Abstract
Objectives
This study aimed to evaluate the efficacy of gemcitabine-based chemoradiotherapy followed by surgery (gem-CRTS) for pancreatic ductal adenocarcinoma (PDAC) for borderline resectable (BR) and locally unresectable (UR) tumors.
Methods
One hundred patients with PDAC who underwent the gem-CRTS protocol were classified into 3 groups, namely, resectable (R; 14), BR (44), and UR (42). After chemoradiotherapy, the patients were reassessed for curative-intent resection.
Results
At reassessment, distant metastases became apparent in 27% of R patients, in 12% of BR patients, and in 18% of UR patients. The multivariate analysis of preoperative factors indicated that the CA19-9 reduction rate was an independent prognostic factor in the BR group. Among reassessed patients, the resection rate was 63.6% in R, 83.7% in BR, and 50.0% in UR patients. In 63 patients that underwent curative-intent resection, the 3-year survival rate was 83.3% in R, 33.0% in BR, and 7.8% in UR patients. Using multivariate analysis, the independent prognostic factor was found to be the surgical margin in BR patients and human equilibrative nucleoside transporter 1 expression in UR patients.
Conclusions
We consider that our gem-CRTS protocol, even for locally UR PDAC, allows for the identification of candidates for aggressive resection at the time of reassessment and improved prognosis in the patients with positive human equilibrative nucleoside transporter 1 expression.
Key Words/Abbreviations: pancreatic cancer, preoperative chemoradiotherapy, resectabilty, gemcitabine, human equilibrative nucleoside transporter 1 expression, AC - adjuvant chemotherapy, BR - borderline resectable, CA - celiac artery, CRT - chemoradiotherapy, CT - computed tomography, DP - distal pancreatectomy, EMT - epithelial-mesenchymal transition, EUS-FNA - endoscopic ultrasonography–guided fine-needle aspiration, gem-CRTS - gemcitabine-based chemoradiotherapy followed by surgery, hENT-1 - human equilibrative nucleoside transporter-1, NCCN - National Comprehensive Cancer Network, NCRT - neoadjuvant chemoradiotherapy, MDCT - multidetector computed tomography, MSTs - median survival times, PD - pancreaticoduodenectomy, PDAC - pancreatic ductal adenocarcinoma, PV - portal vein, R - resectable, SMA - superior mesenteric artery, SMV - superior mesenteric vein, UICC - Union Internationale Contre le Cancer, UR - unresectable
Pancreatic ductal adenocarcinoma (PDAC) has been suggested to be a systemic disease at the time of diagnosis because of the exceedingly high rates of distant metastatic recurrence even after successful surgical resection of early-stage tumors.1 Therefore, a multimodal treatment of PDAC is required because surgical treatment alone does not greatly improve survival. Chemoradiotherapy (CRT) before surgery for PDAC may provide for the early treatment of a micrometastatic disease, allow for the identification of patients with metastatic disease at the time of reassessment, and increase the R0 resection rate, resulting in a reduced risk for local tumor recurrence.2,3
Nevertheless, surgical resection is the only potentially curative technique for managing PDAC. R0 resection is a strong prognostic indicator for long-term patient survival.4,5 With respect to margin status, the survival benefits of an incomplete resection (R1 or R2) may be comparable to a definitive CRT without surgery.6 The National Comprehensive Cancer Network (NCCN) have developed guidelines to define tumor resectability in PDAC,7 so as to improve patient selection for surgery and increase the likelihood of an R0 resection. Using their criteria, PDAC is classified as resectable (R), borderline resectable (BR), or unresectable (UR), for example, locally advanced or metastatic disease. The probability of achieving an R0 resection is a key criterion for determining whether a patient is a potential candidate for resection. In this setting, a BR tumor can be defined as one that increases the likelihood of an incomplete resection. According to a previous report,8 R and BR tumors have been categorized as potentially resectable tumors. On the contrary, UR tumors are locally advanced PDACs including tumors with superior mesenteric artery (SMA) or celiac artery (CA) encasement greater than 180°, unreconstructable portal vein (PV)/superior mesenteric vein (SMV) occlusion, or aortic invasion or encasement in addition to distant metastases or nodal metastasis beyond the field of resection.
With respect to the recommended treatment of the 3 groups, in group R, initial surgical resection is defined as the standard therapeutic strategy, but some reports have suggested that neoadjuvant CRT (NCRT) for R tumors is feasible9–11 because PDAC is a systematic disease. Even if R0 resection is achieved for an R tumor, 10% to 15% of them will exhibit an early recurrence; thus, NCRT could help select patients who might not benefit from surgical resection. In the BR group, although there is no high-level evidence supporting treatment with NCRT, some institutions prefer an initial approach involving neoadjuvant therapy.7 In the UR group, NCRT is not usually used but it has been reported that UR tumors may be downstaged by CRT to allow for surgical resection.12–14 Consequently, there has been no consensus or clear evidence concerning the indication of NCRT for R, BR, and UR tumors.
Our institution has introduced gemcitabine-based CRT followed by surgery (gem-CRTS) for the treatment of PDAC since February 2005. Gemcitabine (20,20-difluoro-2-2-deoxycitidine analog) inhibits DNA replication and repair. Recently, intratumoral human equilibrative nucleoside transporter 1 (hENT1), which is the major transporter responsible for gemcitabine uptake into cells, has been reported as an important predictive marker of chemosensitivity for gemcitabine-based adjuvant chemotherapy (AC) for PDAC.15 We previously reported that hENT1 expression was an independent predictor of overall survival after gem-CRTS in patients with Union Internationale Contre le Cancer (UICC) T3 to T4 PDAC.16 However, there have been no previous reports describing the significance of gem-CRTS for PDAC according to resectability based on the NCCN guidelines, especially focusing on hENT1 expression.
The aim of the present study was to evaluate the efficacy of gem-CRTS for the treatment of PDAC regarding the 3 resectability groups (R, BR, and UR) defined by the NCCN pancreatic cancer guidelines (2010), with special attention to serum CA19-9 alternation and hENT1 expression.
Materials and Methods
Between February 2005 and October 2010, 100 patients with PDAC who were diagnosed as having UICC-T3 and UICC-T4 tumors using multidetector computed tomography (MDCT) were enrolled for our gem-CRTS protocol. All patients were warned of the risks of treatment, especially concerning the possibility of developing distant metastases after gem-CRT treatment. They all gave their written informed consent for inclusion in the study. The diagnosis of pancreatic cancer was confirmed by means of cytological or histological analysis of biopsy specimens obtained using endoscopic ultrasonography–guided fine-needle aspiration (EUS-FNA). Patients were excluded when the tumor extension determined by MDCT was categorized as UICC-T1 or UICC-T2 and/or when they showed evident distant metastatic lesions. The study protocol was approved by the medical ethics committee of Mie University, and the study was performed in accordance with the ethical standards established in the 1964 Declaration of Helsinki.
All patients underwent pretreatment examination using a 64-slice MDCT. Computed tomography (CT) was performed according to a defined pancreas protocol as 4-phasic contrast-enhanced MDCT with thin slices at intervals of 1 mm. In the present study, all of these 100 patients were reclassified into the 3 groups (R, BR, and UR) according to NCCN guidelines (2010)7 based on MDCT findings at the initial visit to our hospital. The CT criteria of the NCCN guidelines are as follows: R criteria, (1) no evidence of SMV and PV abutment, distortion, tumor thrombus, or venous encasement and (2) clear fat planes around the CA, hepatic artery, and SMA; BR criteria, (1) venous involvement of the PV/SMV demonstrating tumor abutment without impingement and narrowing of the lumen, encasement of the PV/SMV without encasement of the nearby arteries, or short-segment venous occlusion resulting from either tumor thrombus or encasement but with suitable vessel proximal and distal to the area of vessel involvement, allowing for safe resection and reconstruction, (2) gastroduodenal artery encasement up to the hepatic artery with either short-segment encasement or direct abutment of the hepatic artery without extension to the CA, and (3) tumor abutment of the SMA not exceeding greater than 180° of the circumference of the vessel wall; and UR criteria, (1) greater than 180° of SMA encasement, celiac involvement (any abutment of the head with a greater than 180° encasement of the body or tail), (2) unreconstructive PV/SMV occlusion, and (3) aortic invasion. On the basis of the objective CT criteria, the patients enrolled in our study were classified as follows: 14 patients with R, 44 with BR, and 42 with UR tumors.
Treatment Plan and Assessment of Gem-CRTS
Our treatment protocol for gem-CRTS has been reported previously.16 All patients were treated with 3-dimensional conformal radiotherapy using the 4-field box technique from directions that avoided exposure of the kidney, which was an organ at risk. Based on the CT images, the gross tumor volume, which included the main tumor and lymph nodes of more than 1 cm in diameter, was defined. The clinical target volume was defined as the gross tumor volume plus a 5-mm margin in all directions. The planning target volume was basically defined as the clinical target volume plus a 5-mm margin, and an additional 10-mm margin was added in the cranial-caudal direction. The total radiation dose delivered was 45 Gy in 25 fractions (5 fractions/wk). Patients were administered an infusion of gemcitabine at a dosage of 800 mg/m2 on days 1, 8, 22, and 29. The patients underwent reassessment at 4 to 6 weeks after the completion of gem-CRTS; when we determined that curative-intent resection was possible, they were scheduled to undergo pancreatectomy.
Radiographic responses were determined by means of a comparison of pretreatment CT and post-CRT scans. Response was judged according to the Response Evaluation Criteria in Solid Tumors.17 We used serum CA19-9 levels as an index of response to gem-CRT. Serum CA19-9 levels were measured just before the initiation of gem-CRT (pre–CA19-9) and every 4 weeks thereafter. In the patients with obstructive jaundice, drainage was achieved using endoscopic retrograde biliary drainage, endoscopic nasobiliary drainage, or percutaneous transhepatic cholangiodrainage before gem-CRT. We compared the level of pre–CA19-9 with that measured at the time of reassessment (post–CA19-9). The reduction rate was calculated as follows: (pre–CA19-9 − post–CA19-9) / (pre–CA19-9) (%). When the reduction rate was greater than 50% regardless of the pre–CA19-9 level, gem-CRT was defined as being effective. The numbers of patients within the limit of the normal value (<37 U/mL) were 4 (36%) in the R group, 8 (19%) in the BR group, and 9 (23%) in the UR group. CA19-9 level may not be an accurate reflection of disease status in patients who express the Le(a−b−) genotype.18 However, genotyping was not performed routinely, and thus, we could not determine the patients with Le(a−b−); these patients usually presented with values lower than the assay sensitivity threshold (1 U/mL). There was 1 (9.1%) patient who possessed no detectable serum CA19-9 in the R group, 2 (4.7%) in the BR group, and 1 (2.5%) in the UR group, and all of them were determined as noneffective (CA19-9 reduction rate, <50%).
Indication of Resection, Surgical Procedure, and Postoperative Complications
At the time of reassessment, especially in the case of UR patients, we determined that curative-intent resection was possible when the following findings on MDCT were observed: no stenosis or change of shape in the celiac trunk and SMA as well as the absence of metastatic lesions in other distant organs. Intraoperatively, curative-intent resection was avoided when distant metastatic disease was detected on histological examination of frozen sections of suspicious lesions and of distant lymph nodes, including paraaortal lymph nodes. Curative-intent resection was also avoided when the tumor was found to be appreciably locally advanced, showing unreconstructable PV/SMV occlusion even if an external iliac vein graft had been used and/or a severe tumor invasion around the SMA (which was impossible to dissect without a remnant tumor) was evident.
Pancreaticoduodenectomy (PD) or distal pancreatectomy (DP) was performed as previously described.19 Resection and reconstruction of the PV/SMV were performed when the surgeon could not separate the pancreatic head or the uncinate process from these vessels without leaving gross tumor on the vessel. When limited involvement of the common hepatic artery was identified, a segmental resection of this vessel was performed with primary anastomosis. The patients who had an unresectable disease at surgery, which was usually due to the presence of distant metastasis, underwent surgical bypass as clinically indicated.
Postoperative complications including morbidity and mortality were graded according to the Clavien-Dindo classification.20 Postoperative pancreatic fistula, which is a complication specific to pancreatectomy, was graded according to the International Study Group on Pancreatic Fistula classification.21 Postoperative mortality was defined as all causes of death in the hospital.
Postoperative Chemotherapy and Follow-Up
From 6 weeks after resection, we planned to start the postoperative chemotherapy regimen consisting of gemcitabine at a dosage of 800 mg/m2 biweekly for at least 6 months. After pancreatectomy, all patients were evaluated as follows: physical examination every month, laboratory tests including carcinoembryonic antigen serum level measurements (normal level, <5 ng/mL) and CA19-9 level measurements (normal level, <37 U/mL) every 2 or 3 months, as well as MDCT every 3 months within 2 years and every 6 months thereafter. However, when the serum levels of the tumor markers increased, they were immediately evaluated using MDCT.
Evaluation of Histological Response
The resected specimens were fixed in a formalin solution, sliced into 5-mm sections, and embedded in paraffin blocks. A 3-μm section was obtained from each block and stained with hematoxylin and eosin. Sections were routinely examined for lymph node status, degree of lymphatic invasion (ly0, ly1, ly2, and ly3), degree of venous invasion (v0, v1, v2, and v3), degree of intrapancreatic nerve invasion (ne0, ne1, ne2, and ne3), and status of the surgical margin (R0, R1, and R2). Histological response to gem-CRT was evaluated according to the Evan histological criteria.22 We defined patients with grade IIb or higher (51%–100% tumor destruction) as high responders and patients with grade I or IIa (0%–50% tumor destruction) as low responders.23
Immunohistochemical Analysis and Evaluation of hENT1 Expression
The paraffin-embedded blocks were also used for the assessment of intratumoral hENT1 expressions using immunohistochemical analysis. Staining of hENT1 was carried out as detailed in a previous report.16 The scoring for hENT1 was carried out on the basis of the relative intensities of staining of the pancreatic tumor, with reference to the normally strong hENT1 staining of cell membranes within the islets of Langerhans cells as internal controls. The degree of hENT1 expression was defined by the intensity and extent of positive staining as high, intermediate, and low. All of the patients were classified into 2 groups, namely, positive hENT1 expression (high and intermediate) and negative hENT1 expression (low) groups.
Statistical Analyses
In all patients who came for reassessment, the date of the initial treatment was chosen as the starting point for the measurement of survival time. Disease-free survival time was calculated in the patients that underwent resection, and defined as the time from the date of initial treatment to the date of first relapse or death. Survival was calculated using the Kaplan-Meier method and was compared between the groups using the log-rank test. The day of final follow-up was July 31, 2012, and there was no loss of follow-up. All variables were dichotomized for analyses. A multivariate analysis was performed using Cox proportional hazard model. Variables with a significance of P < 0.1 in the univariate analysis were entered into the multivariate analysis. Comparisons were performed using the χ2 test with Yates correction in the univariate analysis. All statistical analyses were performed using the SPSS version 18 (SPSS Inc, Chicago, Ill) software. A P value less than 0.05 was considered statistically significant.
RESULTS
The flow of all 100 patients through the treatment protocol is illustrated in Figure 1. The gem-CRT was completed in all 14 (100%) of the R patients, in all 44 (100%) of the BR patients, and in 40 (95.2%) of the 42 UR patients. Two (2.0%) of the 100 patients could not complete gem-CRT because of a decline in performance status related to disease progression. During gem-CRT, grade 3 gastrointestinal toxicities occurred in 1 of the R patients and in 3 of the UR patients. Thirty-eight patients (R, 6; BR, 12; UR, 20) had grade 3 hematological toxicities, and 5 patients (R, 1; BR, 3; UR, 1) had grade 4 hematological toxicities. Three R patients and 1 BR patient who completed gem-CRT did not return to the hospital for surgery. Finally, 94 patients (R, 11; BR, 43; UR, 40) could be reassessed; 73 were operable (R, 8; BR, 38; UR, 27), and 21 were inoperable (R, 3; BR, 5; UR, 13). The reasons that patients were inoperable in the R, BR, and UR groups were distant metastases in 3 (3/11, 27%), 5 (5/43, 12%), and 7 (7/40, 18%), respectively, and local tumor factors in 0, 0, and 6 (6/40, 15%), respectively. Among the 73 patients who presented for surgery, 10 patients (R, 1; BR, 2; UR, 7) were found to have an unresectable disease owing to the presence of radiographically occult distant metastases (R, 1; BR, 1; UR, 4) or local tumor factors (BR, 1; UR, 3), and thus, the curative-intent resection rate was 87.5% (7/8) in R patients, 94.7% (36/38) in BR patients, and 74.0% (20/27) in UR patients.
FIGURE 1.
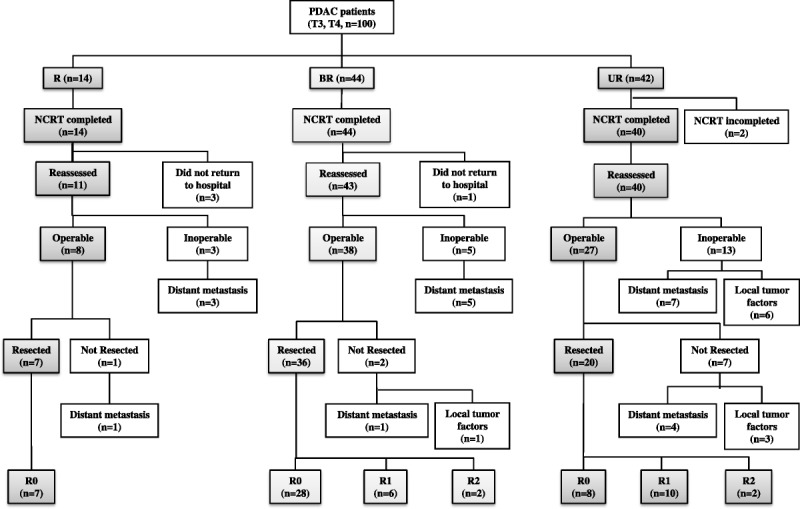
Algorithm illustrating patient flow through gem-CRTS. One hundred patients with stage T3 to T4 PDAC were classified into R, BR, and locally UR groups according to the NCCN guidelines.
The types of pancreatectomy in the R, BR, and UR groups were PD in 4, 32, and 16 patients, respectively, as well as DP in 3, 4, and 4 patients, respectively. The combined resection rate of PV/SMV was significantly higher in the BR (32/36, 88.9%) and UR (19/20, 95.0%) groups, as compared with that in the R group (2/7, 28.6%) (P < 0.001). In 5 patients (3 in the BR and 2 in the UR group), an external iliac vein graft was used as an interpositional venous graft to reconstruct the PV/SMV. A combined resection of the celiac trunk was performed in 3 UR patients, and resection of the hepatic artery, in 2 BR and 2 UR patients. The R0 resection rate was significantly higher in R (7/7, 100%) and BR patients (32/36, 77.8%), as compared with that in UR patients (8/20, 40.0%) (P = 0.0023).
Patient characteristics and the outcomes of gem-CRT treatment are summarized in Table 1. There were no statistically significant differences in age and size of tumor before gem-CRT therapy among the 3 groups. According to the evaluation using MDCT before gem-CRT, all of the BR and UR patients had cancer involvement in large vessels but no R patient did. The response status after gem-CRT did not differ among the 3 groups. Although CA19-9 levels (median) decreased after gem-CRT in all 3 groups, the pre– and post–CA19-9 levels did not differ among the 3 groups. The incidence of patients with a greater than 50% reduction in CA19-9 level was 27.3% (3/11) in the R, 53.5% (23/43) in the BR, and 35.0% (14/40) in the UR groups (these values did not differ significantly).
TABLE 1.
Characteristics of Patients Enrolled for the Gem-CRT Protocol and the Outcome of Gem-CRT
The cumulative survival curves for 94 patients in the 3 groups who were reassessed are shown in Figure 2A. The 3-year survival rates of R, BR, and UR patients were 60.6%, 27.4%, and 4.6%, respectively (R vs UR, P = 0.0115).
FIGURE 2.
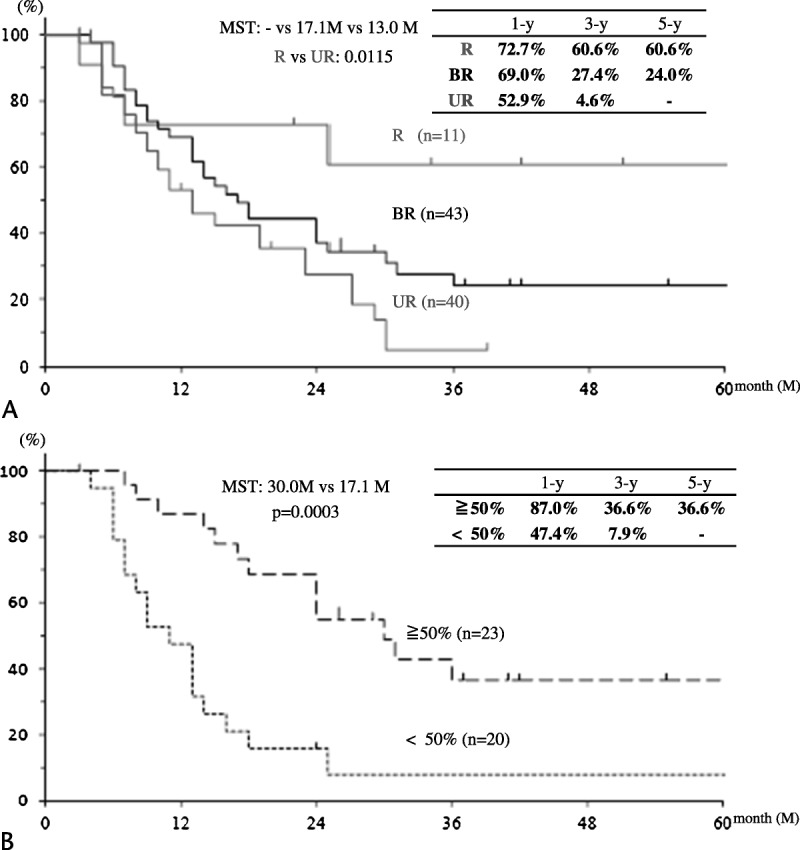
Survival curves after initial treatment in reassessed patients. A, Cumulative survival curves according to the 3 groups in 94 patients who were reassessed. B, Cumulative survival curves according to the CA19-9 reduction rate in 43 BR patients who were reassessed.
The univariable and multivariable analyses of the effect of preoperative factors on survival time are summarized in Table 2. The statistically significant variables in the univariable analyses were the CA19-9 reduction rate (P = 0.0003) and cancer involvement of a major artery (P = 0.0421) in the BR group as well as sex (P = 0.0306) in the UR group. The multivariable analysis indicated the CA19-9 reduction rate in the BR group as the single significant independent factor.
TABLE 2.
Univariable and Multivariable Analyses of the Effect of Preoperative Factors on Survival Time in Reassessed Cases
Figure 2B shows the survival curves for the BR group according to the CA19-9 reduction rate. The 3-year survival rate was significantly higher in patients with a CA19-9 reduction rate greater than 50% than that in patients with a CA19-9 reduction rate less than 50% (36.6% vs 7.9%).
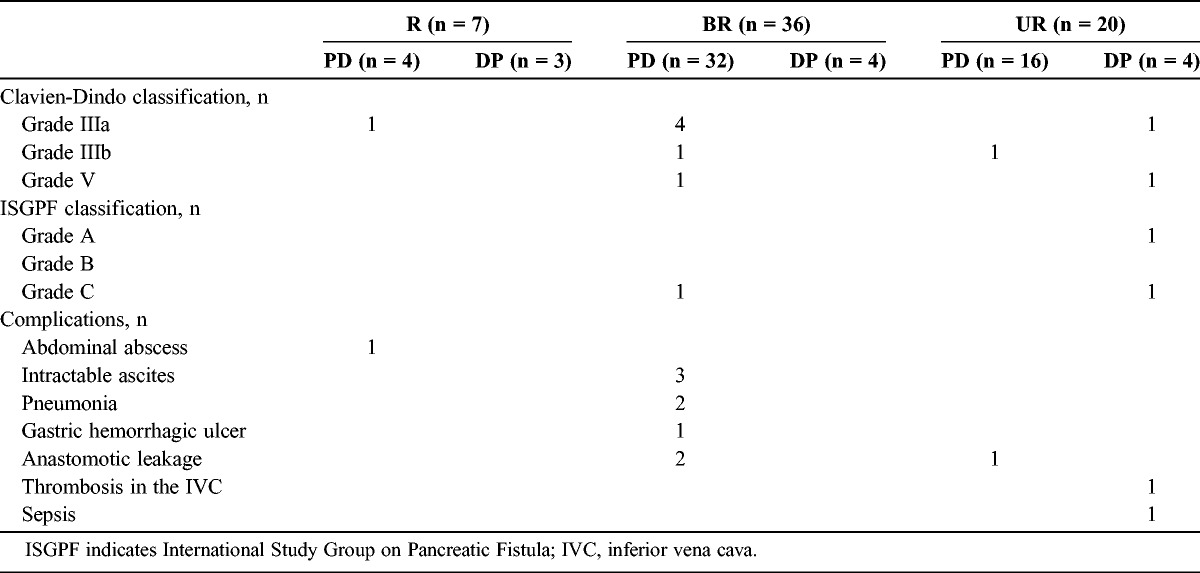
Postoperative complications (Clavien grades IIIa-V) occurred in 1 (14.3%) of 7 R patients, in 6 (16.7%) of 36 BR patients, and in 3 (15.0%) of 20 UR patients (Table 3). There were no significant differences in postoperative complications (Clavien grades IIIa–V) between the 3 groups. Postoperative 30-day mortality occurred in 2 patients owing to pneumonia (a BR patient who underwent PD) and sepsis (a UR patient who underwent DP) caused by grade C pancreatic fistula according to the International Study Group on Pancreatic Fistula criteria.
TABLE 3.
Postoperative Mortality and Morbidity
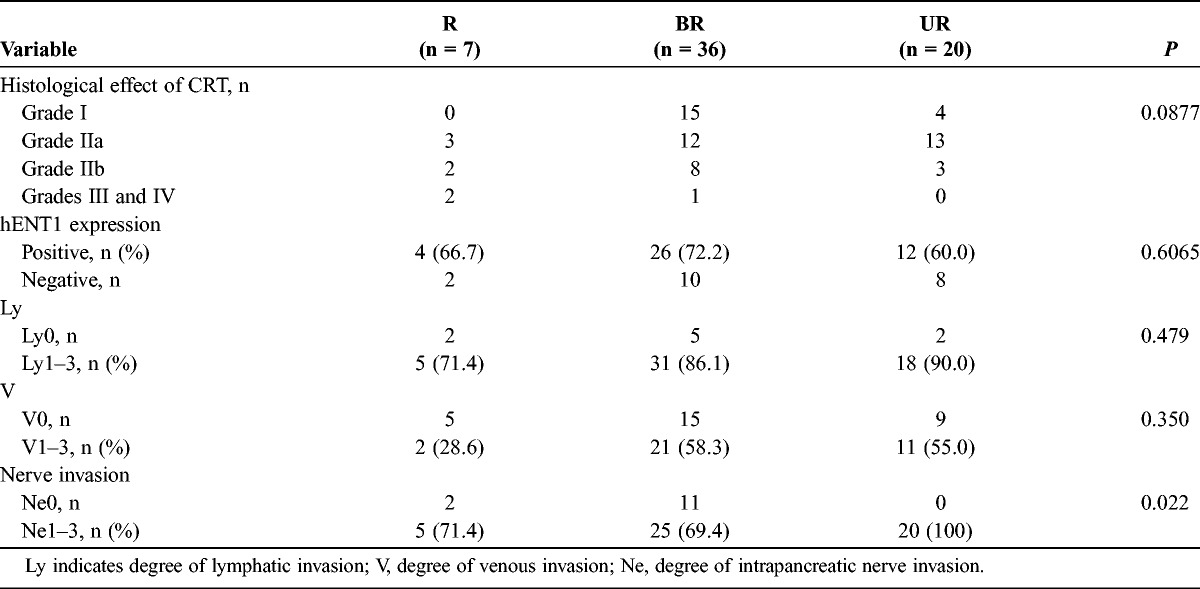
When we compared pathological factors according to resectability groups (Table 4), a high response was observed in 4 R patients (50%), 9 BR patients (25%), and 3 UR patients (15%). The rate of positive hENT1 expression was almost the same in each of the 3 groups, as follows: 67% in the R, 72% in the BR, and 60% in the UR group. There were no significant differences in the degree of lymphatic and venous invasion, whereas for nerve invasion, the incidence of ne1-3 was significantly higher in the UR relative with those in the R and BR groups (P = 0.022).
TABLE 4.
Pathological Findings Regarding the Resectability Groups
The cumulative survival curves for 63 patients in the 3 groups who completed the gem-CRTS treatment are shown in Figure 3A. The 3-year survival rates of the R, BR, and UR patients were 83.3%, 33.0%, and 7.8%, respectively (R vs BR, P = 0.0208; R vs UR, P = 0.0022). The disease-free survival curves for the 3 groups after initial treatment are presented in Figure 3B. The 3-year disease-free survival rates for R, BR, and UR patients were 83.3%, 31.8%, and 7.8%, respectively (without any statistical difference).
Figure 3.
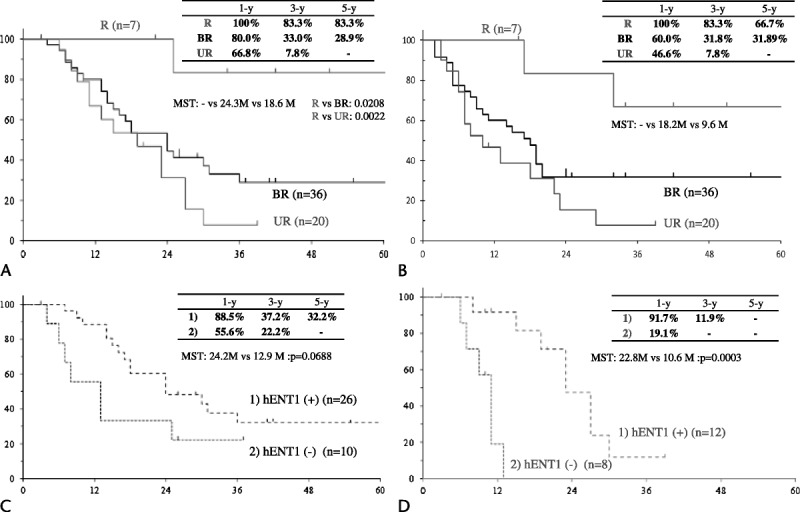
Survival curves after initial treatment in patients who completed gem-CRTS. A, Cumulative survival curves for the R, BR, and locally UR groups (63 patients in total) who completed gem-CRTS (resected cases). B, Disease-free survival curves for the 3 groups involving the 63 patients who completed gem-CRTS (resected cases). C, Cumulative survival curves according to hENT1 expression in the 36 BR patients who completed gem-CRTS. D, Cumulative survival curves according to hENT1 expression in the 20 UR patients who completed gem-CRTS.
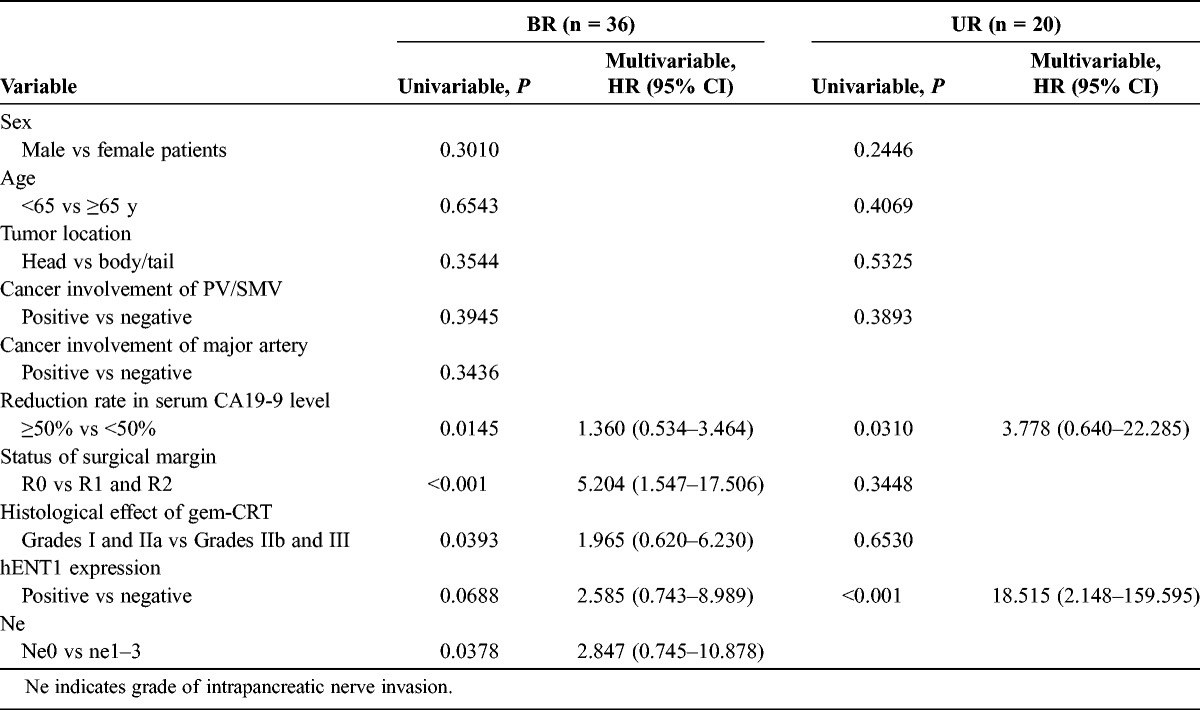
Table 5 shows the univariable and multivariable analyses of preoperative and postoperative factors regarding survival time after gem-CRTS (excluding R patients because 6 of the 7 patients remain alive). The statistically significant factors in the univariable analyses in BR patients were the CA19-9 reduction rate, status of the surgical margin, histological effect of gem-CRT, and nerve invasion; those in UR patients were the CA19-9 reduction rate and hENT1 expression. The multivariable analyses indicated the status of the surgical margin in the BR group and positive hENT1 expression in the UR group as the single independent significant factors.
TABLE 5.
Univariable and Multivariable Analyses of Preoperative and Postoperative Factors on Survival Time After Gem-CRTS
We compared survival curves according to hENT1 expression in BR (Fig. 3C) and UR patients (Fig. 3D). The 3-year survival rate was not significantly different between positive and negative hENT1 expressions (37.2% vs 22.2%) in the BR group (median survival time [MST], 24.2 months vs 12.9 months; P = 0.0688), whereas in the UR group, there was a significant difference (11.9% vs 0%; MST, 22.8 months vs 10.6 months; P = 0.003).
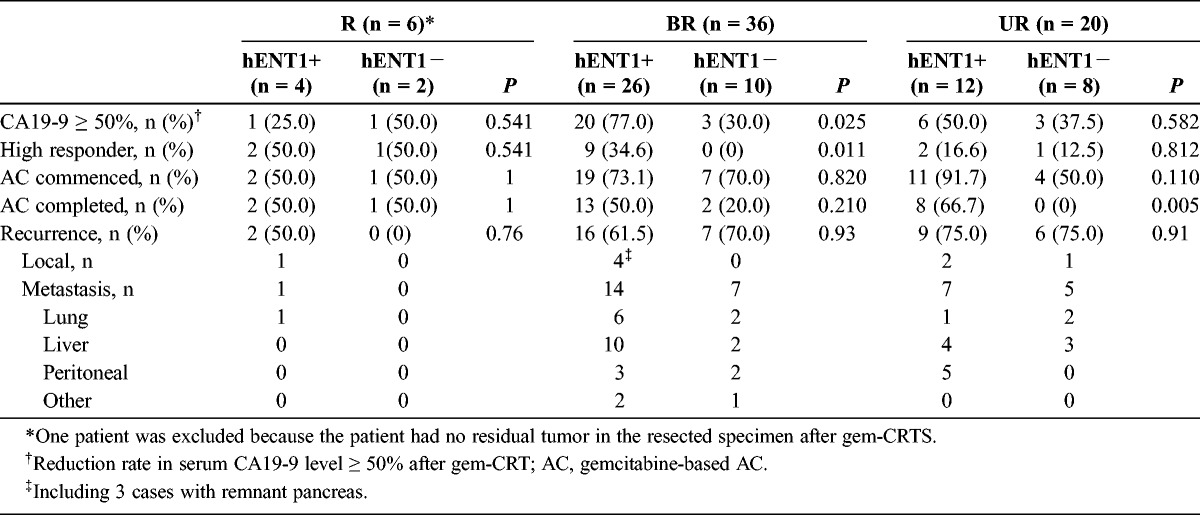
Table 6 shows the CA19-9 reduction rate, histological response, status of AC, and sites of tumor recurrence according to hENT1 expression among the 3 groups. In the R group, we excluded 1 patient from the examination of hENT1 expression because the patient had no residual tumor in the resected specimen after gem-CRTs. In the BR patients, the CA19-9 reduction rate and histological response, which were considered as indicators of the gem-CRT effect, showed significantly higher incidences of CA19-9 reduction greater than 50%. In addition, there was a higher rate of positive hENT1 expression than negative hENT1 expression (77% vs 30%, respectively [P = 0.025], and 35% vs 0%, respectively [P = 0.011]). In R and UR patients, however, there was no significant difference between positive and negative hENT1 expressions. It was possible to commence the planned gem-AC in 44 patients, but the remaining 18 patients could not be treated owing to a prolonged recovery time. Although the hENT1 expression did not influence the commencement of gem-AC among the 3 groups because there was no significant difference in the incidence of patients who commenced gem-AC, it significantly influenced the gem-AC completion rate in UR patients (66.7% with positive expression vs 0% with negative expression, P = 0.005). The incidences of local recurrence and distant metastasis were not significantly different between the patients with positive and negative hENT1 expression in each group. When we compared the incidences of the CA19-9 reduction rate of greater than 50.0% in all 62 patients and in patients selected and treated with gem-AC according to hENT1 expression, there was a significantly higher positive expression than negative expression in the former group (64.3% [27/42] vs 35.0% [7/20] [P = 0.030] and 54.8% [23/42] vs 15.0% [3/20] [P = 0.003]). Furthermore, the 15 BR patients who completed gem-AC survived longer than the 21 who did not complete gem-AC; the MSTs were 24.4 and 18.8 months, respectively (P = 0.014). Eight UR patients who completed gem-AC survived longer than the 12 who did not complete gem-AC; the MSTs were 26.8 and 10.8 months, respectively (P = 0.0002).
TABLE 6.
CA19-9 Reduction Rates, Histological Response, Status of Adjuvant Chemotherapy, and Sites of Tumor Recurrence According to hENT1 Expression Among the 3 Groups
DISCUSSION
Unexpectedly, at the time of reassessment in our study, distant metastases had occurred at a similar frequency among the 3 groups, as follows: 27% in the R group, 12% in the BR group, and 18% in UR group. To our knowledge, there have been no reports regarding the frequency of occurrence of distant metastasis after completion of gem-CRT according to resectability based on the NCCN guidelines. Previous studies have reported that the occurrence rate of distant metastasis at the time of reassessment after NCRT was 12.8% in BR patients24 and 12.5% in potentially resectable tumors (including R and BR tumors).25 However, there have been no reports concerning UR. Our results suggest that PDAC is a systematic disease regardless its resectability status. A subgroup analysis carried out in the CONKO-001 randomized controlled trial regarding AC after curative-intent resection1 also suggested that PDAC is a systemic disease even in early-stage tumors at the time of diagnosis. This was because the median disease-free survival time among patients with T1 to T2 tumors in the observation group, which was almost the same as that among patients with T3 to T4 tumors in the gemcitabine group (12.9 months), was significantly shorter than that in the gemcitabine group (10.0 vs 48.2 months). One of the advantages of NCRT is the identification of a subset of patients for whom resection will not offer a survival benefit. In fact, all of the R patients that underwent resection in our study did not experience recurrence within 2 years after gem-CRTS treatment. Although NCRT is recommended for the treatment of BR tumors as an option,7 it may also be recommended for R tumors.
Although CA19-9 has been accepted as a measure of pancreatic cancer burden, the role of CA19-9 in the evaluation of patients with NCRT before planned surgical resection has not been well evaluated. Recently, there have been 2 studies that have underscored CA19-9 as a marker of resectability and survival in patients with potentially R PDAC treated with NCRT. One of these studies26 indicated that a pre–CA19-9 level of less than 37 U/mL had a positive predictive value of 86% for completing PD but a negative predictive value of 33%; in addition, a post–CA19-9 level of less than 61 U/mL had a positive predictive value of 93% and a negative predictive value of 28%. The other study25 used more complicated criteria by dividing the patients into 3 categories (I, increased; MD, modestly decreased; SD, substantially decreased) and using pre–CA19-9 level and post–CA19-9 reduction rate as end points; the authors suggested that alteration in CA19-9 status was a single independent factor associated with prognosis. In the present study, the multivariable analysis of the effect of preoperative factors on survival time in reassessed cases indicated that a CA19-9 reduction rate of more than 50% was the single significant prognostic factor in BR patients but not in R and UR patients. In our study, all of the 15 patients (3 in the R group, 5 in the BR group, and 7 in the UR group) who developed distant metastases at reassessment did not show a CA19-9 reduction rate of greater than 50%, suggesting that CA19-9 reduction rate is associated with the systematic progression of PDAC.
Several previous studies have indicated that margin resection status is a very important prognostic factor and that the survival benefits of R1 resection may be comparable to palliative CRT without surgery.27 There have been few studies that have evaluated the R0 resection rates in BR according to the NCCN guidelines, and we could find only 2 studies that had evaluated the R0 resection rates in BR patients. One of these studies28 emphasized the effect of NCRT on the R0 resection rate by retrospectively comparing patients that had and had not undergone NCRT for BR tumors; the R0 resection rate was significantly higher in patients who had received NCRT than in those who had not (59% vs 11%). The other study, which was Japanese,29 compared the R0 resection rate between R (n = 109) and BR (n = 24) patients who were retrospectively classified according to NCCN guidelines and reported rates of 81% and 71%, respectively. In our study, the R0 resection rate was 100% in R, 78% in BR, and 40% in UR patients. Our results suggested that gem-CRT increased the R0 resection rate in R and BR patients and moreover, that the R0 resection rate was an independent prognostic risk factor in BR patients. As for the R0 resection rate in UR patients who underwent curative-intent resection after CRT, there have been no reports because UR patients are usually not candidates for resection. Only a few studies12–14 have reported that 8.6% to 32% of locally advanced patients (including BR and UR patients) who received CRT had undergone resection. To the best of our knowledge, the present study is the first to have evaluated the R0 resection rate by performing curative-intent resection after gem-CRT in UR patients. Among 42 UR patients enrolled for gem-CRTS, curative-intent resection could be performed in 20 (48%), of whom only 8 (40%) had undergone an R0 resection.
The rate of high responders to gem-CRT has been getting worse in line with resectability (57% in R, 25% in BR, and 15% in UR patients). Although the mechanism for resistance to CRT has not been fully explored, it has been reported that pancreatic cancer stem cells are a fundamental reason for this resistance.30–32 A recent study33 has revealed that chemoradioresistant pancreatic cancer cells are rich in stem-cell–like tumor cells and undergo epithelial-mesenchymal transition (EMT). It is known that N-cadherin is associated with a high invasiveness potential in cancer. A previous study34 has also demonstrated that overexpression of N-cadherin in PDAC, which was significantly correlated with the degree of nerve invasion, was involved in EMT. In the present study, the degree of nerve invasion was significantly higher in UR than in R and BR patients. Based on these findings, we hypothesize that pancreatic cancer cells in UR patients had become chemoradioresistant by undergoing EMT.
Several previous reports have demonstrated that hENT1 expression in the resected specimen is a significant predictive marker of chemosensitivity for gemcitabine-based AC in PDAC.15,35,36 As for neoadjuvant therapy, we could find only 2 previous reports, 1 of which was ours, that have described the relationship between hENT1 expression and prognosis for PDAC. Our previous study16 demonstrated that hENT1 expression was the independent predictor of overall survival after gem-CRTS in 55 patients with UICC-T3 to UICC-T4 PDAC. On the contrary, the other study37 found that hENT1 expression was not associated with prognosis in 63 patients who underwent gem-CRTS. The significant difference between the 2 studies involving gem-CRTS was the type of AC, gem-AC in the former and postoperative liver perfusion chemotherapy using continuous infusion of 5-fluorouracil in the latter. In our previous study,16 the percentage of patients who completed gem-AC had significantly lower negative hENT1 expression than positive expression, and in addition, the patients who completed gem-AC had a longer MST than those who did not complete gem-AC (24.9 vs 12.8 months). In the present study, the survival rate of BR patients did not differ significantly between those with positive and negative hENT1 expression, whereas UR patients with positive hENT1 expression had a significantly higher survival rate. Furthermore, the rate of completion of gem-AC treatment in UR patients was significantly higher in those with positive hENT1 expression than in those with negative expression (66.7% vs 0%), whereas in BR patients, there was no significant difference between the 2 groups. These results suggested that the status of hENT1 expression highly influenced the gem-AC completion rate, which in turn affected patient survival.
Pretreatment evaluation of hENT1 expression in pancreatic cancer tissue can be beneficial in predicting the efficacy of gemcitabine therapy before initial treatment. The EUS-FNA specimens that we used for cytological/histological diagnosis might be suitable for evaluating hENT1 expression; however, the analysis of hENT1 expression in the pancreatic tumor tissue taken by EUS-FNA has not been established. Recently, we evaluated the availability of EUS-FNA samples for hENT1 expression and compared the status of hENT1 expression in the resected specimen in the 55 patients with PDAC treated with gem-CRTS.38 Among the 55 patients, only 23 (41.8%) who were histologically diagnosed as having PDAC could be evaluated for hENT1 expression in the EUS-FNA samples, positive for hENT1 expression in 16 (69.6%) samples and negative for hENT1 expression in 7 (30.4%) samples. The expression of hENT1 in 87% of EUS-FNA samples was identical with that in resected specimens after gem-CRT. The 16 patients with positive hENT1 expression in the EUS-FNA samples had significantly longer overall and recurrence-free survival rates than the 7 with negative hENT1 expression (2-year survival and recurrence-free survival rates, 67.5% and 29.2%, respectively, vs 35.7% and 0%, respectively). Our data provide the evidence that intratumoral hENT1 expression in EUS-FNA samples can be used to predict the treatment outcome before gem-CRT. However, improvement in the rate of acquisition of specimens by EUS-FNB and further modification of the protocol for the assay of hENT1 are needed.
In conclusion, a CA19-9 reduction rate of more than 50% after gem-CRT and R0 status were the significant prognostic factors in BR PDAC. Positive expression of hENT1 in the resected specimen was the significant prognostic factor in UR PDAC. We consider that our gem-CRTS protocol, even for locally UR PDAC, allows for the identification of candidates for aggressive resection at the time of reassessment, thus facilitating an increase in the R0 resection rate and improving prognosis in patients with positive hENT1 expression.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1. Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007; 297: 267– 277 [DOI] [PubMed] [Google Scholar]
- 2. Crane CH, Varadhachary G, Settle SH, et al. The argument for pre-operative chemoradiation for localized, radiographically resectable pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006; 20: 365– 382 [DOI] [PubMed] [Google Scholar]
- 3. Spitz FR, Abbruzzese JL, Lee JE, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol. 1997; 15: 928– 937 [DOI] [PubMed] [Google Scholar]
- 4. Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000; 4: 567– 579 [DOI] [PubMed] [Google Scholar]
- 5. Howard TJ, Krug JE, Yu J, et al. A margin-negative R0 resection accomplished with minimal postoperative complications is the surgeon’s contribution to long-term survival in pancreatic cancer. J Gastrointest Surg. 2006; 10: 1338– 1345 [DOI] [PubMed] [Google Scholar]
- 6. Zervos EE, Rosemurgy AS, Al-Saif O, et al. Surgical management of early-stage pamcreatic cancer. Cancer Control. 2004; 11: 23– 31 [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network (NCCN) Practice Guidelines for Pancreatic Cancer. 2010. Available : http://www.nccn.org Accessed April 1, 2012.
- 8. Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006; 13: 1035– 1046 [DOI] [PubMed] [Google Scholar]
- 9. Breslin TM, Hess KR, Harbison DB, et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol. 2001; 8: 123– 132 [DOI] [PubMed] [Google Scholar]
- 10. Hoffman JP, Lipsitz S, Pisansky T, et al. Phase II trial of preoperative radiation therapy and chemotherapy for patients with localized, resectable adenocarcinoma of the pancreas: an Eastern Cooperative Oncology Group Study. J Clin Oncol. 1998; 16: 317– 323 [DOI] [PubMed] [Google Scholar]
- 11. Pisters PW, Abbruzzese JL, Janjan NA, et al. Rapid-fractionation preoperative chemoradiation, pancreaticoduodenectomy, and intraoperative radiation therapy for resectable pancreatic adenocarcinoma. J Clin Oncol. 1998; 16: 3843– 3850 [DOI] [PubMed] [Google Scholar]
- 12. Sa Cunha A, Rault A, Laurent C, et al. Surgical resection after radiochemotherapy in patients with unresectable adenocarcinoma of the pancreas. J Am Coll Surg. 2005; 201: 359– 365 [DOI] [PubMed] [Google Scholar]
- 13. Maximous DW, Abdel-Wanis ME, El-Sayed MI, et al. Preoperative gemcitabine based chemo-radiotherapy in locally advanced non metastatic pancreatic adenocarcinoma. Int Arch Med. 2009; 2: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Polistina F, Costantin G, Casamassima F, et al. Unresectable locally advanced pancreatic cancer: a multimodal treatment using neoadjuvant chemoradiotherapy (gemcitabine plus stereotactic radiosurgery) and subsequent surgical exploration. Ann Surg Oncol. 2010; 17: 2092– 2101 [DOI] [PubMed] [Google Scholar]
- 15. Marechal R, Bachet JB, Mackey JR, et al. Levels of gemcitabine transport and metabolism proteins predict survival times of patients treated with gemcitabine for pancreatic adenocarcinoma. Gastroenterology. 2012; 143: 664– 674 [DOI] [PubMed] [Google Scholar]
- 16. Murata Y, Hamada T, Kishiwada M, et al. Human equilibrative nucleoside transporter 1 expression is a strong independent prognostic factor in UICC T3-T4 pancreatic cancer patients treated with preoperative gemcitabine-based chemoradiotherapy. J Hepatobiliary Pancreat Sci. 2012; 19: 413– 425 [DOI] [PubMed] [Google Scholar]
- 17. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92: 205– 216 [DOI] [PubMed] [Google Scholar]
- 18. Tempero MA, Uchida E, Takasaki H, et al. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res. 1987; 47: 5501– 5503 [PubMed] [Google Scholar]
- 19. Strasberg SM, Drebin JA, Linehan D. Radical antegrade modular pancreatosplenectomy. Surgery. 2003; 133: 521– 527 [DOI] [PubMed] [Google Scholar]
- 20. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009; 250 (2): 187– 196 [DOI] [PubMed] [Google Scholar]
- 21. Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005; 138 (1): 8– 13 [DOI] [PubMed] [Google Scholar]
- 22. Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992; 127 (11): 1335– 1339 [DOI] [PubMed] [Google Scholar]
- 23. Murata Y, Mizuno S, Kishiwada M, et al. Impact of histological response after neoadjuvant chemoradiotherapy on recurrence-free survival in UICC-T3 pancreatic adenocarcinoma but not in UICC-T4. Pancreas. 2012; 41: 130– 136 [DOI] [PubMed] [Google Scholar]
- 24. Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008; 206: 833– 846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takahashi H, Ohigashi H, Ishikawa O, et al. Serum CA19-9 alterations during preoperative gemcitabine-based chemoradiation therapy for resectable invasive ductal carcinoma of the pancreas as an indicator for therapeutic selection and survival. Ann Surg. 2010; 251: 461– 469 [DOI] [PubMed] [Google Scholar]
- 26. Katz MH, Varadhachary GR, Fleming JB, et al. Serum CA 19-9 as a marker of resectability and survival in patients with potentially resectable pancreatic cancer treated with neoadjuvant chemoradiation. Ann Surg Oncol. 2010; 17 (7): 1794– 1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004; 350: 1200– 1210 [DOI] [PubMed] [Google Scholar]
- 28. Chun YS, Milestone BN, Watson JC, et al. Defining venous involvement in borderline resectable pancreatic cancer. Ann Surg Oncol. 2010; 17: 2832– 2838 [DOI] [PubMed] [Google Scholar]
- 29. Takahashi S, Kinoshita T, Konishi M, et al. Borderline resectable pancreatic cancer: rationale for multidisciplinary treatment. J Hepatobiliary Pancreat Sci. 2011; 18 (4): 567– 574 [DOI] [PubMed] [Google Scholar]
- 30. Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007; 67: 1030– 1037 [DOI] [PubMed] [Google Scholar]
- 31. Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007; 1: 313– 323 [DOI] [PubMed] [Google Scholar]
- 32. Lee CJ, Dosch J, Simeone DM. Pancreatic cancer stem cells. J Clin Oncol. 2008; 26: 2806– 2812 [DOI] [PubMed] [Google Scholar]
- 33. Du Z, Qin R, Wei C, et al. Pancreatic cancer cells resistant to chemoradiotherapy rich in “stem-cell-like” tumor cells. Dig Dis Sci. 2011; 56: 741– 750 [DOI] [PubMed] [Google Scholar]
- 34. Nakajima S, Doi R, Toyoda E. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clin Cancer Res. 2004; 10: 4125– 4133 [DOI] [PubMed] [Google Scholar]
- 35. Kondo N, Murakami Y, Uemura K, et al. Combined analysis of dihydropyrimidine dehydrogenase and human equilibrative nucleoside transporter 1 expression predicts survival of pancreatic carcinoma patients treated with adjuvant gemcitabine plus S-1 chemotherapy after surgical resection. Ann Surg Oncol. 2012; 19 (suppl 3): S646– S655 [DOI] [PubMed] [Google Scholar]
- 36. Okazaki T, Javle M, Tanaka M, et al. Single nucleotide polymorphisms of gemcitabine metabolic genes and pancreatic cancer survival and drug toxicity. Clin Cancer Res. 2010; 16: 320– 329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kawada N, Uehara H, Katayama K, et al. Human equilibrative nucleoside transporter 1 level does not predict prognosis in pancreatic cancer patients treated with neoadjuvant chemoradiation including gemcitabine. J Hepatobiliary Pancreat Sci. 2012; 19: 717– 722 [DOI] [PubMed] [Google Scholar]
- 38.Murata Y, Isaji S, Kishiwada M, et al. Prognostic significance of human equilibrative nucreoside transporter 1 (hENT1) expression in pancreatic cancer patients treated with gemcitabine based chemoradiotherapy and availability of endoscopic ultrasonography guided fine needle biopsy samples. 46th annual meeting of the pancreas club. Available: http://pancreasclub.com/wp-content /uploads/2012finalprogram.pdf# Accessed October 1, 2012.


