Vaccines have been medicine’s most successful weapon against disease since their first use in the early 19th century; they have saved countless lives, drastically decreased mortality in particular among children and helped to eradicate smallpox, one of the greatest scourges of humankind. Vaccine research is still ongoing to fight HIV/AIDS, malaria and a range of other diseases, and it has recently yielded another efficient tool against human papilloma virus to protect women from virus-induced cervical cancer.
Meanwhile, vaccine research has opened up a second front by applying vaccination as a therapy for people already suffering from disease, which is not susceptible to a preventative approach. Such therapeutic vaccines could work against both infectious diseases and cancer and some are already being used to treat infections. However, the strategy of harnessing the patient’s own immune system to fight cancer, although it is appealing and theoretically possible, has been facing a number of roadblocks in clinical practice.
Therapeutic vaccination to treat an infection is only effective against pathogens with a relatively long incubation period, for otherwise the immune system does not have enough time to mount a proper response. One example is the zoonotic viral disease rabies, often caught from dog bites, which still causes thousands of deaths mostly in developing countries (http://centerforvaccineethicsandpolicy.net/2013/10/12/rabies-kills-24000-a-year-in-africa-because-vaccine-costly-experts/). Effective vaccines against the virus have been available for more than 20 years but the cost of vaccinating all people at risk in developing countries would be prohibitive. A more cost-efficient alternative is to administer the vaccine after potential infection, such as a dog bite, exploiting the fact that the incubation period for rabies varies between 2 weeks and 6 years.
It is, however, the theoretical possibility of curing cancer that has created the greatest excitement and interest in therapeutic vaccine research. In fact, therapeutic vaccines could become an alternative to the “slash, burn and poison” triumvirate—surgery, radiotherapy, and chemotherapy—with much less severe side effects. It could help oncologists to deal with hitherto nearly untreatable tumors, notably pancreatic or esophageal cancer. Despite some setbacks and failures in early clinical trials, there is growing confidence in the underlying science buoyed by some evidence of efficacy.
One major difference between cancer and an infectious pathogen as a vaccine target—and one of the major problems for developing cancer vaccines—is that pathogens naturally trigger a strong immune response while cancer cells often elicit only a weak reaction or none at all. To obtain an efficient vaccine, it is therefore not sufficient to present the immune system appropriate target molecules that are expressed on tumor cells but not normal cells, but it is also necessary to ensure an adequate response.
The difficulties of achieving both aims were demonstrated by a candidate therapy to treat melanoma that showed promise in early trials but then failed in a larger-scale phase III trial. The vaccine was developed by GlaxoSmithKline (GSK) and targets a tumor-specific antigen called MAGE-A3 that is expressed in a variety of cancers, including melanoma. GSK announced in September 2013 that the phase III trial, called DERMA, had failed to demonstrate that its vaccine significantly extended disease-free survival compared to placebo.
The fate of GSK’s trial highlights that there is still some way to go before therapeutic vaccines can enter routine clinical practice. To date, the US Food and Drug Administration has approved only one therapeutic vaccine, called Provenge, developed by the Seattle-based biotech firm Dendreon for treatment of advanced prostate cancer that has metastasized and become resistant to androgen-deprivation treatment. FDA approval in April 2010 came after a phase III double-blind, placebo-controlled trial involving 512 patients showed an increase in overall survival of approximately 4 months compared to the control group.
The Provenge treatment involves several steps (Fig 1), starting by taking blood from the patient to collect immune cells, including T cells, B cells, natural killer (NK) cells, and antigen-presenting cells (APCs). (http://www.fda.gov/biologicsbloodvaccines/cellulargenetherapyproducts/approvedproducts/ucm210037.htm). These cells are then exposed to a recombinant human protein called PAP-GM-CSF. It consists of prostatic acid phosphatase (PAP), an antigen expressed in prostate cancer tissue, linked to activator granulocyte-macrophage colony-stimulating factor (GM–CSF), which activates immune cells. Following this exposure, the cells are then injected back into the patient in three doses at 2 weekly intervals. Dendreon has not responded to requests for comment, but the FDA indicates that each dose can only be prepared up to around 3 days before administration so that it is impossible to develop a complete treatment course in one step.
Figure 1. Vaccination strategies to treat cancer.
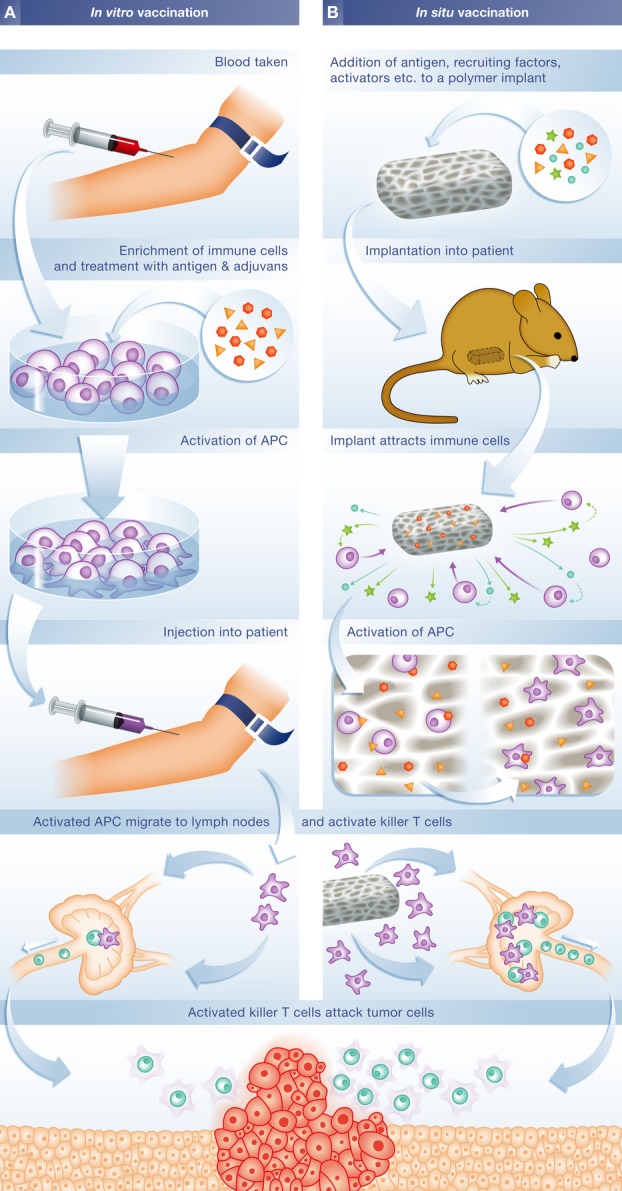
(A) In vivo approaches such as Provenge take blood from a patient to enrich for immune cells, which are then exposed to tumor-specific antigens and other factors. After injection back into the patient, activated antigen-presenting cells migrate to the lymph nodes where they activate T cells which then destroy tumor cells. (B) In situ vaccination uses a polymer-based implant loaded with antigens and other molecules required to attract and activate APC. After transplantation, the implant recruits APC by releasing molecules that signal bacterial infection. Upon entering the implant, APCs become activated against the tumor antigen, after which they migrate to lymph nodes to activate T cells. APC = antigen-presenting cells.
Since its approval, the drug has had mixed fortunes. It was heralded as a breakthrough in cancer therapy, as a proof of principle that the immune system can be mobilized to attack tumors by manipulating the APCs, primarily dendritic cells, which act as messengers between the innate and adaptive immune systems. However, the process is highly labor-intensive and expensive, and it merely extends survival by just a few months. Each dose costs US$31,000 to prepare and the total of US$93,000 is considerably higher than the average of US$62,000 that had been previously anticipated by biotech analysts during development. This led to lower sales than expected and sustained losses for Dendreon during the time Provenge has been available. The combination of high cost and modest efficacy means that Provenge is unlikely to be the model for future cancer vaccines, according to Alan Melcher, a specialist in cancer immune therapy at the University of Leeds, UK. “Efficacy is not that great with just a few months extra survival,” he said. “If it was 100% successful, everyone would be using it despite the cost.”
Other biotech companies thus seek to improve the laboratory processing step so that only one round of collecting and activating immune cells is required per patient. ImmunoCellular for instance claims to have developed a process that yields 20 doses of its dendritic therapeutic cancer vaccine, which targets glioblastoma, one of the most aggressive form of brain cancer, from a single blood collection (http://www.imuc.com/technology/immunocellular-advantages).
Yet, to become economically viable, personalized therapeutic vaccines will have to completely eliminate the need for external processing of the patient’s blood. A team under David Mooney at Harvard University in the USA set out to solve this and another problem simultaneously: Avoiding the cumbersome and expensive manipulation of cells in vitro and generating a stronger immune response. Their approach tries to mimic key aspects of bacterial infections, since these cause a strong immune response against whole cells and to instigate the appropriate activation and transport of dendritic cells to the lymph nodes where they present the target antigen material to T cells and B cells. The objective, according to Mooney, was to develop an implant that by itself would serve as a vaccine and eliminate the expense and regulatory burden inherent to cell-based therapies such as Provenge.
“… the strategy of harnessing the patient’s own immune system to fight cancer, although it is appealing and theoretically possible, has been facing a number of roadblocks…”
The starting point was the observation that a suitable microenvironment that exposes ATC to exogenous cytokines such as GM-CSF along with molecules known to alert the immune system to the presence of invading pathogens, such as the DNA molecule CpG-ODN, efficiently activates ATC and controls their migration to the lymph nodes 1. This could be combined with cancer antigens to generate dendritic cells that would then alert T cells and B cells to fight tumor cells 2. Mooney and his colleagues developed a polymer-based system that, if implanted, recruits dendritic cells and activates them through the presentation of suitable antigens and other factors (Fig 1). Mooney pointed out that his team had built on a large body of previous work formulating vaccines that contain a wide range of molecular signals as adjuvants. “That work suggested it would be possible to do what we proposed,” he said.
Their approach has shown convincing results in mice injected with highly aggressive and metastatic melanoma cells. Mice who received only placebo or the inert polymer developed significant tumors within 18 days and had to be put down by day 23. The group with the polymer-based vaccine did significantly better: 50% of mice given the optimum doses of molecules were free of tumors after 40 days and 23% were cured altogether 3. Their vaccine is now undergoing phase 1 clinical trials (http://clinicaltrials.gov/ct2/show/NCT01753089) to assess safety in patients with advanced stage 4 melanoma, where the cancer has metastasized to multiple skin areas and other sites in the body such as the lung, liver, or brain.
In practice, cancer vaccines will be likely used in combination with other compounds to further manipulate and optimize the immune reaction. Dendritic cell-based vaccines for instance would require supporting therapies that offset the tumor’s immune suppression, for example, by antagonizing pathways that induce immune tolerance to tumors. This is particularly relevant for cancers at advanced stage, because by then tumors have effectively suppressed many aspects of the immune system. In the shorter term, though, therapeutic vaccines will most likely be used alongside rather than as a replacement for existing therapies to reduce doses of chemotherapy or radiotherapy and therefore side effects. There is also the potential for using therapeutic vaccination in combination with surgery to induce an immune response against recurrent or smaller secondary tumors that cannot be removed physically.
“… therapeutic vaccines could become an alternative to the “slash, burn and poison” triumvirate—surgery, radiotherapy and chemotherapy—with much less severe side effects”
In particular, therapeutic vaccines can enhance the efficacy of conventional treatments by overcoming the immune-suppression effect of tumors, commented Angus Dalgleish, Professor of Oncology at St George’s Hospital in London, UK. “My own group showed that even early colorectal cancer patients induce marked suppression of cell-mediated immunity and that this was restored 1 month after resection,” he said. “It has become clear that in the absence of resection this has to be addressed early and not after all other therapies.” In fact, there is growing evidence that therapeutic vaccines can enhance the response of tumors to both chemo- and radiotherapy, according to Dalgleish. “Following correction of the immune response then tumor kill can be induced by chemo, RT (radiotherapy) or ablation and this will lead to tumor-specific auto vaccination, which can be further enhanced by adding low-dose IL-2 [interleukin-2],” he explained.
Not all therapeutic cancer vaccines would target tumors directly. As many as 16% of cancers diagnosed in 2008 were induced by an infectious agent 4. Most of these are associated with hepatitis B and C (liver cancer), Helicobacter pylori (stomach cancer) and human papilloma viruses (HPVs), which cause a variety of tumors but particularly cervical cancer in women. The infectious agent was at first regarded as a complicating factor but then became a potential vaccine target. In fact, pathogens make easier targets because they elicit a stronger immune response and avoid the issue of having to break the immune system’s tolerance to self-antigens in tumors. Prophylactic HPV vaccines have been given to teenage girls for some years now and significantly reduced the incidence of cervical cancer 5.
Continuing demand for treatment has also led to the development of therapeutic vaccines that target HPV viral antigens. Early findings suggest that administration of the HPV vaccine can clear HPV-precancerous lesions. A phase II clinical trial started in March 2014 across Europe for a therapeutic vaccine against HPV called ProCervix, which was developed by the French pharmaceutical company Genticel. ProCervix exploits the fact that the immune system is already primed by the original virus to recognize antigens in the tumor cells.
Another therapeutic vaccine for prostate cancer—ProstVac, developed by the Denmark-based biotech company Bavarian Nordic—is made by adding prostate-specific antigen to the vaccinia virus that was commonly used for smallpox vaccines, with the objective of stimulating an immune response against the tumor. Currently, patients with advanced hormone-resistant cancer are being recruited for a phase III trial. “We did see a big difference in overall survival in phase II with an increase of 8.5 months,” Jennifer Harris, Medical Science Liaison Officer at Bavarian Nordic, commented on earlier results.
“In particular therapeutic vaccines can enhance the efficacy of conventional treatments by overcoming the immune-suppression effect of tumors…”
Harris pointed out that therapeutic vaccines have most promise against slow-growing cancers, given that the immune system takes time to ramp up. She added that the approach could be combined with chemotherapy, but only if the vaccine is used first because chemotherapy tends to weaken the immune system, which would then be less able to respond effectively to stimulation by the vaccine. Such virus-based treatments where the immune system appears to respond to tumor antigens after being prompted by familiar antigens hold greater promise than approaches that require development of personalized vaccines, Melcher commented, partly because of their potential for larger-scale manufacture and therefore lower cost. “For this reason they are more attractive to the big pharmaceutical companies,” he said.
Another approach that has also shown some success in clinical trials is to target the tumor directly with a virus, which is the strategy behind a treatment called TVEC (talimogene laherparepvec) for treating melanoma. TVEC is a herpes simplex virus re-engineered to replicate in tumor tissue, where it destroys the cells by rupturing their membranes, while amplifying the white blood cell growth factor GM-CSF. In a phase III trial, 16% of the patients who had the treatment experienced a significant shrinkage of their tumors that lasted at least 6 months, compared with 2% in a control group.
Melcher pointed out that treatments like TVEC have started being referred to as oncolytic vaccines rather than oncolytic viruses as their mode of action has become clearer. In a sense, they blur the line between vaccine and drug as they have a much more active role. By attacking and destroying tumor cells, the viruses release a tumor antigen material that then triggers an immune response and so has an indirect vaccination effect.
The existing vaccines against infectious diseases made a huge difference for human health and life expectancy, and they were made possible by the growing understanding of bacteria, viruses, and the immune system. The ultimate vaccine against cancer that would recruit the body’s immune system to fight tumors is very appealing given the low side effects compared to slash, burn and poison but it is still years if not decades away from clinical reality. However, as the underlying knowledge expands, so does the development of anti-cancer vaccines improve.
Conflict of interest
The author declares that he has no conflict of interest.
References
- Weiner GJ, Liu HM, Wooldridge JE, Dahle CE, Krieg AM. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. PNAS. 1997;94:10833–10837. doi: 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- Ali OA, Emerich D, Dranoff G, Mooney DJ. In situ regulation of DC subsets and T cells mediates tumor regression in mice. Sci Transl Med. 2009;1:8ra19. doi: 10.1126/scitranslmed.3000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- Morrow MP, Yan J, Sardesai NY. Human papillomavirus therapeutic vaccines: targeting viral antigens as immunotherapy for precancerous disease and cancer. Expert Rev Vaccines. 2013;12:271–283. doi: 10.1586/erv.13.23. [DOI] [PubMed] [Google Scholar]


