Table 1. Synthesis of (dtbbpy)NiII(COR1)Br and Selected Characterization Dataa.
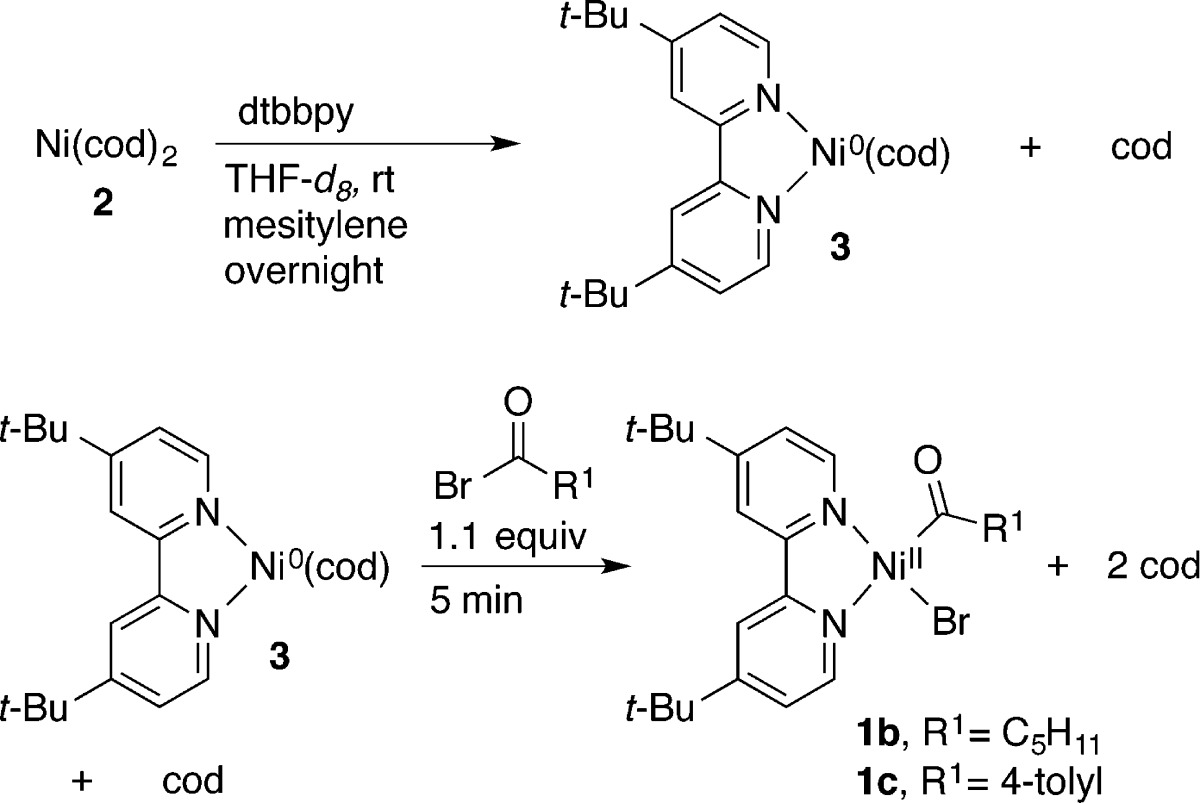
| entryb | [Ni] | yield (%)c | IR (cm–1)d | NMR (ppm)e | UV–vis (cm–1) (103 M–1 cm–1)f |
|---|---|---|---|---|---|
| 1 | 1b | 74 | 1612 | 3.12 (t) | 20325 (5) |
| 2 | 1c | 89 | 1617 | N/A | 20576 (7) |
dtbbpy = 4,4′-di-tert-butyl-2,2′-bipyridine.
See the Supporting Information for full characterization data and procedures.
1H NMR yield vs liberated cyclooctadiene; average of two runs.
C=O stretches reported that are characteristic of M–C(O)R complexes.15e
1H NMR chemical shift and multiplicity for α proton of M–C(O)CH2R.15c N/A = not applicable.
UV–vis absorbances reported as wavenumbers (ε values are given in parentheses).
