Table 4. Optimization and Controlsa.
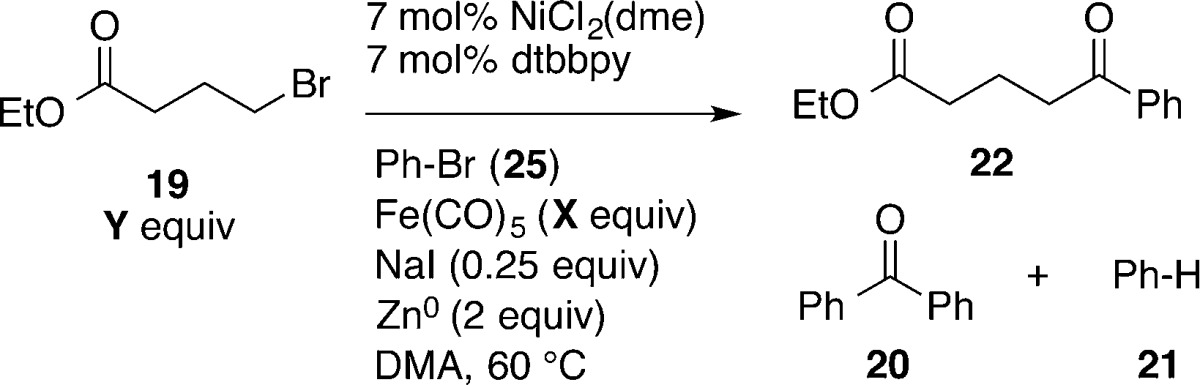
| entry | notes | X | Y | 22 | 20 | 21 |
|---|---|---|---|---|---|---|
| 1 | 0.35 | 2.50 | 69 | 5 | 4 | |
| 2 | 0.35 | 2.25 | 72 (60) | 6 | 6 | |
| 3 | 0.35 | 2.0 | 56 | 7 | 8 | |
| 4 | 0.35 | 1.5 | 50 | 9 | 15 | |
| 5b | 0.40 | 2.0 | 51 | 7 | 8 | |
| 6c | 0.45 | 2.0 | 42 | 3 | 5 | |
| 7c | 0.50 | 2.0 | 32 | 3 | 4 | |
| 8d | no NaI | 0.35 | 2.0 | 0 | 0 | 0 |
| 9 | LiI for NaI | 0.35 | 2.0 | 49 | 7 | 7 |
| 10 | KI for NaI | 0.35 | 2.0 | 48 | 4 | 8 |
| 11 | 5 mol % catalyst | 0.35 | 2.0 | 42 | 6 | 9 |
| 12e | no dtbbpy | 0.35 | 2.25 | 0 | 0 | 2 |
| 13e,f | no Ni or Zn | 0.35 | 2.0 | 0 | 0 | 1 |
| 14e,g | no Ni | 0.35 | 2.0 | <1 | <1 | 0 |
| 15c,g | no Zn | 0.35 | 2.25 | 25 | <1 | 4 |
| 16 | 6/1 DMA/THF | 0.35 | 2.25 | 76 | 7 | 0 |
| 17 | 1/1 DMA/THF | 0.35 | 2.25 | 52 | 7 | 3 |
| 18e,g | 1/2 DMA/THF | 0.35 | 2.25 | <1 | 0 | 1 |
Reactions were run on a 0.75 mmol scale in 3 mL of DMA. Yields are uncorrected GC yields vs dodecane. The yield in parentheses is an isolated yield of purified product.
PhBr (8%) remained.
PhBr (27%–47%) remained.
Starting materials were unchanged after 40 h, except for a 13% yield of hydrodehalogenated 19.
PhBr (75%) remained.
Alkyl-Br 19 not consumed.
Dialkyl ketone (12–23%) was formed.
