Abstract
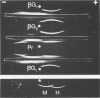
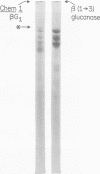
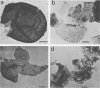
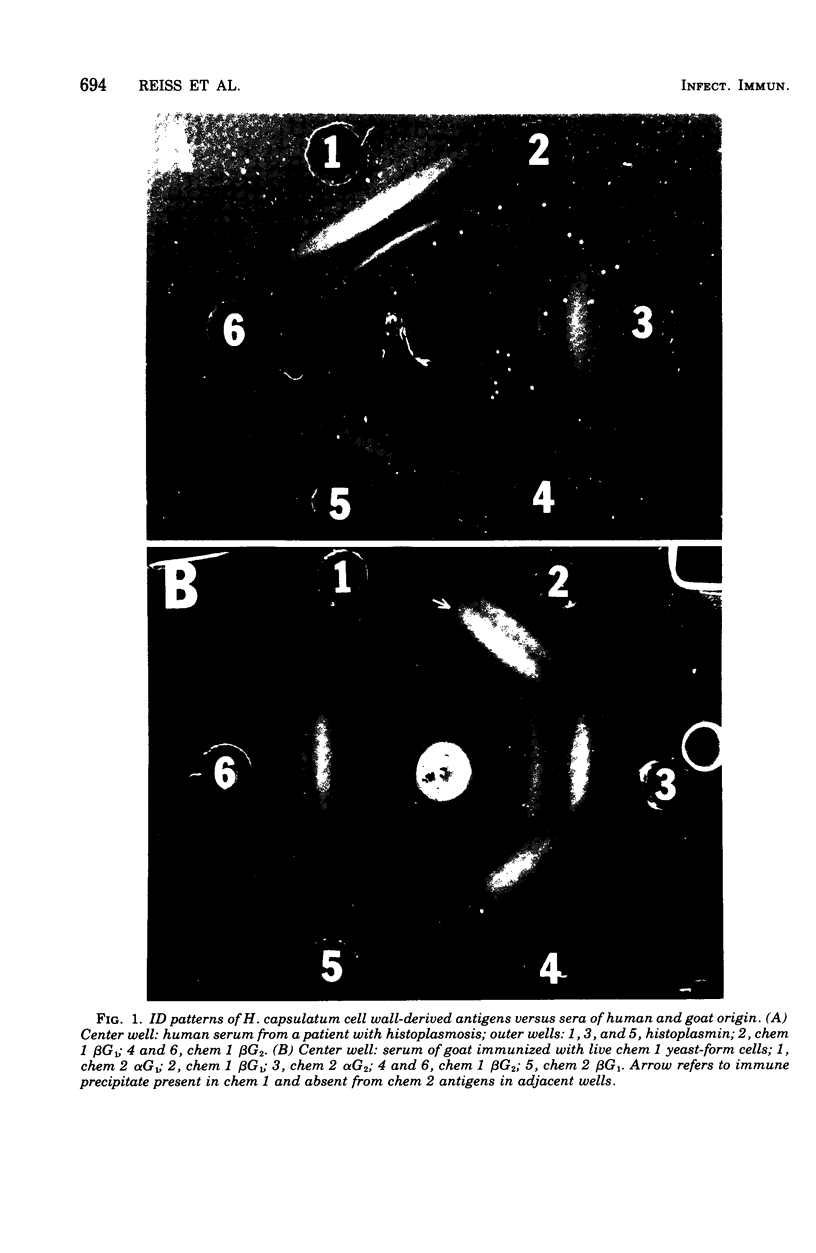
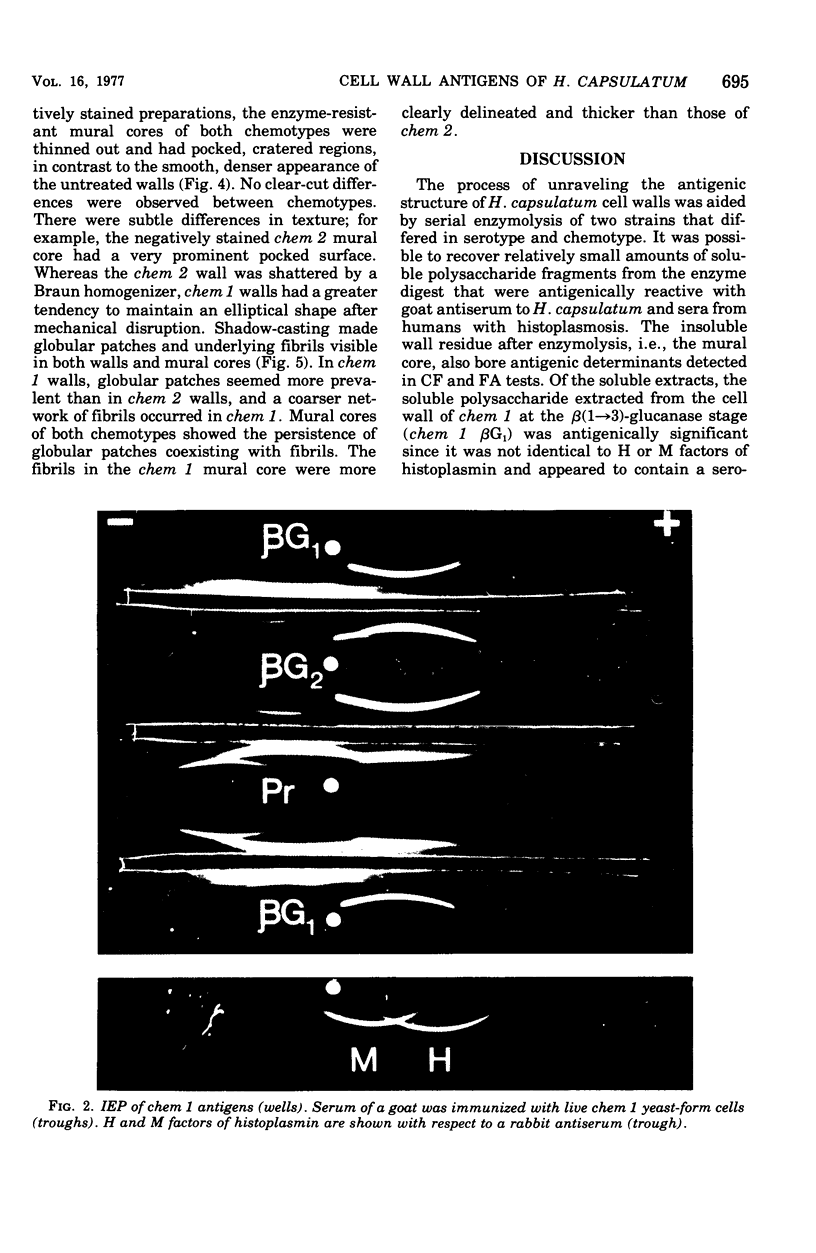
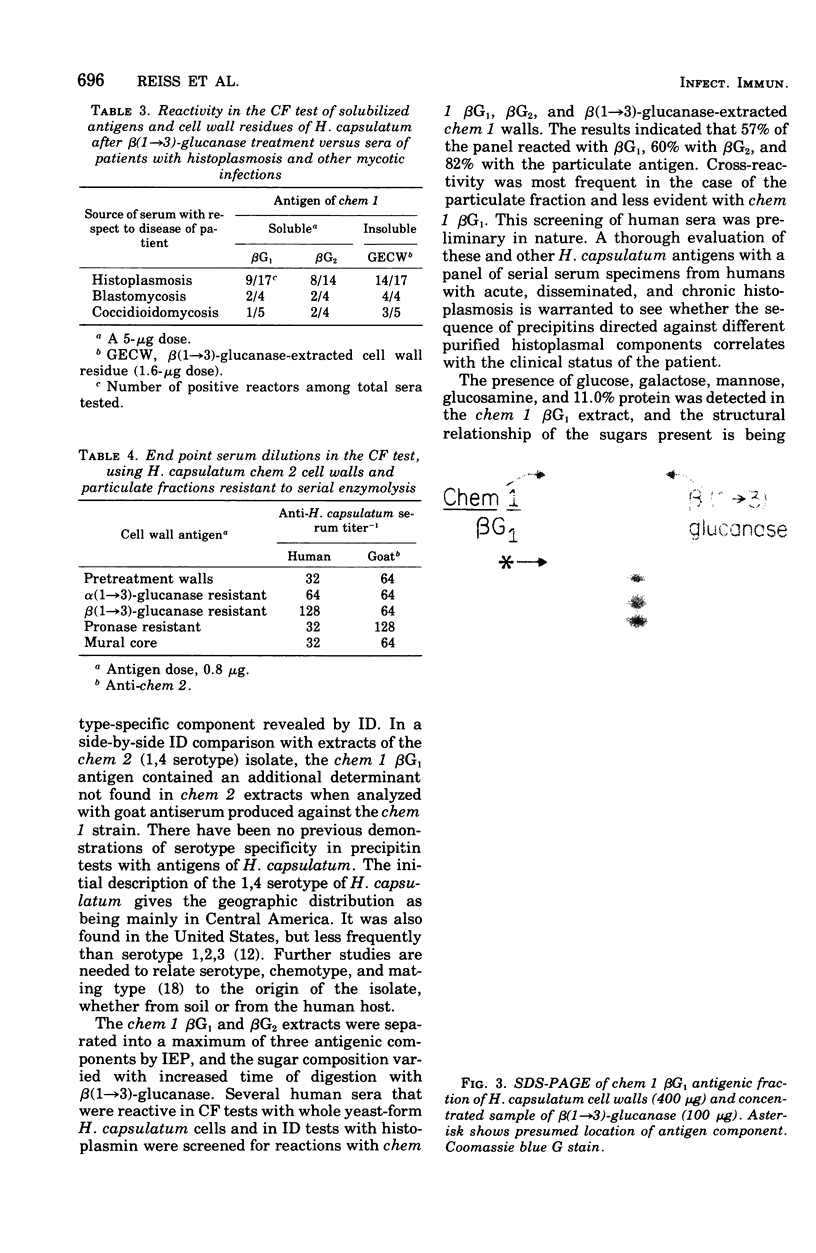
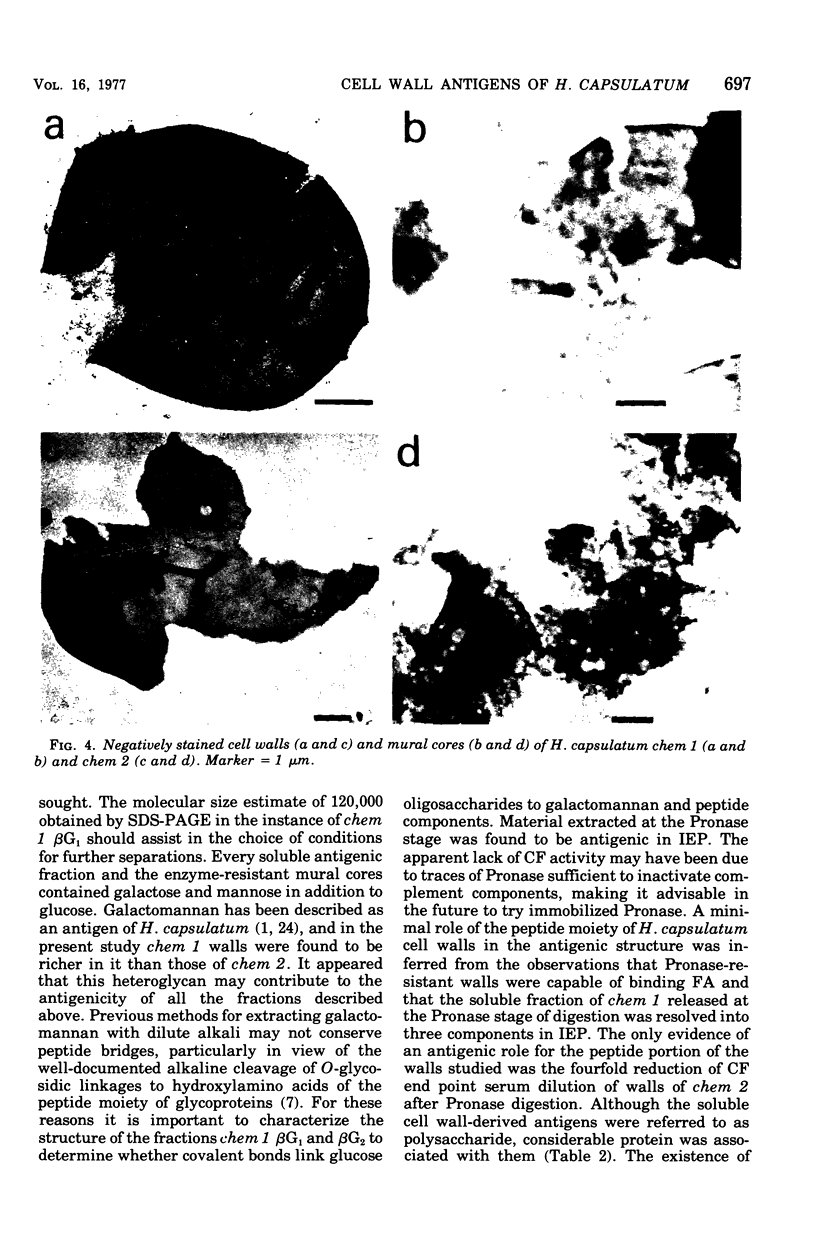
Cell walls of Histoplasma capsulatum yeast-form chemotypes 1 (chem 1) and 2 (chem 2) treated sequentially with several polysaccharolytic enzymes and Pronase yielded soluble, nondialyzable polysaccharides at each step, which were analyzed for monosaccharides, protein composition, and serological activity. Polysaccharide recovered after digestion of chem 1 walls with β(1→3)-glucanase contained glucose > mannose > glucosamine > galactose. This fraction (chem 1 βG1) was analyzed by polyacrylamide gel electrophoresis and contained a component having an apparent molecular weight of 120,000. The chem 1 βG1 fraction was reactive in immunodiffusion (ID), producing an immune precipitate not identical to the H and M factors of histoplasmin. In a side-by-side ID comparison with extracts of chem 2, the chem 1 βG1 antigen contained an additional determinant not found in chem 2 extracts when tested with goat antiserum to H. capsulatum. Therefore, the chem 1 antigen gave preliminary ID evidence of antigenic group specificity. A chemical difference observed was the absence of glucosamine from chem 2 polysaccharide. In complement fixation (CF) tests, 9 of 17 sera from human histoplasmosis patients reacted with chem 1 βG1, but some cross-reactivity with sera of patients with other systemic mycoses occurred. The immunoelectrophoretic patterns of chem 1 wall-derived polysaccharides showed a marked shift in mobility after Pronase digestion, implying the presence of covalent peptides. The ultrastructural appearance and serological activity of intact walls and enzyme-resistant mural cores were also studied. The surface of the mural cores of both chemotypes was perforated and frayed. In shadow-cast preparations both fibrillar and globular areas persisted in the mural cores. The CF end point serum dilutions showed an increase after α- and β-glucanase extractions of chem 2 walls and fourfold reduction after Pronase digestion. The mural cores of both chemotypes were still reactive in CF tests and retained some ability to bind fluorescent antibody. The chem 1 mural core reacted with specific fluorescein-labeled H. capsulatum antiglobulins produced by adsorption with Blastomyces dermatitidis, thus indicating at least partial retention of H. capsulatum-specific factors. The presence of galactose, mannose, and glucose was detected in the mural cores as well as enriched levels of amino sugar, despite exposure to chitinase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma I., Kanetsuna F., Tanaka Y., Yamamura Y., Carbonell L. M. Chemical and immunological properties of galactomannans obtained from Histoplasma duboisii, Histoplasma capsulatum, Paracoccidioides brasiliensis and Blasomyces dermatitidis. Mycopathol Mycol Appl. 1974 Oct 15;54(1):111–125. doi: 10.1007/BF02055979. [DOI] [PubMed] [Google Scholar]
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- Colonna W. J., Lampen J. O. Structure of the mannan form Saccharomyces strain FH4C, a mutant constitutive for invertase biosynthesis. I. Significance of phosphate to the structure and refractoriness of the molecule. Biochemistry. 1974 Jun 18;13(13):2741–2748. doi: 10.1021/bi00710a013. [DOI] [PubMed] [Google Scholar]
- Domer J. E. Monosaccharide and chitin content of cell walls of Histoplasma capsulatum and Blastomyces dermatitidis. J Bacteriol. 1971 Sep;107(3):870–877. doi: 10.1128/jb.107.3.870-877.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J. P., Howard D. H. Characterization of antigens from the yeast phase of Histoplasma capsulatum. Infect Immun. 1971 Aug;4(2):116–125. doi: 10.1128/iai.4.2.116-125.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEINER D. C. Diagnosis of histoplasmosis using precipitin reactions in agargel. Pediatrics. 1958 Oct;22(4 Pt 1):616–627. [PubMed] [Google Scholar]
- KAUFMAN L., KAPLAN W. Preparation of a fluorescent antibody specific for the yeast phase of Histoplasma capsulatum. J Bacteriol. 1961 Nov;82:729–735. doi: 10.1128/jb.82.5.729-735.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanetsuna F., Carbonell L. M., Gil F., Azuma I. Chemical and ultrastructural studies on the cell walls of the yeastlike and mycelial forms of Histoplasma capsulatum. Mycopathol Mycol Appl. 1974 Oct 15;54(1):1–13. doi: 10.1007/BF02055967. [DOI] [PubMed] [Google Scholar]
- Kaufman L., Blumer S. Occurrence of serotypes among Histoplasma capsulatum strains. J Bacteriol. 1966 Apr;91(4):1434–1439. doi: 10.1128/jb.91.4.1434-1439.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi G. S., Guiliacci P. L. Cell wall studies of Histoplasma capsulatum. Sabouraudia. 1967 Feb;5(3):180–188. [PubMed] [Google Scholar]
- Kopecká M., Phaff H. J., Fleet G. H. Demonstration of a fibrillar component in the cell wall of the yeast Saccharomyces cerevisiae and its chemical nature. J Cell Biol. 1974 Jul;62(1):66–76. doi: 10.1083/jcb.62.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Weeks R. J., Larsh H. W. Studies on Emmonsiella capsulata (Histoplasma capsulatum). II. Distribution of the two mating types in 13 endemic states of the United States. Am J Epidemiol. 1974 Jan;99(1):44–49. doi: 10.1093/oxfordjournals.aje.a121583. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. Permanganate fixation of plant cells. J Biophys Biochem Cytol. 1959 Dec;6:431–436. doi: 10.1083/jcb.6.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obijeski J. F., Marchenko A. T., Bishop D. H., Cann B. W., Murphy F. A. Comparative electrophoretic analysis of the virus proteins of four rhabdoviruses. J Gen Virol. 1974 Jan;22(1):21–33. doi: 10.1099/0022-1317-22-1-21. [DOI] [PubMed] [Google Scholar]
- Pine L., Boone C. J. Cell wall composition and serological reactivity of Histoplasma capsulatum serotypes and related species. J Bacteriol. 1968 Sep;96(3):789–798. doi: 10.1128/jb.96.3.789-798.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss E., Mitchell W. O., Stone S. H., Hasenclever H. F. Cellular immune activity of a galactomannan-protein complex from mycelia of Histoplasma capsulatum. Infect Immun. 1974 Oct;10(4):802–809. doi: 10.1128/iai.10.4.802-809.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss E. Serial enzymatic hydrolysis of cell walls of two serotypes of yeast-form Histoplasma capsulatum with alpha(1 leads to 3)-glucanase, beta(1 leads to 3)-glucanase, pronase, and chitinase. Infect Immun. 1977 Apr;16(1):181–188. doi: 10.1128/iai.16.1.181-188.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San-Blas G., Carbonell L. M. Chemical and ultrastructural studies on the cell walls of the yeastlike and mycelial forms of Histoplasma farciminosum. J Bacteriol. 1974 Aug;119(2):602–611. doi: 10.1128/jb.119.2.602-611.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]