Abstract
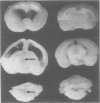
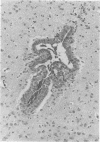
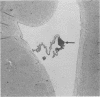
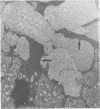
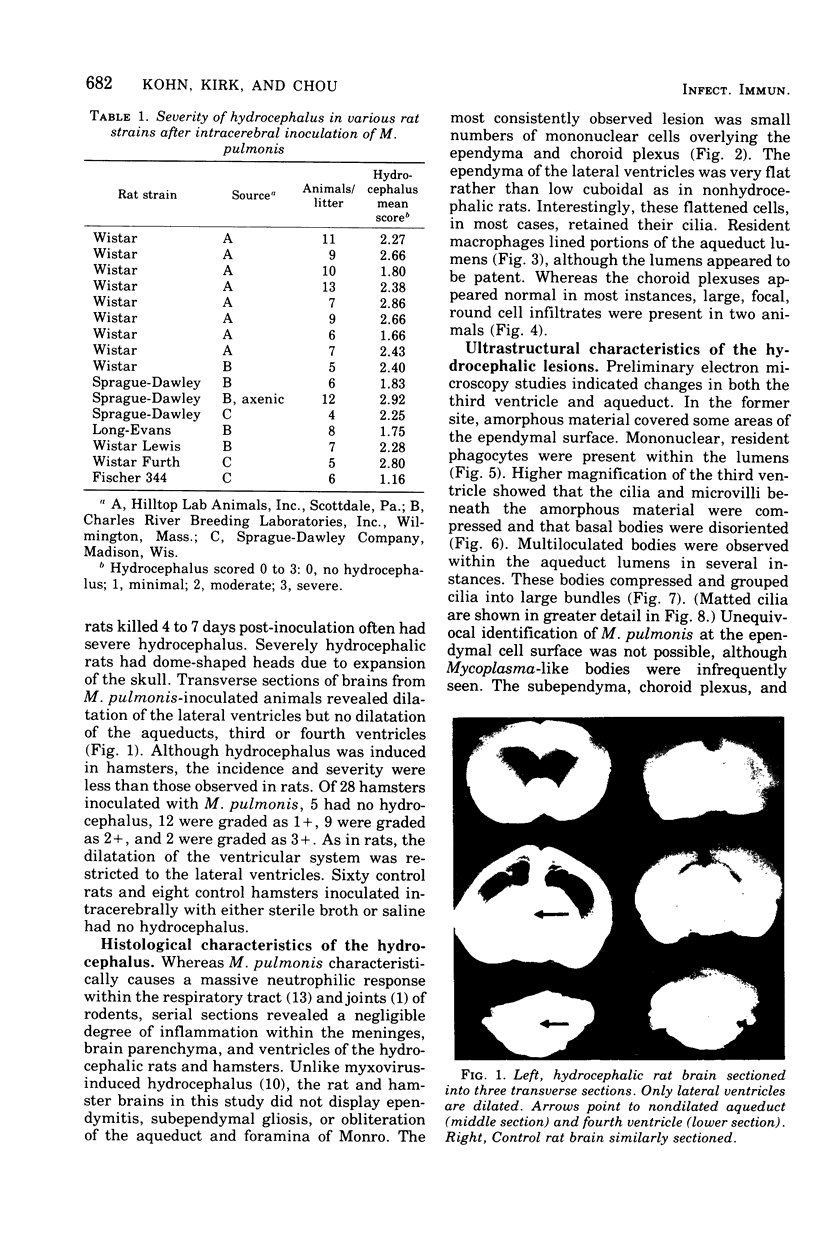
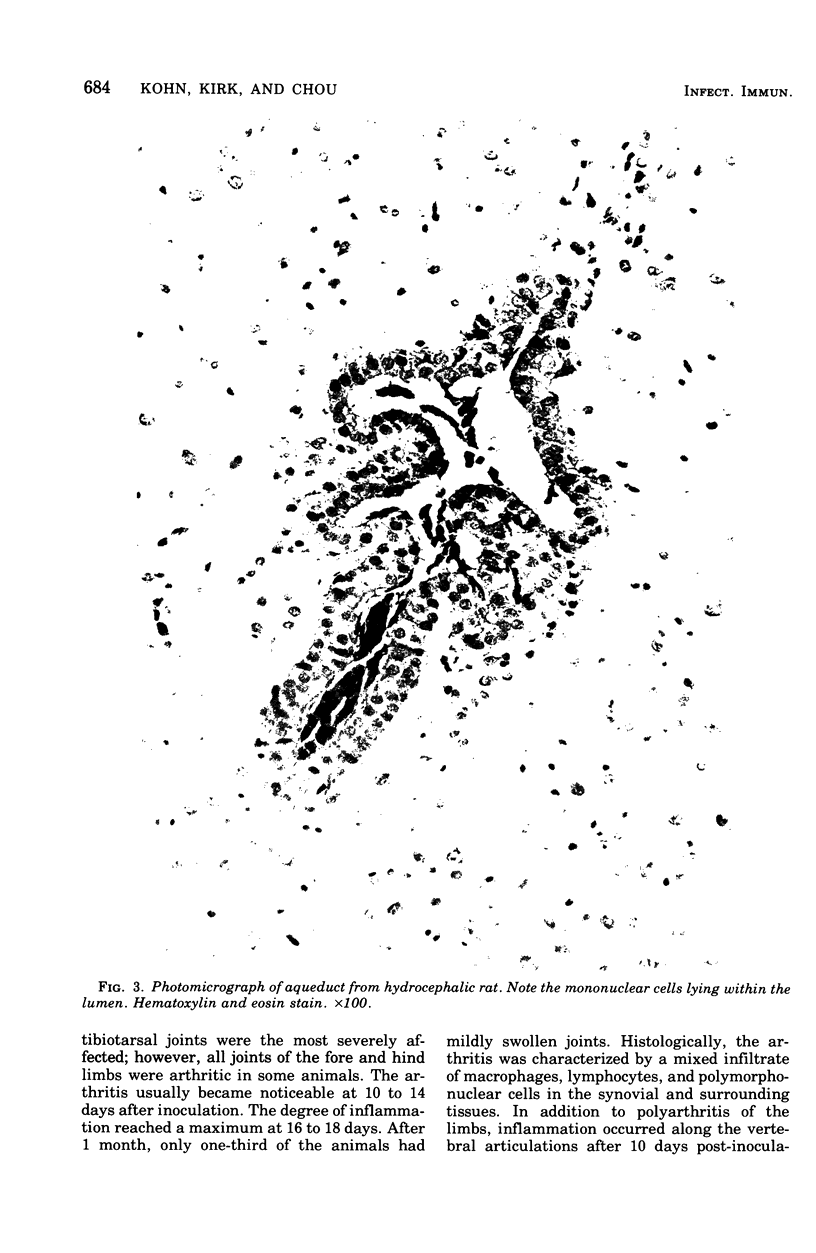
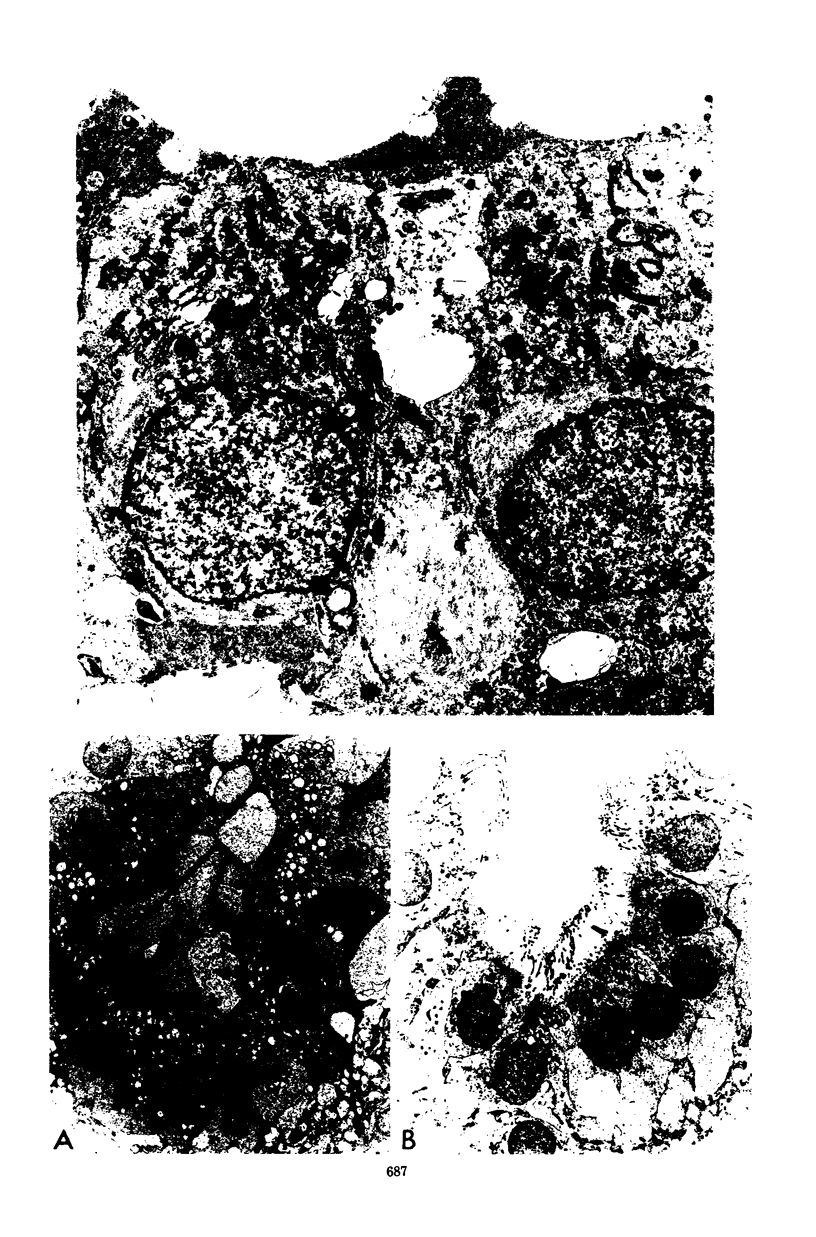
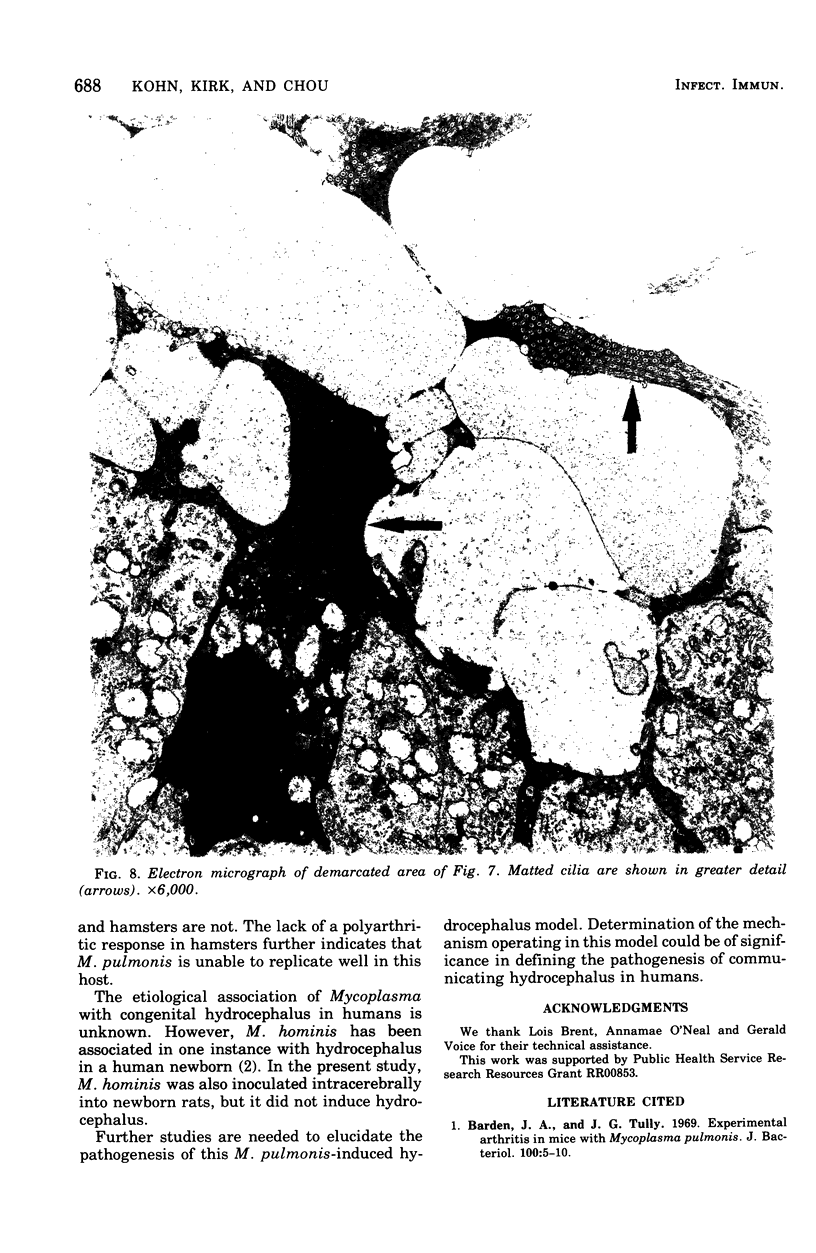
Mycoplasma pulmonis, a pathogen of the respiratory tract in rats, was inoculated intracerebrally into neonate rats and hamsters to determine if it would induce lesions in the ependyma. Hydrocephalus was induced in 116 of 120 rats and in 23 of 28 hamsters. The severity of hydrocephalus was greater in the rats than in the hamsters. Hydrocephalus induction occurred only subsequent to inoculation of viable M. pulmonis. At 2 weeks of age, rats became refractory to induction of hydrocephalus. Light microscopy indicated that the hydrocephalus was communicating without an inflammatory response in the ventricles and meninges. Preliminary electron microscopy revealed that amorphous material covered portions of the ependymal surface and that cilia were sometimes matted together. It was suggested that the hydrocephalus was due to ciliary dysfunction or to an imbalance of cerebrospinal fluid secretion and absorption. This M. pulmonis-induced hydrocephalus may be a useful model for elucidating the pathogenesis of certain types of congenital hydrocephalus in humans.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barden J. A., Tully J. G. Experimental arthritis in mice with Mycoplasma pulmonis. J Bacteriol. 1969 Oct;100(1):5–10. doi: 10.1128/jb.100.1.5-10.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boe O., Diderichsen J., Matre R. Isolation of Mycoplasma hominis from cerebrospinal fluid. Scand J Infect Dis. 1973;5(4):285–288. doi: 10.3109/inf.1973.5.issue-4.09. [DOI] [PubMed] [Google Scholar]
- Borit A., Sidman R. L. New mutant mouse with communicating hydrocephalus and secondary aqueductal stenosis. Acta Neuropathol. 1972;21(4):316–331. doi: 10.1007/BF00685139. [DOI] [PubMed] [Google Scholar]
- CLYDE W. A., Jr MYCOPLASMA SPECIES IDENTIFICATION BASED UPON GROWTH INHIBITION BY SPECIFIC ANTISERA. J Immunol. 1964 Jun;92:958–965. [PubMed] [Google Scholar]
- Duckett S. The morphology of tellurium-induced hydrocephalus. Exp Neurol. 1971 Apr;31(1):1–16. doi: 10.1016/0014-4886(71)90172-5. [DOI] [PubMed] [Google Scholar]
- Elizan T. S., Fabiyi A., Clark H. F. Suckling mouse cataract agent (SMCA)-induced hydrocephalus and chronic brain infection in newborn rats. Proc Soc Exp Biol Med. 1972 Jan;139(1):51–55. doi: 10.3181/00379727-139-36074. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Gamble D. D. Independence of leukemoid potential and toxigenicity of Mycoplasma fermentans. J Infect Dis. 1974 Dec;130(6):664–668. doi: 10.1093/infdis/130.6.664. [DOI] [PubMed] [Google Scholar]
- Hochwald G. M., Epstein F., Malhan C., Ransohoff J. The rôle of the skull and dura in experimental feline hydrocephalus. Dev Med Child Neurol Suppl. 1972;27:65–69. doi: 10.1111/j.1469-8749.1972.tb09776.x. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Johnson K. P. Hydrocephalus as a sequela of experimental myxovirus infections. Exp Mol Pathol. 1969 Feb;10(1):68–80. doi: 10.1016/0014-4800(69)90049-5. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Johnson K. P. Hydrocephalus following viral infection: the pathology of aqueductal stenosis developing after experimental mumps virus infection. J Neuropathol Exp Neurol. 1968 Oct;27(4):591–606. [PubMed] [Google Scholar]
- Kohn D. F. Bronchiectasis in rats infected with Mycoplasma pulmonis: an electron microscopy study. Lab Anim Sci. 1971 Dec;21(6):856–861. [PubMed] [Google Scholar]
- Kohn D. F., Kirk B. E. Pathogenicity of Mycoplasma pulmonis in laboratory rats. Lab Anim Care. 1969 Jun;19(3):321–330. [PubMed] [Google Scholar]
- Nielsen S. L., Baringer J. R. Reovirus-induced aqueductal stenosis in hamsters. Phase contrast and electron microscopic studies. Lab Invest. 1972 Dec;27(6):531–537. [PubMed] [Google Scholar]
- O'DELL B. L., WHITLEY J. R., HOGAN A. G. Vitamin B12, a factor in prevention of hydrocephalus in infant rats. Proc Soc Exp Biol Med. 1951 Feb;76(2):349–353. doi: 10.3181/00379727-76-18487. [DOI] [PubMed] [Google Scholar]
- Olson N. O., Kerr K. M., Campbell A. Control of infectious synovitis. 12. Preparation of an agglutination test antigen. Avian Dis. 1963 Aug;7(3):310–317. [PubMed] [Google Scholar]
- Phillips P. A., Alpers M. P., Stanley N. F. Hydrocephalus in mice inoculated neonatally by the oronasal route with reovirus type 1. Science. 1970 May 15;168(3933):858–859. doi: 10.1126/science.168.3933.858. [DOI] [PubMed] [Google Scholar]
- ROKKONES T. Experimental hydrocephalus in young rats. Int Z Vitaminforsch Beih. 1955;26(1-2):1–10. [PubMed] [Google Scholar]
- Tully J. G., Whitcomb R. F., Williamson D. L., Clark H. F. Suckling mouse cataract agent is a helical wall-free prokaryote (spiroplasma) pathogenic for vertebrates. Nature. 1976 Jan 15;259(5539):117–120. doi: 10.1038/259117a0. [DOI] [PubMed] [Google Scholar]