory, thrombogenic and angiogenic potential, cytoskeleton structure, and organization to accelerate CVD progression during diabetes mellitus. Combining the effect of all waveforms and glycation extents, our data suggest that pathological shear stress and glycated albumin deteriorate EC functions to promote cardiovascular complications during diabetes mellitus. However, there are some limitations to the present study. All observations are based on the assumption that the glycated albumin formed in vitro is similar to that during diabetic conditions. The duration of shear stress application is limited to the early adaptation phase, and there could be longer adaptation functions that return these findings to normal levels or promote CVD mechanisms further. However, with these limitations, we still showed that additive interactions exist between glycated albumin and physiological shear stress that promote endothelial dysfunction during diabetic CVDs .
Acknowledgments
The authors thank the Oklahoma Center for the Advancement of Science and Technology (Award Number HR09‐158) for supporting this research project. There are no conflicts of interests to declare associated with this work.
J Diabetes Invest 2014; 5: 372–381
References
- 1.Schwartz C, Valente A, Sprague E, et al. Pathogenesis of the atherosclerotic lesion. Implications for diabetes mellitus. Diabetes Care 1992; 15: 1156–1167 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association . Role of cardiovascular risk factors in prevention and treatment of macrovascular disease in diabetes. Diabetes Care 1989; 12: 573–579 [DOI] [PubMed] [Google Scholar]
- 3.Schmidt AM, Yan SD, Wautier JL, et al. Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ Res 1999; 84: 489–497 [DOI] [PubMed] [Google Scholar]
- 4.Goldin A, Beckman JA, Schmidt AM, et al. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 2006; 114: 597–605 [DOI] [PubMed] [Google Scholar]
- 5.Tan KC, Chow WS, Ai VH, et al. Advanced glycation end products and endothelial dysfunction in type 2 diabetes. Diabetes Care 2002; 25: 1055–1059 [DOI] [PubMed] [Google Scholar]
- 6.Basta G, Lazzerini G, Del TS, et al. At least 2 distinct pathways generating reactive oxygen species mediate vascular cell adhesion molecule‐1 induction by advanced glycation end products. Arterioscler Thromb Vasc Biol 2005; 25: 1401–1407 [DOI] [PubMed] [Google Scholar]
- 7.Fry DL. Acute vascular endothelial changes associated with increased blood velocity gradients. Circ Res 1968; 22: 165–197 [DOI] [PubMed] [Google Scholar]
- 8.DePaola N, Gimbrone MA Jr, Davies PF, et al. Vascular endothelium responds to fluid shear stress gradients. Arterioscler Thromb 1992; 12: 1254–1257 [DOI] [PubMed] [Google Scholar]
- 9.Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol 1998; 18: 677–685 [DOI] [PubMed] [Google Scholar]
- 10.Moore JE Jr, Xu C, Glagov S, et al. Fluid wall shear stress measurements in a model of the human abdominal aorta: oscillatory behavior and relationship to atherosclerosis. Atherosclerosis 1994; 110: 225–240 [DOI] [PubMed] [Google Scholar]
- 11.Chiu JJ, Usami S, Chien S. Vascular endothelial responses to altered shear stress: pathologic implications for atherosclerosis. Ann Med 2009; 41: 19–28 [DOI] [PubMed] [Google Scholar]
- 12.Makino A, Platoshyn O, Suarez J, et al. Downregulation of connexin40 is associated with coronary endothelial cell dysfunction in streptozotocin‐induced diabetic mice. Am J Physiol Cell Physiol 2008; 295: C221–C230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maria Z, Yin W, Rubenstein D. Glycated albumin and pathological shear stress alters endothelial cell thrombogenic potential, pro‐inflammatory state and cytoskeletal dynamics. J Diabetes Metab 2011; S4: 1–9 [Google Scholar]
- 14.Rubenstein DA, Maria Z, Yin W. Glycated albumin modulates endothelial cell thrombogenic and inflammatory responses. J Diabetes Sci Technol 2011; 5: 703–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubenstein DA, Yin W. Glycated albumin modulates platelet susceptibility to flow induced activation and aggregation. Platelets 2009; 20: 206–215 [DOI] [PubMed] [Google Scholar]
- 16.Yin W, Shanmugavelayudam SK, Rubenstein DA. The effect of physiologically relevant dynamic shear stress on platelet and endothelial cell activation. Thromb Res 2011; 127: 235–241 [DOI] [PubMed] [Google Scholar]
- 17.Frangos JA, Eskin SG, McIntire LV, et al. Flow effects on prostacyclin production by cultured human endothelial cells. Science 1985; 227: 1477–1479 [DOI] [PubMed] [Google Scholar]
- 18.Davies PF. Flow‐mediated endothelial mechanotransduction. Physiol Rev 1995; 75: 519–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frangos JA, McIntire LV, Eskin SG. Shear stress induced stimulation of mammalian cell metabolism. Biotechnol Bioeng 1988; 32: 1053–1060 [DOI] [PubMed] [Google Scholar]
- 20.Chiu JJ, Lee PL, Chen CN, et al. Shear stress increases ICAM‐1 and decreases VCAM‐1 and E‐selectin expressions induced by tumor necrosis factor‐[alpha] in endothelial cells. Arterioscler Thromb Vasc Biol 2004; 24: 73–79 [DOI] [PubMed] [Google Scholar]
- 21.Malek AM, Jackman R, Rosenberg RD, et al. Endothelial expression of thrombomodulin is reversibly regulated by fluid shear stress. Circ Res 1994; 74: 852–860 [DOI] [PubMed] [Google Scholar]
- 22.Lin MC, Almus‐Jacobs F, Chen HH, et al. Shear stress induction of the tissue factor gene. J Clin Invest 1997; 99: 737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grabowski EF, Zuckerman DB, Nemerson Y. The functional expression of tissue factor by fibroblasts and endothelial cells under flow conditions. Blood 1993; 81: 3265–3270 [PubMed] [Google Scholar]
- 24.Wright JA, Richards T, Becker DL. Connexins and diabetes. Cardiol Res Pract 2012; 2012: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato T, Haimovici R, Kao R, et al. Downregulation of connexin 43 expression by high glucose reduces gap junction activity in microvascular endothelial cells. Diabetes 2002; 51: 1565–1571 [DOI] [PubMed] [Google Scholar]
- 26.DePaola N, Davies PF, Pritchard WF Jr, et al. Spatial and temporal regulation of gap junction connexin43 in vascular endothelial cells exposed to controlled disturbed flows in vitro. Proc Natl Acad Sci USA 1999; 96: 3154–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwak BR, Mulhaupt F, Veillard N, et al. Altered pattern of vascular connexin expression in atherosclerotic plaques. Arterioscler Thromb Vasc Biol 2002; 22: 225–230 [DOI] [PubMed] [Google Scholar]
- 28.Gabriels JE, Paul DL. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circ Res 1998; 83: 636–643 [DOI] [PubMed] [Google Scholar]
- 29.Minshall RD, Sessa WC, Stan RV, et al. Caveolin regulation of endothelial function. Am J Physiol Lung Cell Mol Physiol 2003; 285: L1179–L1183 [DOI] [PubMed] [Google Scholar]
- 30.Stitt AW, Burke GA, Chen F, et al. Advanced glycation end‐product receptor interactions on microvascular cells occur within caveolin‐rich membrane domains. FASEB J 2000; 14: 2390–2392 [DOI] [PubMed] [Google Scholar]
- 31.Sonveaux P, Martinive P, DeWever J, et al. Caveolin‐1 expression is critical for vascular endothelial growth factor‐induced ischemic hindlimb collateralization and nitric oxide‐mediated angiogenesis. Circ Res 2004; 95: 154–161 [DOI] [PubMed] [Google Scholar]
- 32.Schubert W, Frank PG, Woodman SE, et al. Microvascular hyperpermeability in caveolin‐1 (‐/‐) knock‐out mice. Treatment with a specific nitric‐oxide synthase inhibitor, L‐NAME, restores normal microvascular permeability in Cav‐1 null mice. J Biol Chem 2002; 277: 40091–40098 [DOI] [PubMed] [Google Scholar]
- 33.Langille BL, Graham JJ, Kim D, et al. Dynamics of shear‐induced redistribution of F‐actin in endothelial cells in vivo. Arterioscler Thromb 1991; 11: 1814–1820 [DOI] [PubMed] [Google Scholar]
- 34.Salameh A, Zinn M, Dhein S. High D‐glucose induces alterations of endothelial cell structure in a cell‐culture model. J Cardiovasc Pharmacol 1997; 30: 182–190 [DOI] [PubMed] [Google Scholar]
- 35.Mott RE, Helmke BP. Mapping the dynamics of shear stress‐induced structural changes in endothelial cells. Am J Physiol Cell Physiol 2007; 293: C1616–C1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malek AM, Izumo S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J Cell Sci 1996; 109: 713–726 [DOI] [PubMed] [Google Scholar]


