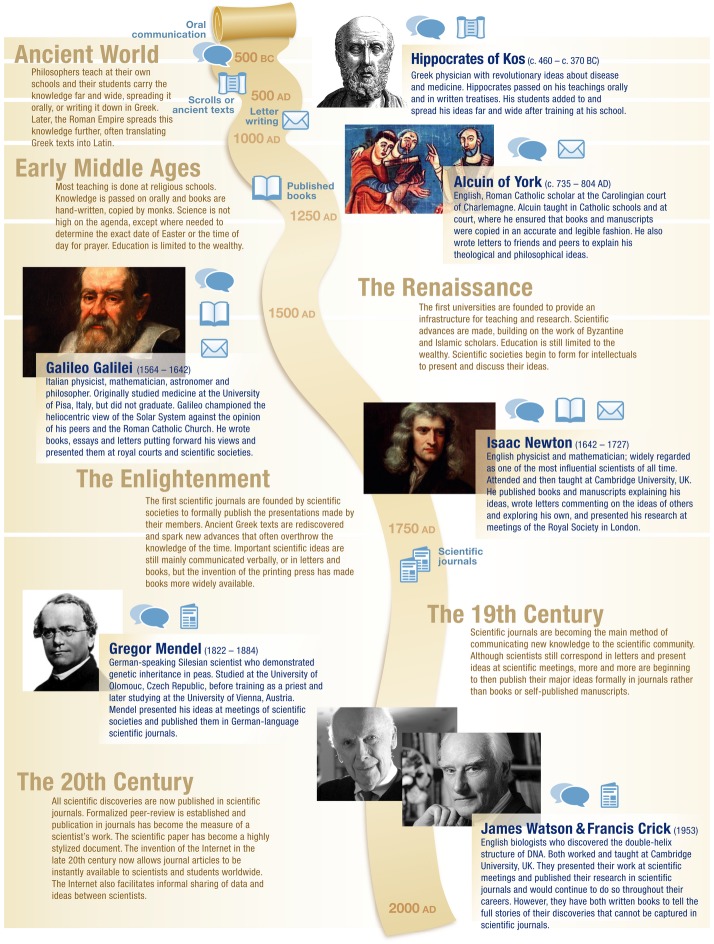
In 1963, Peter Medawar gave a talk, Is the scientific paper a fraud?, in which he argued that scientific journal articles give a false impression of the real process of scientific discovery 1. In answering his question, he argued that, “The scientific paper in its orthodox form does embody a totally mistaken conception, even a travesty, of the nature of scientific thought.” His main concern was that the highly formalized structure gives only a sanitized version of how scientists come to a conclusion and that it leaves no room for authors to discuss the thought processes that led to the experiments.
Medawar explained that papers were presented to appear as if the scientists had no pre-conceived expectations about the outcome and that they followed an inductive process in a logical fashion. In fact, scientists do have expectations and their observations and analysis are made in light of those expectations. Although today’s scientific papers are increasingly presented as being hypothesis-driven, the underlying thought processes remain hidden; scientists appear to follow a logical and deductive process to test their idea and the results of these tests lead them to support or reject the hypothesis. However, even the trend toward more explicit framing of a hypothesis is often misleading, as hypotheses may be framed to explain a set of observations post hoc, suggesting a linear process that does not describe the actual discovery.
There is, of course, a good reason why the scientific paper is highly formalized and structured. Its purpose is to communicate a finding and it is important to do this as clearly as possible. Even if the actual process of discovery had been messy, a good paper presents a logical argument, provides supporting evidence, and comes to a conclusion. The reader usually does not need or want to know about false starts, failed experiments, and changes of direction.
This approach to scientific communication has implications for teaching undergraduates the nature and practice of science as it creates a completely wrong impression of how science actually works and perpetuates a stereotype of scientists as logical and rational beings, doggedly adhering to the scientific method. Students may confuse the presentation of a logical argument with an accurate representation of what was actually done. This leads to a view of science that is unrealistic and may even be damaging as it implies that failure, serendipity, and unexpected results are not a normal part of research.
“Students may confuse the presentation of a logical argument with an accurate representation of what was actually done”
Textbooks further reinforce this view. They typically present a discovery as having been made by a single scientist, or small group of scientists, with little explanation of the fact that these scientists were building on the work of others. In addition, the discovery is often presented as apparently logically emerging from a crucial experiment or observation. This completely conceals both the process of discovery and the thought that preceded it.
A case in point is the discovery of the double helical structure of DNA by James Watson and Francis Crick. Their Nature paper reporting the discovery is famous for its elegance and brevity 2. A typical textbook account mentions that Watson and Crick used models to generate the double helix structure accommodating complementary base pairs. It usually also mentions the X-ray data of Rosalind Franklin and Maurice Wilkins but says little beyond this. As with a scientific paper, this is a question of purpose; students read textbooks to “learn facts,” rather than to learn about scientific discovery.
As Watson’s book, The Double Helix 3, makes clear, the actual process of discovery was anything but straightforward. In fact, Watson says in the preface that his reason for writing the book was because he was concerned about the general public’s impression of scientific progress: “There remains general ignorance about how science is ‘done’. That is not to say that all science is done in the manner described here. This is far from the case, for styles of scientific research vary almost as much as human personalities. On the other hand, I do not believe that the way DNA came out constitutes an odd exception to a scientific world complicated by the contradictory pulls of ambition and the sense of fair play.”
By way of example, two crucial mistakes were made during the discovery. The first resulted from Watson misunderstanding the X-ray data, which he described as a humiliating experience when he presented an incorrect model, with the bases on the outside, to Franklin and Wilkins. The other mistake was made by Linus Pauling, who published a triple helix structure with the bases on the outside, having inexplicably neglected some basic chemistry. In both cases, it was feedback from others, owing to formal and informal interactions within the scientific community, that corrected these mistakes. Furthermore, the base structures Watson was using—taken from a textbook—showed tautomeric forms that would not have formed complementary base pairs. This error was only corrected because Watson shared an office with a chemist, Jerry Donohue, who saw what Watson was working on and provided the correct tautomeric structures 3, a crucial piece of luck.
This presents a completely different view than that presented in either a standard textbook or Watson and Crick’s Nature paper. It shows science as an activity whereby ideas are tried out, discussed, and modified and where mistakes are all too common. It reinforces the views expressed by Ernst Mayr 4, “All interpretations made by a scientist are hypotheses, and all hypotheses are tentative. They must forever be tested and they must be revised if found to be unsatisfactory. Hence, a change of mind in a scientist, and particularly in a great scientist, is not only not a sign of weakness but rather evidence for continuing attention to the respective problem and an ability to test the hypothesis again and again.”
As practicing scientists, we know that many of our ideas, experiments, and results will never be published: troubleshooting and optimization become unimportant the moment the problem is solved; negative results and false directions are rarely reported; much data that is collected is simply uninteresting. A crucial aspect of science is trying out new ideas, using imagination and remaining open to the unexpected. Many of us subscribe to Max Delbrück’s principle of limited sloppiness 5, “If you are too sloppy, you never get any reproducible results and you can never draw any conclusions, but if you are just a little bit sloppy, then when you see something startling you say, ‘Oh my God, what did I do, what did I do different this time?’ and if you really accidentally varied just one parameter, you nail it down.”
The contrast between The Double Helix, the Nature paper, and the textbooks is not problematic in themselves, because these different accounts have different purposes and audiences. The problem arises because undergraduates are more likely to read textbooks and papers during their education than autobiographies. It is possible to go through a complete science degree reading nothing else than the sanitized view of science. This is especially true when students are introduced to journal articles early in their degree, either as models for how to write laboratory reports or to introduce cutting-edge research well before they experience the reality of research.
“It is possible to go through a complete science degree reading nothing else than the sanitized view of science”
As undergraduates view science largely through textbooks and papers, it is not surprising that many stick to a stereotypical view of scientists and scientific research. In fact, the literature on students’ conceptions of the nature of science bemoans the fact that these naïve conceptions are very hard to change. In his 1992 review of research in this area, Norman Lederman traces the desire to improve students’ understanding of the nature of science as far back as 1907 and discusses the lack of success 6; his 2007 review of the subsequent literature comes to nearly identical conclusions 7, suggesting that little progress has been made. The belief that the scientific method is a clearly defined series of steps is particularly resistant to change, even after students experience the reality of research 8.
Science is a social activity and we do students a disservice if we maintain that it is only about facts and objective truths. While individual scientists make personal judgments about their research and what they publish, the ultimate arbiter of the value of research is the scientific community. A discovery will be accepted only if the evidence is sufficient to persuade the larger community, and this may not happen immediately. Evidence is always subject to interpretation and interpretations change in light of new information. An individual scientist therefore has to convince his or her peers that a hypothesis is right, rather than simply presenting results from experiments or observations. This does not imply a relativist position: scientific progress is ultimately based on evidence, but it is a community decision whether or not the evidence is sufficient to accept a new hypothesis.
It is therefore important to distinguish between the individual scientist and the community. While science attains objectivity through continuous assessment, scrutiny, and reproducing results, individual scientists are not necessarily objective. As Stephen Jay Gould commented 9, “[…] we scientists are no different from anyone else. We are passionate human beings, enmeshed in a web of personal and social circumstances. Our field does recognize canons of procedure designed to give nature the long shot of asserting herself in the face of such biases, but unless scientists understand their hopes and engage in vigorous self-scrutiny, they will not be able to sort out unacknowledged preference from nature’s weak and imperfect message.”
“The belief that the scientific method is a clearly defined series of steps is particularly resistant to change, even after students experience the reality of research”
One needs only to look at the history of science to see that it is fraught with arguments and changing perspectives. Scientists are individuals and have different levels of tolerance for new ideas, different standards by which they evaluate evidence, and different levels of awareness of their own biases. Part of Peter Medawar’s problem with the scientific paper was that it presents a spurious objectivity at the level of the individual.
Understanding the difference between active science and communicating science matters, because of the important role of science in modern societies. We increasingly depend on science and technology to address societal problems, and we need some scientific literacy to develop informed opinions on issues such as global warming, medical research, or genetically modified organisms. An important aspect is the ability to use judgment. If science is seen as a completely objective enterprise, there is no need for judgment because “the facts speak for themselves.” However, scientists make judgments all the time; what techniques to use, what model system might be appropriate, choosing a particular experimental design, which parameters should be modified, accepting or ignoring data, or deciding on what statistical tests and significance levels are appropriate.
Introducing undergraduate and high school students to a more realistic view of science and how it is done is just as important for recruiting future scientists as for producing scientifically literate citizens. The counterargument is that those who become scientists eventually develop a better understanding of research once they start their undergraduate research projects or during postgraduate study. However, there are at least two reasons why this is unsatisfactory. First, it is surely helpful to have a better idea of what scientific research is like before making a career choice. Martin Schwartz, in a personal essay 10, describes his realization as a postgraduate student that being good at exams was not a good preparation for the “immersion into the unknown” required to do scientific research. He relates his attempts to solve a problem by looking for someone who knew the answer. It was only when he realized that no one knew the answer—and that that was the whole point of research—that he was able to find his own solution. An education that introduces students to the uncertainty of research before their postgraduate work would better prepare them to face these challenges.
The second reason for teaching the process of scientific research early on is that it has implications for recruiting scientists. Much science education at both secondary and tertiary level gives students the impression that science is about learning facts. Many students are put off by this approach and the perception that there is no scope within science for discussion, creativity, and imagination 11. Girls and ethnic minority groups in particular might be deterred from science because of the factual and authoritarian focus of much science education 12. A more balanced approach to teaching science might improve recruitment and increase the diversity and talent within the scientific community.
Despite repeated calls to adapt science education at both secondary and tertiary level, much remains to be done. Developing the ability to make judgment calls requires that students face challenges where they have some control over process and outcomes. Moreover, students need to learn that mistakes or false starts are not time wasted, but are an essential part of making progress. They also need to understand that the scientific method is not a series of well-defined steps that always produces an answer, but a dynamic process that requires intellectual engagement and judgment.
“…students need to learn that mistakes or false starts are not time wasted, but are an essential part of making progress”
As two scientists who have been teaching a first-year university course about the nature and practice of science, we have first-hand experience of the shock students experience when they discover that the process of science is influenced by scientists’ personalities and expectations. After reading and discussing Medawar’s essay 1, many students agree that they have been misled to believe in a particular image of science, which does not reflect reality. The Double Helix 3 is shocking in a different way as it highlights the crucial role of social interactions and how the different personalities of scientists are such an important factor for discovery.
Many discussions about how to develop science education recommend introducing students to authentic research through experience and inquiry-learning, but just doing a research project and submitting a formal report at the end can perpetuate the myth that the only good science is what goes into the report. Research experiences, including failures, are important for students, not just because they get to do research, but also as an opportunity to discuss what it is like to be a scientist and to help them see that “real” science is just as much the bits that don’t go into the report as the bits that do. Doing science and communicating science are quite different things; in the fifty years since Peter Medawar expressed his concern about the scientific paper, little has changed.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Medawar P. Is the scientific paper a fraud? Listener. 1963;70:377–378. [Google Scholar]
- Watson J, Crick F. A structure for deoxyribose nucleic acid. Nature. 1953;171:737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- Watson J. The Double Helix. New York, NY:: Atheneum Publishers; 1968. [Google Scholar]
- Mayr E. The Growth of Biological Thought: Diversity, Evolution and Inheritance. Cambridge, MA: The Belknap Press of Harvard University Press; 1982. [Google Scholar]
- Delbrück M. Interview by Carolyn Harding. Pasadena, California, July 14-September 11, 1978. Oral History Project, California Institute of Technology Archives. Retrieved Nov 15th 2013 from the World Wide Web: http://resolver.caltech.edu/CaltechOH:OH_Delbruck_M.
- Lederman NG. Students’ and teachers’ conceptions of the nature of science: a review of the research. J Res Sci Teach. 1992;29:331–359. [Google Scholar]
- Lederman NG. Nature of science: past, present and future. In: Abell SK, Lederman NG, editors. Handbook of Research on Science Education. Mahwah, NJ: Lawrence Erlbaum Associates; 2007. pp. 831–880. [Google Scholar]
- Cartrette D, Melroe-Lehrman B. Describing changes in undergraduate students’ preconceptions of research activities. Res Sci Educ. 2012;42:1073–1100. [Google Scholar]
- Gould S. An Urchin in the Storm. London: Collins Harvill; 1988. Nurturing nature. [Google Scholar]
- Schwartz M. The importance of stupidity in scientific research. J Cell Sci. 2008;121:1771. doi: 10.1242/jcs.033340. [DOI] [PubMed] [Google Scholar]
- Lyons T. Different countries, same science classes: students’ experiences of school science in their own words. Int J Sci Educ. 2006;28:591–613. [Google Scholar]
- Hodson D. Going beyond cultural pluralism: science education for sociopolitical action. Sci Educ. 1999;83:775–796. [Google Scholar]