Abstract
Aims/Introduction
Dysregulated inflammatory response is believed to be an important factor in the pathogenesis of several late complications of diabetes mellitus. β‐Glucans are potent inducers of immune function. The present randomized, double blind, two‐center, placebo‐controlled study was undertaken to explore safety, tolerability and efficacy of soluble β‐1,3/1,6‐glucan (SBG) as a local treatment of diabetic foot ulcers.
Materials and Methods
A total of 60 patients with type 1 or 2 diabetes and lower extremity ulcers (Wagner grade 1–2, Ankle/Brachial Index ≥0.7) received SBG or a comparator product (methylcellulose) locally three times weekly up to 12 weeks in addition to conventional management scheme. A total of 54 patients completed the study.
Results
A tendency for shorter median time to complete healing in the SBG group was observed (36 vs 63 days, P = 0.130). Weekly percentage reduction in ulcer size was significantly higher in the SBG group than in the methylcellulose group between weeks 1–2, 3–4 and 5–6 (P < 0.05). The proportion of ulcers healed by week 12 was also in favor of SBG (59% vs 37%, P = 0.09), with a significantly higher healing incidence in the SBG group at week 8 (44% vs 17%, P = 0.03). SBG was safe and well tolerated. There was a clinically significant difference regarding the incidence of serious adverse events in favor of the SBG treatment.
Conclusions
Local treatment of diabetic lower extremity ulcers with β‐1,3/1,6‐polyglucose shows good safety results. This β‐glucan preparation shows promising potential as a treatment accelerating cutaneous healing. Further studies are required to confirm this effect. This trial was registered with ClinicalTrials.gov (no. NCT00288392).
Keywords: Diabetic foot, Wound healing, β‐Glucans
Introduction
Foot ulcers are among the main complications of diabetes, with a 25% lifetime risk in all diabetic patients1. In their review of diabetic foot and leg ulcers in several populations, Reiber et al.2 found a prevalence of between 4.4% and 10.5%. Many of the diabetic foot ulcers need intensive treatment and lead to hospitalization. Healing can take months, and amputation is a frequent outcome. Given the high prevalence of diabetes and the high costs associated with these ulcers, the treatment of this affliction is not only a major burden to the patients, but also to the healthcare system3.
Conventional management of diabetic ulcers is based on regular cleansing and debridement, off‐loading and treatment of intercurrent infections with antibiotics4. Under standard care, between 20 and 40% of all chronic diabetic foot ulcers normally heal within 12 weeks, and just over 50% of the ulcers are healed by 6 months5. At least 30% of the patients require surgical intervention in order to improve healing9. There are still very few alternative interventions that have been shown to enhance ulcer healing, although some treatments, such as the application of platelet‐derived growth factors (PDGFs) and tissue‐engineered artificial skin graft products, are now available10. However, the effectiveness of these products has not been perceived as convincing10. Furthermore, the use of growth factors are complicated by safety concerns related to the development of malignancy and an increased mortality13. Thus, a drug with a more favorable risk–benefit profile would be welcome.
Wound and ulcer healing involves a coordinated series of partly overlapping events14 where tissue macrophages play a crucial role15, especially in the initial inflammatory phase. Apparently, diabetic ulcers do not follow an orderly and predictable progression of healing7. Although the pathogenesis of ulcers is complex and only partly understood, it is clear that the macrophage plays a major role in the overall healing17. It has been shown that macrophages from diabetic mice and humans are dysfunctional with respect to the production of cytokines and growth factors essential for the healing of wounds and ulcers18. Disturbed wound healing in diabetic mice can, however, be improved by exposure to immunomodulators, such as β‐glucans20.
β‐Glucans – the principal component of many natural medicinal products used in Asia since the ancient times – are now known to activate macrophages. The ability of yeast β‐glucans to promote wound repair was first described by Leibovich and Danon21, and later confirmed by others22. β‐1,3‐glucans are components of the cell walls of bacteria and fungi. In their natural state, they are insoluble in aqueous solutions, and therefore impractical to apply in animal experiments or in clinical situations, be it locally or systemically. The production of water‐soluble β‐1,3/1,6‐glucan (SBG; a backbone of β 1‐3‐D‐polyglucose with side chains of the same structure linked to the backbone with β 1‐6 bonds) without chemical derivatization has been achieved by Biotec Pharmacon ASA, Norway. It is extracted from Saccharomyces cerevisiae by a patented method23. SBG modulates immune processes, and acts on cells of the innate immune system, where the initial step is the interaction with cellular receptors leading to an altered state of the cells involved24. SBG has been shown to be a potent enhancer of immune functions in different animal models25. Based on promising results in animal experiments, where solubilized β‐glucan was applied to surgical wounds in diabetic mice20, it was considered an obvious further development to test it in clinical situations.
The present study was therefore undertaken to explore the efficacy and safety of SBG as a potential topical treatment of chronic foot and leg ulcers in diabetic patients.
Materials and Methods
A double blind, placebo‐controlled phase II study was undertaken at two clinical centers in the Russian Federation. The initially expected prevalence of healed ulcer in the comparator group and the treatment group was estimated to be 50 and 80%, respectively. The clinically relevant difference in time until healed was assessed to be 21 days. With a significance level of 5%, a power of 90% and an estimated rate of dropouts of 10%, at least 30 patients in each group had to be included in the study.
Eligibility for participation was determined at a screening visit and was as follows: type 1 or 2 diabetes mellitus; age above 18 years; wound with partial (Wagner grade 1) or full (Wagner grade 2) thickness skin involvement, but not including tendon, joints or bone; wound located on the foot or lower leg, and had been present for at least 4 weeks, but not more than 2 years; adequate blood supply determined as the presence of a palpable pulse at the corresponding foot; and wound area greater than 1 cm2. Exclusion criteria were as follows: pregnancy; breast feeding; women of child‐bearing age not using birth control; ankle‐brachial index <0.7; malnutrition; clinical evidence of gangrene at any location; active or extensive cellulitis extending more than 1 cm beyond wound margin or purulent discharge; medical conditions (e.g., diabetic nephropathy) that in the justified opinion of the investigator would make the patient an inappropriate candidate for the study; active osteomyelitis; necrotic toes on the study ulcer foot; surgical procedure other than debridement on the study ulcer foot within 3 weeks before screening; study ulcer over Charcot's joint; deep tissue infection of the study ulcer at the day of enrolment; non‐study ulcer within 5.0 cm from the study ulcer at enrolment; random blood sugar reading >450 mg/dL; alcohol or drug abuse; and participation in other clinical studies in the last 4 weeks. All the patients were recruited from the outpatient department.
The patients were randomized (simple block randomization 1:1 method with random block size) into two groups receiving 2% aqueous solution of either SBG (Lot FE102; Biotec Pharmacon ASA, Tromsø, Norway) or comparator, methylcellulose (Lot FE101; Dow Chemical/Colorcon Ltd, Dartford, UK) locally to the wound three times weekly for a maximum of 12 weeks, in addition to the conventional therapy. The choice of methylcellulose as a suitable comparator product to the β‐1,3/1,6‐glucan product SBG was based on the assessment that the two products had equal physical and chemical characteristics, but where methylcellulose is known to lack any immunomodulating qualities. The study drugs were applied by the surgeon as an evenly distributed layer on the ulcer surface. The conventional therapy comprised of surgical wound debridement, removal of hyperkeratoses and systemic antibiotics. Dressings were of pure cotton, and were soaked with 0.9% saline solution before removal to prevent damage to tissues. The patients were instructed to wear comfortable waterproof shoes with orthopedic insoles and to keep their feet warm. Ulcer size was measured weekly and documented with digital photography. Clinical examinations were carried out at each visit by surgeons and endocrinologists. In the event of complete wound healing, the patient would reach the primary end‐point and thus participation in the study ended. The study progress was audited by Evidence‐CPR (Saint Petersburg, Russia).
Blinding of patients and investigators was achieved by delivering the test substances in physically indistinguishable forms and vials labeled with randomization numbers. Both solutions were without any smell and taste, and were supplied as single‐dose vials containing 5 mL 2% SBG or methylcellulose. Information about the study treatment for each individual (as identified by the patient number) was available to the sponsor and the principal investigator in sealed emergency envelopes. Unblinding could only occur in clinical emergencies, and did not take place in the present study. Unused medicines were returned to Evidence‐CPR to be destroyed. The study was carried out in compliance with Good Clinical Practice, according to the ICH Tripartite Guideline E6. Written informed consent was obtained from each patient before any study procedures or assessments were carried out, and after the aims, methods, anticipated benefits and potential hazards were explained. The protocol for the research project has been approved by the Local Independent Ethics Committees and conformed to the provisions of the Declaration of Helsinki.
The primary end‐point was time to complete ulcer healing. At visits two to 13, the investigators were asked to give their opinion on the response to treatment as one of the following alternatives: complete response, partial response, no response and progressive disease. The time to complete ulcer healing was determined based on the time‐point of achieved complete response.
Secondary end‐points included: number of ulcers that had completely healed; percentage weekly change in ulcer size; treatment response defined by investigator (last visit – baseline); percentage change in ulcer size (last visit – visit 1) and safety.
The number of healed ulcers was determined as the number of ulcers with complete response achieved by week 12. Two methods of defining responses were used: investigator's opinion and calculation based on percentage reduction of ulcer area from baseline to week 12. The latter was divided into five types of response: (i) complete (≥0% total ulcer area <1% of the baseline); (ii) moderate (≥1% total ulcer area <51% of the baseline); (iii) mild (≥51% total ulcer area <91% of the baseline); (iv) no response (≥91% total ulcer area <110% of the baseline); and (v) progressive disease (total ulcer area ≥110% of the baseline). Ulcer size (area and depth) was determined at each study visit. These measurements were used for calculation of percentage changes in ulcer size from baseline to week 12.
Safety evaluation was based on monitoring clinical and laboratory parameters and recording adverse events. An adverse event was any unfavorable change in general condition or concomitant diseases, symptoms, new concomitant diseases or accidents and clinically relevant changes in laboratory parameters. All adverse events were recorded on the adverse event form with information about the nature, duration, severity, relationship, action taken regarding study products and outcome. All clinical and laboratory adverse events were followed up until resolved or as clinically required. A serious adverse event was defined as an adverse event that was fatal; life‐threatening; resulted in hospitalization or prolongation of hospitalisation; resulted in persistent or significant disability/incapacity; or resulted in a congenital anomaly/birth defect. All serious and unexpected adverse events were reported on the serious adverse event form and faxed to the sponsor no later than 24 h after the investigator was made aware of the event. The reports were forwarded to the regulatory authority in accordance with national regulations.
Statistical Analysis
Statistical analysis was carried out by LINK Medical Research (Oslo, Norway) with SAS for Windows (version 8.2; SAS Institute Inc., Cary, NC, USA). Two‐sided tests were carried out using 5% as the nominal level of significance, and interval estimates were constructed using 95% as the level of confidence. Survival analysis was carried out on variable ‘days’ (time to complete healing) and expressed by Kaplan–Meier plots. Median and mean time until complete ulcer healing (‘survival time’) and 95% confidence intervals were obtained. Ulcer healing responses, as based on the investigator's opinion, were tested by frequency analysis with chi‐squared‐test or with Fisher's exact test, as appropriate. Similar analyses were carried out on treatment response by percentage reduction in total ulcer area from baseline to the final visit. Responses were categorized as: complete (≥0% total ulcer area <1% of the baseline), moderate (≥1% total ulcer area <51% of the baseline), mild (≥51% total ulcer area <91% of the baseline), no response (≥91% total ulcer area <110% of the baseline) and progressive disease (total ulcer area ≥110% of the baseline). Additionally, complete healing vs not‐complete healing were tested by frequency analysis with chi‐squared‐test or with Fisher's exact test, as appropriate. The percentage of change in total ulcer area from week 12 to the baseline was analyzed by an ancova model with percentage area change as a dependent variable. The linear model was applied using SAS PROC MIXED (SAS Institute Inc.) with center and treatment as fixed effects, whereas baseline ulcer area, Wagner's grade, localization of ulcer, sex and age were covariates in the different models tested. Percentage weekly change in the total ulcer area was considered as a repeated measurement of the ulcer area, and was analyzed by SAS PROC GLM (SAS Institute Inc.). A model consisting of factors found significant for the previous analysis was utilized here. Testing of the secondary variables was exploratory, and the results were mainly used for exploratory purposes to obtain more details of ulcer healing. As a result of this, no adjustments for multiple testing were carried out.
The safety population (SP) included all patients who were treated at least once with study medication (n = 60). This population was used to evaluate the safety laboratory variables and adverse events. The intention‐to‐treat (ITT) population was defined as all patients who were randomized and had at least one dose of study medication (n = 60). ITT was equal to SP in this study. Per protocol (PP) population included patients who had completed the treatment period of maximum 12 weeks according to the protocol (n = 52). Patients who did not have at least one study ulcer with an area >1.0 cm2 at baseline were considered protocol violators, and were not included into the PP population (two patients). Other patients that were not included in the PP population were patients that withdrew consent (four patients) or had an amputation (two patients).
Before database lock, it was decided to also analyze populations at ulcer level as the primary population for the efficacy analyses, as some patients had more than one ulcer at baseline. These populations are referred to as ‘PP Ulcer’ or ‘ITT Ulcer’ The reasons for this were that ulcer population was considered more relevant for evaluation of treatment effects, and as ‘time to ulcer healing’ was the primary end‐point, it was considered correct to include every ulcer present at baseline in addition to single patients in the analyses. Therefore, in patients with multiple ulcers at baseline, each ulcer was considered individually as healed or non‐healed by the end of the observation period. The PP Ulcer analyses were supported by supplementary analyses on ITT population.
Results
Patients
A total of 60 patients were randomized, and 54 (27 in each group) completed the study (Figure 1). There were two dropouts in the SBG‐group and four dropouts in the methylcellulose group. There were two patients with a protocol violation (ulcer area <1.0 cm2 at baseline) that were not withdrawn from the study, but excluded from the PP population. Demographic characteristics, vital signs, laboratory parameters and ulcer data at baseline are presented in Table 1.
Figure 1.
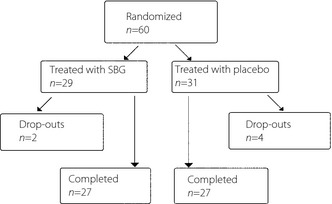
Disposition of patients.
Table 1. Demographic characteristics, vital signs, laboratory parameters and ulcer data at baseline.
| Variable | SBG | Methylcellulose | |
|---|---|---|---|
| ITT age (years) | Mean | 60.6 | 60.8 |
| Range | 28.8 –84.5 | 24.4 –87.9 | |
| PP age (years) | Mean | 61.2 | 62.2 |
| Range | 28.8 –84.5 | 37.5 –87.9 | |
| ITT sex | Female% (n) | 62.1 (18) | 58.1 (18) |
| Male% (n) | 37.9 (11) | 41.9 (13) | |
| PP sex | Female% (n) | 61.5 (16) | 53.8 (14) |
| Male% (n) | 38.5 (10) | 46.2 (12) | |
| ITT diabetes mellitus | Type 1% | 20.7 | 32.3 |
| Type 2% | 79.3 | 67.7 | |
| ITT blood glucose (mmol/L) | Mean | 11 | 12 |
| Range | 9 –14 | 8 –18 | |
| ITT blood CRP (mg/L) | Mean | 6 | 6 |
| Range | 5 –12 | 3 –11 | |
| ITT blood leukocytes (×109/L) | Mean | 7 | 7 |
| Range | 5 –8 | 5 –8 | |
| ITT ulcer area at start (cm2) | Mean | 4.11 | 2.65 |
| Upper–lower CI | 1.70 –6.53 | 2.0 –3.29 | |
| PP ulcer area at start (cm2) | Mean | 4.39 | 2.87 |
| Upper–lower CI | 1.71 –7.07 | 2.09 –3.64 | |
CI, confidence interval; CRP, C‐reactive protein; ITT, intention‐to‐treat; PP, per protocol; SBG, soluble yeast β‐1,3/1,6‐glucan; SP, safety population.
Primary Efficacy End‐Point: Time to Complete Healing
As per the investigators' assessments, 26 out of 57 ulcers in the PP ulcer population were completely healed during the study, of which 15 of 27 ulcers in the SBG group and 11 of 30 in the methylcellulose group (Figure 2). The difference between groups on the time to healing was not statistically significant, although a tendency in favor of SBG was seen; mean 41.8 vs 56 days (P = 0.0793) and median 36 vs 63 days (P = 0.1298) in the PP ulcer population (Table S1). When analyzing the time to ulcer healing with survival curves, a tendency that SBG treatment had a shorter time to complete healing compared with placebo was observed (Figure 2; P = 0.0944).
Figure 2.
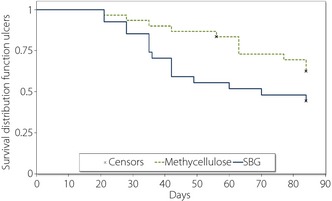
Cumulative rate of complete ulcer healing in response to local treatment with soluble β‐1,3/1,6‐glucan (SBG) or methylcellulose (placebo). Differences between the curves were tested by a log–rank test (P = 0.0944).
Secondary Efficacy End‐Points
The dynamics of ulcer healing was analyzed by comparing percentage weekly reduction in ulcer area between the groups (Table S2). The reduction in wound area was found to be significantly larger in SBG‐treated patients as compared with methylcellulose in the PP Ulcer population in the earlier weeks of treatment (week 1–2, P = 0.0445; week 3–4, P = 0.0375; week 5–6, P = 0.0496). The accumulated ulcer area reduction in the two treatment groups from baseline to week 12 is shown in Figure 3.
Figure 3.
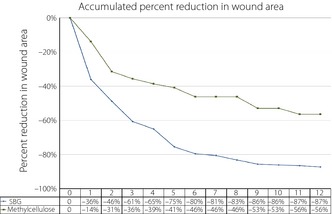
Accumulated median percentage wound size reduction during the 12‐week treatment period (per protocol ulcer). SBG, soluble β‐1,3/1,6‐glucan.
In the SBG group, 56% of the ulcers in the PP population had a complete response to treatment by week 12 compared with 37% in the methylcellulose group (P = 0.1528) when carrying out the analysis based on the investigator's clinical assessments (Table S3). A somewhat stronger tendency was seen when doing calculations based on ulcer area measurements. A complete response, defined as the remaining ulcer area being 0–1% of the initial area, was recorded in 59% of ulcers in the SBG arm vs 37% in the control arm (P = 0.0881). Also a near‐significant tendency for more pronounced reduction in ulcer depth (P = 0.0580) in patients treated with SBG compared with controls was seen (data not shown).
As data showed an early response of the SBG treatment (as shown in wound reduction in Table S2), additional analysis was carried out on the complete healing at week 8 of treatment. A significant difference was observed in healing efficacy between the two treatment arms in both ulcer and patient populations, as shown in Figure 4, where 44% of the ulcers in the PP‐ulcer population had healed vs 17% in the control group (P = 0.0269). The figures for the ITT‐ulcer population were 43% completely healed in the SBG group vs 17% in the methylcellulose‐treated group (P = 0.017). Similar results were recorded in the per patient population (Figure 4).
Figure 4.
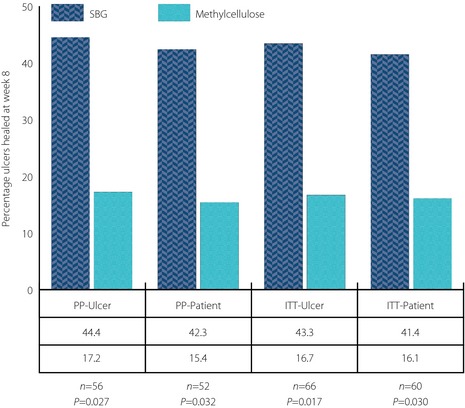
Percentage of completely healed wounds at week 8 of treatment in the different patient populations. Equality of response between the two treatment arms was tested with chi‐squared‐test. P‐values as shown in the figure. ITT‐Patient, intention‐to‐treat patient population; ITT‐Ulcer, intention‐to‐treat ulcer population; PP‐Patient, per protocol patient population; PP‐Ulcer, per protocol ulcer population.
Analysis of Safety Variables
Local treatment of wounds with either SBG or methylcellulose preparations was well tolerated. Out of a total of 10 adverse events, four were classified as serious (Table S4). There was no significant difference between the treatments regarding the incidence of adverse events. However, a clinically significant difference between the groups for the incidence of serious adverse events was noted in favor of SBG treatment. There were four serious adverse events (three toe amputations/resections of metatarsal bones and one other surgical operation) in the control group, whereas there were none in the SBG group. No clinically significant changes in laboratory variables were detected.
Discussion
The results from the present study show a consistent tendency that SBG has potential as a treatment of chronic diabetic foot and leg ulcers. SBG appears to induce a rapid onset of action, and promotes a shorter time to healing compared with standard wound care5. Furthermore, SBG seems to have a favorable risk–benefit profile, although the material in the present study is limited.
When evaluating the fact that the primary end‐point was not reached, a better than anticipated healing rate in the control group as compared with what would be expected from other studies27, together with a relatively small sample size, should be taken into consideration. In future studies, identifying a target or index ulcer at baseline; that is, the largest or the ‘most clinically important’, in patients with more than one ulcer would be preferred in order to avoid the need for defining ulcer populations. The credibility of such ulcer populations might be questioned, as they could introduce a mixture of inter‐ and intrapatient variables. In the present study though, both the ulcer populations and the patient populations showed the same trends in favor of SBG.
The present study did not have a follow‐up period to investigate the frequency of ulcer relapse that could confirm long‐term ulcer healing, such a follow‐up period could have added validity of the healing frequency.
As the present study showed a significant result in favor of SBG, with healing rates between 40 and 45% at as early as 8 weeks of therapy, the healing incidence at week 8 should be further investigated. In comparison, the PDGF becaplermin gel showed a healing rate of approximately 14% on average in four studies after 8 weeks of treatment in a similar patient group27; and in a study with a collagen/oxidized regenerated cellulose dressing, the healing rate was 26% at week 830. After 12 weeks of treatment, the healing rate in these two latter studies were 27 and 37%, respectively; and at 20 weeks of treatment, the overall healing rate in the becaplermin‐treated patient groups were approximately 45%27. The corresponding healing rate seen in the present study after 12 weeks of treatment with SBG was 56% in the PP ulcer population. Thus, the healing rates seen with SBG in the present study at 12 weeks seem to be more in line with the healing incidence seen after 20 weeks of treatment in the becaplermin studies. This figure probably approaches the maximal, because as the study progresses, the percentage of remaining ulcers fundamentally refractory to treatment will increase. However, caution should be exercised, as the numbers might not be directly comparable because the target ulcer identification and definition of the two study populations could be different, which would impact on the expected healing rate31.
Apparently, SBG treatment promotes ulcer healing more rapidly than observed in the becaplermin studies, and the relative ulcer size reduction seems to be even more pronounced during the very first weeks of treatment. Thus, targeting and modulating macrophage function by an immunomodulator, such as SBG, seems to be a promising approach to promote healing of diabetic ulcers.
It is known that SBG stimulates the secretion of an array of cytokines from human monocytes that modulate inflammation24. Macrophages are not only important during the inflammatory phase, but also coordinate later events in wound healing, suggesting that SBG might have a positive impact on several stages of the process. Indeed, a soluble β‐glucan from the medical mushroom, Sparassis crispa, have been reported to promote wound healing after oral administration in rats with streptozotocin‐induced diabetes32. Furthermore, supernatants from macrophages stimulated in vitro with a soluble β‐glucan from S. cerevisiae induced cross‐linking of collagen after topical administration to wounds in rats22. β‐Glucan can also directly induce production of collagen by dermal fibroblasts in vitro33, suggesting that SBG might work through fibroblasts as well. Thus, SBG might possibly exert its effect through action on several cell types and processes in the wound bed. The complete mechanism of action remains, however, to be elucidated.
We have also observed that there was a considerable difference in the results between the two study centers. We can offer no apparent explanation for this difference. Possible contributing factors could be differences in the details of routine ulcer care, patient hygiene, storing of the test substances or adjuvant medications.
Conclusion
The present study shows that SBG has potential as an effective, safe, and well‐tolerated treatment of diabetic foot and leg ulcers. It might speed up closure of diabetic leg ulcers. Further studies are warranted to confirm the clinical utility of this treatment.
Supplementary Material
Table S1 | Primary end‐point result – time to complete ulcer healing.
Table S2 | Secondary end‐point result – weekly percentage reduction in ulcer size.
Table S3 | Secondary end‐point result – percentage healed ulcers, and ulcer depth reduction.
Table S4 | Summary of adverse events.
Acknowledgments
We thank patients for their participation in the study. We are grateful to the Norwegian Research Council for postdoctoral fellowship to SNZ and to Professor Rolf Seljelid for his work with primary sources, data analysis and writing of manuscript. The study was sponsored by Biotec Pharmacon ASA. Dr SNZ received research grants from Biotec Pharmacon ASA; however, not directly related to the current study.
J Diabetes Invest 2014; 5: 392–399
References
- 1.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005; 293: 217–228 [DOI] [PubMed] [Google Scholar]
- 2.Reiber GE, Vileikyte L, Boyko EJ, et al. Causal pathways for incident lower‐extremity ulcers in patients with diabetes from two settings. Diabetes Care 1999; 22: 157–162 [DOI] [PubMed] [Google Scholar]
- 3.Langer A, Rogowski W. Systematic review of economic evaluations of human cell‐derived wound care products for the treatment of venous leg and diabetic foot ulcers. BMC Health Serv Res 2009; 9: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White R, McIntosh C. Topical therapies for diabetic foot ulcers: standard treatments. J Wound Care 2008; 17: 426, 428–432. [DOI] [PubMed] [Google Scholar]
- 5.Jeffcoate WJ, Price P, Harding KG. Wound healing and treatments for people with diabetic foot ulcers. Diabetes Metab Res Rev 2004; 20(Suppl 1): S78–S89 [DOI] [PubMed] [Google Scholar]
- 6.Treece KA, Macfarlane RM, Pound N, et al. Validation of a system of foot ulcer classification in diabetes mellitus. Diabet Med 2004; 21: 987–991 [DOI] [PubMed] [Google Scholar]
- 7.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005; 366: 1736–1743 [DOI] [PubMed] [Google Scholar]
- 8.Kurd SK, Hoffstad OJ, Bilker WB, et al. Evaluation of the use of prognostic information for the care of individuals with venous leg ulcers or diabetic neuropathic foot ulcers. Wound Repair Regen 2009; 17: 318–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berendt AR, Peters EJ, Bakker K, et al. Specific guidelines for treatment of diabetic foot osteomyelitis. Diabetes Metab Res Rev 2008; 24(Suppl 1): S190–S191 [DOI] [PubMed] [Google Scholar]
- 10.White R, McIntosh C. A review of the literature on topical therapies for diabetic foot ulcers. Part 2: advanced treatments. J Wound Care 2009; 18: 335–341 [DOI] [PubMed] [Google Scholar]
- 11.Papanas N, Maltezos E. Becaplermin gel in the treatment of diabetic neuropathic foot ulcers. Clin Interv Aging 2008; 3: 233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinchliffe RJ, Valk GD, Apelqvist J, et al. A systematic review of the effectiveness of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev 2008; 24(Suppl 1): S119–S144 [DOI] [PubMed] [Google Scholar]
- 13.Hart JC, Rosenthal N. Regranex_DHP_Letter. Available at: http://www.regranex.com/REGRANEX_DHP_Letter.pdf 2008.
- 14.Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature 2008; 453: 314–321 [DOI] [PubMed] [Google Scholar]
- 15.Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol 1975; 78: 71–100 [PMC free article] [PubMed] [Google Scholar]
- 16.Blakytny R, Jude EB. Altered molecular mechanisms of diabetic foot ulcers. Int J Low Extrem Wounds 2009; 8: 95–104 [DOI] [PubMed] [Google Scholar]
- 17.Tesch GH. Role of macrophages in complications of type 2 diabetes. Clin Exp Pharmacol Physiol 2007; 34: 1016–1019 [DOI] [PubMed] [Google Scholar]
- 18.Zykova SN, Jenssen TG, Berdal M, et al. Altered cytokine and nitric oxide secretion in vitro by macrophages from diabetic type II‐like db/db mice. Diabetes 2000; 49: 1451–1458 [DOI] [PubMed] [Google Scholar]
- 19.Zykova SN, Svartberg J, Seljelid R, et al. Release of TNF‐alpha from in vitro‐stimulated monocytes is negatively associated with serum levels of apolipoprotein B in patients with type 2 diabetes. Scand J Immunol 2004; 60: 535–542 [DOI] [PubMed] [Google Scholar]
- 20.Berdal M, Appelbom HI, Eikrem JH, et al. Aminated beta‐1,3‐D‐glucan improves wound healing in diabetic db/db mice. Wound Repair Regen 2007; 15: 825–832 [DOI] [PubMed] [Google Scholar]
- 21.Leibovich SJ, Danon D. Promotion of wound repair in mice by application of glucan. J Reticuloendothel Soc 1980; 27: 1–11 [PubMed] [Google Scholar]
- 22.Browder W, Williams D, Lucore P, et al. Effect of enhanced macrophage function on early wound healing. Surgery 1988; 104: 224–230 [PubMed] [Google Scholar]
- 23.Engstad R, Kortner F, Robertsen B, et al. Enzyme treatment of glucans. Patent EP0759089. 1995
- 24.Engstad CS, Engstad RE, Olsen JO, et al. The effect of soluble beta‐1,3‐glucan and lipopolysaccharide on cytokine production and coagulation activation in whole blood. Int Immunopharmacol 2002; 2: 1585–1597 [DOI] [PubMed] [Google Scholar]
- 25.Sandvik A, Wang YY, Morton HC, et al. Oral and systemic administration of beta‐glucan protects against lipopolysaccharide‐induced shock and organ injury in rats. Clin Exp Immunol 2007; 148: 168–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breivik T, Opstad PK, Engstad R, et al. Soluble beta‐1,3/1,6‐glucan from yeast inhibits experimental periodontal disease in Wistar rats. J Clin Periodontol 2005; 32: 347–352 [DOI] [PubMed] [Google Scholar]
- 27.Smiell JM, Wieman TJ, Steed DL, et al. Efficacy and safety of becaplermin (recombinant human platelet‐derived growth factor‐BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomized studies. Wound Repair Regen 1999; 7: 335–346 [DOI] [PubMed] [Google Scholar]
- 28.Marston WA, Hanft J, Norwood P, et al. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 2003; 26: 1701–1705 [DOI] [PubMed] [Google Scholar]
- 29.Veves A, Falanga V, Armstrong DG, et al. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 2001; 24: 290–295 [DOI] [PubMed] [Google Scholar]
- 30.Veves A, Sheehan P, Pham HT. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg 2002; 137: 822–827 [DOI] [PubMed] [Google Scholar]
- 31.Ince P, Game FL, Jeffcoate WJ. Rate of healing of neuropathic ulcers of the foot in diabetes and its relationship to ulcer duration and ulcer area. Diabetes Care 2007; 30: 660–663 [DOI] [PubMed] [Google Scholar]
- 32.Kwon AH, Qiu Z, Hashimoto M, et al. Effects of medicinal mushroom (Sparassis crispa) on wound healing in streptozotocin‐induced diabetic rats. Am J Surg 2009; 197: 503–509 [DOI] [PubMed] [Google Scholar]
- 33.Wei D, Zhang L, Williams DL, et al. Glucan stimulates human dermal fibroblast collagen biosynthesis through a nuclear factor‐1 dependent mechanism. Wound Repair Regen 2002; 10: 161–168 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Primary end‐point result – time to complete ulcer healing.
Table S2 | Secondary end‐point result – weekly percentage reduction in ulcer size.
Table S3 | Secondary end‐point result – percentage healed ulcers, and ulcer depth reduction.
Table S4 | Summary of adverse events.


