Abstract
Primary sclerosing cholangitis (PSC) is a chronic, fibroinflammatory, cholestatic liver disease of unknown etiopathogenesis. PSC generally progresses to liver cirrhosis, is a major risk factor for hepatobiliary and colonic neoplasia, and confers a median survival to death or liver transplantation of only 12 years. Although it is well recognized that approximately 75% of patients with PSC also have inflammatory bowel disease (IBD), the significance of this association remains elusive. Accumulating evidence now suggests a potentially important role for the intestinal microbiota, and enterohepatic circulation of molecules derived there from, as a putative mechanistic link between PSC and IBD and a central pathobiological driver of PSC. In this concise review, we provide a summary of and perspectives regarding the relevant basic, translational, and clinical data, which, taken together, encourage further investigation of the role of the microbiota and microbial metabolites in the etiopathogenesis of PSC and as a potential target for novel pharmacotherapies.
Keywords: cholestatic, etiopathogenesis, metabolites
Introduction
Primary sclerosing cholangitis (PSC) is a chronic, fibroinflammatory, cholestatic liver disease of unknown etiopathogenesis [1,2]. PSC leads to end-stage cirrhosis, is a major risk factor for hepatobiliary and colonic neoplasia, and carries a median liver transplant (LT)-free survival of approximately 12 years [3–6]. Despite ongoing research over several decades, medical therapy for PSC has yet to be established [1,7]. Consequently, PSC remains a common indication for LT in many parts of the world [5]. Although operative treatment with LT is effective for PSC, it is only performed in select patients and centers, and even suitable candidates can have recurrent PSC or malignancy post-LT [8,9]. Therefore, given the morbidity and mortality of PSC, the lack of established pharmacotherapy [1,7,10], and the challenges associated with LT [1,11,12], a better understanding of its etiopathogenesis and novel therapies are critically needed.
PSC is now generally appreciated to be a heterogeneous, complex disorder with genetic, immunologic, and environmental components [1,4,7]. Accumulating data suggest that one such component, and potentially a modifiable one, is the enteric microbiota – which encompasses 100 trillion bacteria from over 2000 species – and its metabolites. In this concise review, we provide (1) a primer on the gut–liver axis; (2) a synopsis of biliary epithelial pathobiology; and (3) an overview of basic, translational, and clinical research regarding the potential role of the microbiota in the etiopathogenesis and treatment of PSC.
Primer on the relationship between the gut and PSC
An association between the gut, specifically inflammatory bowel disease (IBD), and PSC was first described approximately five decades ago. Since then, it has been recognized that not only do nearly 75% of patients with PSC have IBD, but PSC in the context of IBD confers a fourfold increased risk of colon cancer compared to IBD alone. Furthermore, the presence of IBD and an intact colon before LT are predictors of recurrent PSC post-LT [13].
One hypothesis regarding the PSC–IBD association is that it may be related to enterohepatic circulation of gut-derived molecules and possibly facilitated by increased intestinal permeability [14,15]. Although the identity of these gut-derived molecules (or how they trigger hepatobiliary pathobiology) has yet to be determined, microbes and microbial metabolites or derivatives such as lipopolysaccharide (LPS), lipoteichoic acid, and peptidoglycan have been suggested as potential candidates [16,17]. This concept forms the basis of the “leaky gut” hypothesis. More recently, the “PSC microbiota” hypothesis, which incorporates the observations that some patients with PSC have no detectable bowel disease and/or normal intestinal permeability [18] but may have an abnormal repertoire of microbial metabolites or aberrant response to them, has also been proposed [19,20].
The immunobiologically dynamic biliary epithelium
The hepatic portal areas, which are affected in the earliest and through all stages of PSC, are comprised by a variety of resident hepatic cells including fibroblasts and smooth muscle, endothelial, and epithelial cells, including biliary epithelial cells (i.e. cholangiocytes). In addition to these, multiple other cells, particularly leukocytes, are recruited to portal areas following biliary injury. Signaling between these cell types is intricate and critical to biliary injury response processes. We focus here on cholangiocytes and their properties given they are (1) central to periductal fibrosis, ductopenia, and other processes germane to PSC and (2) increasingly believed to play an essential role in the initiation and/or progression of PSC rather than being only a passive target [16].
Cholangiocytes are specialized cells that line the three-dimensional network of conduits, i.e. intra- and extrahepatic bile ducts, which carry bile from hepatic canaliculi to the duodenum. Beyond their role in bile modification and transport [21], cholangiocytes actively sense and respond to endogenous and exogenous molecules, including pathogen-associated molecular patterns (PAMPs). PAMPs are found in bile through either ascending infection or canalicular secretion, among other potential routes, and can modify cholangiocyte function and/or phenotype [16,22–25]. Indeed, cholangiocytes express numerous pathogen recognition receptors, including nucleotide-binding oligomerization domain (NODs) and all known toll-like receptors (TLRs) [16,23]. Binding of microbial molecules and other activators of inflammation (reviewed recently elsewhere [16]) to these receptors results in signaling cascades transduced via MyD88-dependent or -independent pathways, activation of transcription factors (e.g. NFκB, IRF3), increased expression of a variety of innate immune response genes including profibroinflammatory mediators, and ultimately development of hepatobiliary inflammation and fibrosis [16,26].
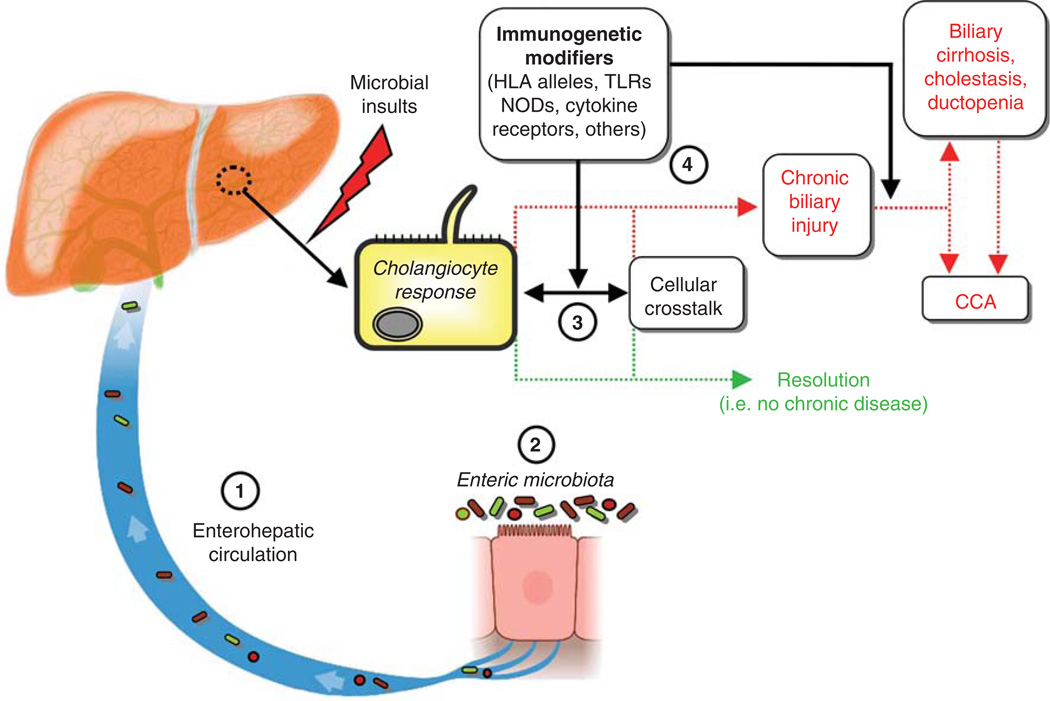
The intricate communication between cholangiocytes, resident hepatic cells, and recruited cells remains an active and important area of research. Such investigation will undoubtedly yield advances in elucidating the uncertainties that exist in biliary pathobiology and the etiopathogenesis of PSC, as summarized in a conceptual working model (Figure 1).
Figure 1.
Conceptual model of the etiopathogenesis and natural history of primary sclerosing cholangitis. Biliary epithelial cells, i.e. cholangiocytes, exist in an environment with multiple potential etiologic mediators of hepatobiliary injury. A growing body of basic, translational, and clinical evidence suggests that among these, microbially derived molecules may be central to hepatobiliary injury in and thus the etiopathogenesis of PSC. However, whether there is (1) increased exposure to microbial molecules (e.g. through the enterohepatic circulation, facilitated by compromised intestinal barrier function), (2) alterations in microbial diversity and/or the repertoire of microbial molecules (e.g. due to intestinal microbial dysbiosis), and/or (3) an aberrant or exaggerated cholangiocyte or other hepatic cell response to microbial molecules (e.g. induction of cholangiocyte senescence and the senescence-associated secretory phenotype) remains uncertain. In addition, host immunogenetics may modulate the impact of any of these variables (4) and thus likely play a role in determining whether hepatobiliary injury resolves or if it persists and results in chronic disease (i.e. PSC). These variables may also determine whether PSC progresses to its associated major adverse endpoints, including development of cholangiocarcinoma (CCA), liver failure, and death. Further investigation of the cellular, molecular, and microbial interactions and phenomena represented in this figure, including through previously unavailable (meta)genomic and bioinformatics techniques, will undoubtedly be pursued in future research and is expected to advance current understanding of the etiopathogenesis of PSC.
Evidence from animal models supporting the gut–liver axis of biliary disease
Several elegant animal model studies have demonstrated that enteric microbial molecules/dysbiosis can lead to hepatobiliary inflammation with features of PSC. For example, rats with small bowel bacterial overgrowth secondary to surgically created jejunal blind loops exhibit extrahepatic and intrahepatic ductal dilatation, irregularity, and beading resembling PSC [27]. Intriguingly, treatment of these rats with mutanolysin (but not ursodeoxycholic acid, prednisone, methotrexate, or cyclosporin) results in significant serologic and histologic improvement [28]. A subsequent study found that administration of peptidoglycan results in irregularities of intrahepatic bile ducts, focal areas of narrowing and fusiform sacculations, and histologic evidence of cholangitis [29]. Similarly, rectal administration of N-formyl L-methionine L-leucine L-tyrosine, an Escherichia coli chemotactic peptide secreted into bile, results in a mixed inflammatory, small-duct cholangitis [30].
More recently, it was shown that inoculation of Balb/c mice with Staphylococcus intermedius results in nonsuppurative cholangitis and anti-biliary epithelial cell and anti-nuclear antibody synthesis [31], as seen in patients with PSC [32]. In addition, several other infectious models, not the least of which is biliary cryptosporidiosis (which also causes sclerosing cholangitis in humans), demonstrate biochemical, histologic, and/or cholangiographic features of PSC; these and other models are reviewed in detail elsewhere [33]. Although these animal model systems do not recapitulate all the findings of PSC, they provide a premise supporting the notion that hepatobiliary disease in PSC may be triggered or modified by microbial molecules.
Human tissue-based translational studies supporting the PSC-microbiota hypothesis
Several intriguing observations have been made regarding the interplay of enterohepatically circulated microbial molecules, the downstream cholangiocyte response, and host immunogenetic susceptibility to aberrant signaling subsequent to microbial recognition. First, TLR and NOD expression and MyD88/IRAK activation are increased in PSC cholangiocytes [20], the former being potentially driven by anti-biliary epithelial cell antibodies [32]. Second, cultured cholangiocytes from patients with PSC exhibit persistent hypersensitivity to LPS and other PAMPs [20]. Third, PSC is not only associated with portal bacteremia, bacterobilia [34], and 16s ribosomal ribonucleic acid in bile [35,36], but cholangiocytes in PSC liver accumulate LPS [37]. Fourth, genome-wide association studies have found immunoregulation-related PSC risk loci [38,39]; one recent example is fucosyltransferase-2, which influences microbial composition, modulates susceptibility to infection, and is associated with IBD[38,40]. Finally, and although not known to be directly supporting the PSC-microbiota hypothesis, it is worth mentioning here the gut lymphocyte homing hypothesis. This hypothesis posits that intestinal T lymphocytes are (1) activated in gut-associated lymph tissue, (2) primed by dendritic cells to express cell-surface receptors integrin α4β7 and CCR9, and recruited to the liver as a result of aberrant hepatic expression of their cognate ligands, namely the addressin MAdCAM-1 and the chemotactic protein CCL25, which are usually restricted to the intestine [2,41]. Although the hepatic (periportal endothelial cell) expression of these ligands and subsequent homing of α4β7+, CCR9+ lymphocytes appears to be specific to PSC [42,43], the pathobiological relevance of this process and how it may relate to enteric microbially derived molecules have not been well defined and merits further study [2]; in this regard, the recent development and clinical application of vedolizumab, a humanized monoclonal antibody that antagonizes integrin α4β7, may offer an avenue for future research regarding mechanisms of disease and therapeutic targets in PSC.
In addition to these observations, we recently described what may be a potentially fundamental cellular phenotype at the intersection of the intestinal microbiota and cholangiocyte injury responses as they pertain to the etiopathogenesis of PSC–cholangiocyte senescence [44]. Cellular senescence, a subject that has seen exponential research growth in the last few years (PubMed.gov), is a state of replicative (G1 phase) arrest, which is generally believed to inhibit propagation or neoplastic transformation of injured cells [45–47]; albeit in replicative arrest, senescent cells remain metabolically active, and, in some cases, can transition to a potentially pathologic state known as a senescence-associated secretory phenotype (SASP) [44,48,49]. SASP cells have been shown to alter their microenvironment (e.g. the extracellular matrix), reinforce and exacerbate the senescent phenotype, initiate pro-fibroinflammatory cellular responses, and accelerate neoplastic transformation [46,48,50–53]. In our studies, we assessed whether cellular senescence and the SASP (1) can be induced in cultured human cholangiocytes by microbialmolecules and (2) are present in cholangiocytes in human PSC liver specimens. Using multiple cellular and molecular techniques, we found that persistent exposure to various microbial molecules (e.g. LPS, flagellin) induced human cholangiocyte senescence and ultimately SASP in vitro; moreover, markers of cholangiocyte senescence and SASP were increased in PSC compared to both normal and diseased controls [44]. In addition, we found evidence of cholangiocyte senescence in an established murine model of PSC [44,54]. These collective findings are currently being investigated further by us and others given their implications regarding novel therapeutic avenues for PSC and other cellular senescence-associated conditions.
Taken together, the aforementioned observations suggest that hepatobiliary injury in PSC may occur as a result of increased enterohepatic circulation of microbial molecules, abnormal microbial (metabolite) composition, aberrant host response to microbial molecules, or (perhaps most likely) a combination of these factors. Furthermore, and given the questions that remain regarding the etiopathogenesis of PSC, these observations (1) provide a framework for more translational studies investigating the relative contributions of microbial molecules and immunogenetic susceptibility to cholangiocyte injury and downstream signaling (Figure 1) [4,55] and (2) serve as a basis for several clinical applications of antibiotics in the treatment of PSC, as reviewed below.
Clinical experience with oral antibiotics in PSC
Since the initial case series of antibiotics in PSC [56], several small studies have been published, the majority within the past 10 years [1,57]. Three of these were prospective clinical trials (Table I), all three of which showed reduction in the biomarker serum alkaline phosphatase (ALK) with antibiotic treatment (weighted average reduction of 41%) [19,58,59]. The most recent of these was a phase II, double-blind study of 35 PSC patients randomized to low-dose vancomycin, high-dose vancomycin, low-dose metronidazole, and high-dose metronidazole. We observed significant improvements in ALK at 12 weeks as well as other endpoints in the low- and high- dose vancomycin groups. Given these findings, and because adverse effects were more frequent with metronidazole, the authors recommended vancomycin for further investigation; a phase III trial is indeed now underway (Clinical-Trials.gov NCT01802073).
Table I.
Clinical trials of oral antibacterial treatment for primary sclerosing cholangitis.
| Change post-therapy | ||||||||
|---|---|---|---|---|---|---|---|---|
| Drug | Year | n | Antibiotic dose | Months of therapy | ALK | AST | ALT | MRS |
| Metronidazole (+UDCA) [53] | 2004 | 39 | 600–800 mg/day | 36 | −52% | −41% | −68% | −0.50 |
| Minocycline [54] | 2009 | 16 | 200 mg/day | 12 | −20% | −3% | NA | −0.53 |
| Vancomycin [19] | 2013 | 18 | 125 or 250 mg qid | 3 | −46%* | −22%* | NA | −0.55* |
| Metronidazole [19] | 2013 | 17 | 250 or 500 mg tid | 3 | −10% | −9% | NA | −0.22 |
There have also been several case series and reports (Table II) [56,60–67]. The most notable of these consisted of 14 pediatric patients with PSC-IBD treated with vancomycin for 54 ± 43 months [66]; significant improvements in serum liver tests and clinical symptoms were noted in nearly all patients. Moreover, when vancomycin was discontinued, there was recurrence of symptoms and laboratory abnormalities in some patients, both of which resolved with vancomycin retreatment [68].
Table II.
Case series and reports of oral antibacterial treatment for primary sclerosing cholangitis.
| Change post-therapy | |||||||
|---|---|---|---|---|---|---|---|
| Drug | Year | n | Antibiotic dose | Months of therapy | ALK | AST | ALT |
| Drug Tetracycline [51]** | 1959 | 5 | 500 mg/day | 1–10 | −45% | −60% | −45% |
| Tetracycline []55† | 1965 | 5 | 500 mg/day | 48 | −21% | NA | NA |
| Metronidazole [62] | 1983 | 1 | 800 mg/day | 0.25 | NA‡‡ | NA‡‡ | NA‡‡ |
| Sulfasalazine (+UDCA) []56†† | 1998 | 2‡ | NA | 39 | −57% | −62% | −82% |
| Vancomycin [57] | 1998 | 3‡ | 375–1000 mg/day | 9 | NA | NA | −89% |
| Sulfasalazine (+UDCA) [58] | 2002 | 1 | 50 mg/kg/day | 37 | NA | NA | −92% |
| Sulfasalazine [59] | 2006 | 1 | 2–4.5 g/day | 24 | −74% | NA | −84% |
| Azithromycin (+UDCA) [60] | 2007 | 1 | 500 mg/day qod | 5 | −72% | −31% | −33% |
| Vancomycin [61] | 2008 | 14‡ | 50 mg/kg/day | 54 ± 43 | NA | NA | −78% |
Abbreviations: ALK = alkaline phosphatase, AST = aspartate aminotransferase; ALT = alanine aminotransferase, GGT = γ-glutamyl transpeptidase; tid = three times a day; qid = four times a day; UDCA = ursodeoxycholic acid.
Months of treatment and follow-up are absolute unless otherwise indicated.
Reflects low-dose vancomycin group only; values for high-dose vancomycin were: −40%, −21%, and −0.03 units, respectively.
Includes one patient who also received prednisone but whose data were not separable from the other four patients.
Does not include two patients who received prednisone.
Does not include a third patient who also received prednisolone and mizoribine.
Pediatric patients.
Original case report indicates dramatic improvement in patient’s condition, including defervescence, return of appetite, reduction of serum bilirubin, and after 2 weeks, becoming completely asymptomatic. Six months later, patient again worsened clinically, but again responded to metronidazole.
In addition to antibiotics, probiotics have been explored in PSC in a few studies over the last several years, including one small, randomized study [69]; because results of studies thus far have been divergent, ostensibly due to different probiotic use and endpoints measured, there are insufficient data at the present to comment on the role of probiotics in treating PSC. Most recently, there has been a case report of successful treatment of recurrent PSC post- LT with vancomycin, representing the first and a promising observation for post-LT disease [68].
Thus, while clinical antibiotic studies in PSC have, to date, been limited in size and number, their collective results are favorable and encourage future research on antibiotics or possibly other means of microbial manipulation as a potentially effective, safe, and minimally disruptive therapy for PSC.
Conclusions and future directions
The accumulating evidence from ongoing basic, translational, and clinical research efforts continues to strengthen the hypothesis regarding a potentially central role for the microbiota in the etiopathogenesis of and as a pharmacotherapeutic target for PSC. With the developments in molecular, high-throughput sequencing, and bioinformatic techniques over the last decade as well as an increasing number of experimental systems facilitating systematic study of the gut–liver axis, there is increasing promise for novel mechanistic and therapeutic advances in PSC (as well as other disorders). In this exciting and evolving research climate, we believe that some of the currently outstanding priorities and areas of future investigation include the following:
examining differences in intestinal microbial structure and function (e.g. metagenomic and metabolomic analyses) between healthy controls and patients with PSC, with and without IBD.
elucidating whether the therapeutic effects of antibacterial agents in PSC are related to (1) effects on microbial (metabolite) diversity, (2) alterations (e.g. decrease) in enterohepatic circulation of profibroinflammatory microbial metabolites, or (3) non-antimicrobial effects (e.g. anti-inflammatory properties [70]).
determining which antibiotic (or combination thereof) is most clinically effective and (1) how it should be dosed in order to provide durable benefits while minimizing risks such as antibiotic resistance and other adverse effects and (2) what the predictors (e.g. IBD status, stage, immunogenetic features) of a therapeutic response to antibiotics may be.
exploring the therapeutic potential and safety of alternative methods of manipulating the intestinal microbiota, including prebiotics (i.e. dietary manipulation or supplementation), probiotics, or fecal microbiota transplant.
Acknowledgments
This work was supported by National Institutes of Health Grants AI089713 (to SPO) and T32DK007198 (fellowship training for JHT).
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Tabibian JH, Lindor KD. Primary sclerosing cholangitis: a review and update on therapeutic developments. Expert Rev Gastroenterol Hepatol. 2013;7:103–114. doi: 10.1586/egh.12.80. [DOI] [PubMed] [Google Scholar]
- 2.Gupta A, Bowlus CL. Primary sclerosing cholangitis: etiopathogenesis and clinical management. Front Biosci. 2012;4:1683–1705. doi: 10.2741/e490. [DOI] [PubMed] [Google Scholar]
- 3.Tabibian JH, Lindor KD. Challenges of cholangiocarcinoma detection in patients with primary sclerosing cholangitis. J Anal Oncol. 2012;1:50–55. doi: 10.6000/1927-7229.2012.01.01.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weismuller TJ, Wedemeyer J, Kubicka S, Strassburg CP, Manns MP. The challenges in primary sclerosing cholangitis–aetiopathogenesis, autoimmunity, management and malignancy. J Hepatol. 2008;48:S38–S57. doi: 10.1016/j.jhep.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Karlsen TH, Schrumpf E, Boberg KM. Update on primary sclerosing cholangitis. Dig Liver Dis. 2010;42:390–400. doi: 10.1016/j.dld.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Jussila A, Virta LJ, Pukkala E, Farkkila MA. Malignancies in patients with inflammatory bowel disease: a nationwide register study in Finland. Scand J Gastroenterol. 2013;48:1405–1413. doi: 10.3109/00365521.2013.846402. [DOI] [PubMed] [Google Scholar]
- 7.Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–678. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 8.Alabraba E, Nightingale P, Gunson B, Hubscher S, Olliff S, Mirza D, et al. A re-evaluation of the risk factors for the recurrence of primary sclerosing cholangitis in liver allografts. Liver Transpl. 2009;15:330–340. doi: 10.1002/lt.21679. [DOI] [PubMed] [Google Scholar]
- 9.Landaverde C, Ng V, Sato A, Tabibian J, Durazo F, Busuttil R. De-novo cholangiocarcinoma in native common bile duct remnant following OLT for primary sclerosing cholangitis. Ann Hepatol. 2009;8:379–383. [PubMed] [Google Scholar]
- 10.Beuers U, Boberg KM, Chapman RW, Chazouillères O, Invernizzi P, Jones DE, et al. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Brandsaeter B, Friman S, Broome U, Isoniemi H, Olausson M, Backman L, et al. Outcome following liver transplantation for primary sclerosing cholangitis in the Nordic countries. Scand J Gastroenterol. 2003;38:1176–1183. doi: 10.1080/00365520310006009. [DOI] [PubMed] [Google Scholar]
- 12.Ali JM, Bonomo L, Brais R, Griffiths WJ, Lomas DJ, Huguet EL, et al. Outcomes and diagnostic challenges posed by incidental cholangiocarcinoma after liver transplantation. Transplantation. 2011;91:1392–1397. doi: 10.1097/TP.0b013e31821aba57. [DOI] [PubMed] [Google Scholar]
- 13.Kugelmas M, Spiegelman P, Osgood MJ, Young DA, Trotter JF, Steinberg T, et al. Different immunosuppressive regimens and recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2003;9:727–732. doi: 10.1053/jlts.2003.50143. [DOI] [PubMed] [Google Scholar]
- 14.Welcker K, Martin A, Kolle P, Siebeck M, Gross M. Increased intestinal permeability in patients with inflammatory bowel disease. Eur J Med Res. 2004;9:456–460. [PubMed] [Google Scholar]
- 15.Buhner S, Buning C, Genschel J, Kling K, Herrmann D, Dignass A, et al. Genetic basis for increased intestinal permeability in families with Crohn’s disease: role of CARD15 3020insC mutation? Gut. 2006;55:342–347. doi: 10.1136/gut.2005.065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Hara SP, Tabibian JH, Splinter PL, Larusso NF. The dynamic biliary epithelia: molecules, pathways, and disease. J Hepatol. 2013;58:575–582. doi: 10.1016/j.jhep.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Mahony CA, Vierling JM. Etiopathogenesis of primary sclerosing cholangitis. Semin Liver Dis. 2006;26:3–21. doi: 10.1055/s-2006-933559. [DOI] [PubMed] [Google Scholar]
- 18.Bjornsson E, Cederborg A, Akvist A, Simren M, Stotzer PO, Bjarnason I. Intestinal permeability and bacterial growth of the small bowel in patients with primary sclerosing cholangitis. Scand J Gastroenterol. 2005;40:1090–1094. doi: 10.1080/00365520510023288. [DOI] [PubMed] [Google Scholar]
- 19.Tabibian JH, Weeding E, Jorgensen RA, Petz JL, Keach JC, Talwalkar JA, et al. Randomised clinical trial: vancomycin or metronidazole in patients with primary sclerosing cholangitis - a pilot study. Aliment Pharmacol Ther. 2013;37:604–612. doi: 10.1111/apt.12232. [DOI] [PubMed] [Google Scholar]
- 20.Mueller T, Beutler C, Pico AH, Shibolet O, Pratt DS, Pascher A, et al. Enhanced innate immune responsiveness and intolerance to intestinal endotoxins in human biliary epithelial cells contributes to chronic cholangitis. Liver Int. 2011;31:1574–1588. doi: 10.1111/j.1478-3231.2011.02635.x. [DOI] [PubMed] [Google Scholar]
- 21.Tabibian JHMA, Masyuk TV, LaRusso NF. Cholangiocyte physiology. Compr Physiol. 2013;3:541–565. doi: 10.1002/cphy.c120019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Hara SP, Splinter PL, Trussoni CE, Gajdos GB, Lineswala PN, LaRusso NF. Cholangiocyte N-Ras protein mediates lipopolysaccharide-induced interleukin 6 secretion and proliferation. J Biol Chem. 2011;286:30352–30360. doi: 10.1074/jbc.M111.269464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokoyama T, Komori A, Nakamura M, Takii Y, Kamihira T, Shimoda S, et al. Human intrahepatic biliary epithelial cells function in innate immunity by producing IL-6 and IL-8 via the TLR4-NF-kappaB and -MAPK signaling pathways. Liver Int. 2006;26:467–476. doi: 10.1111/j.1478-3231.2006.01254.x. [DOI] [PubMed] [Google Scholar]
- 24.Harada K, Nakanuma Y. Biliary innate immunity and cholangiopathy. Hepatol Res. 2007;37:S430–S437. doi: 10.1111/j.1872-034X.2007.00247.x. [DOI] [PubMed] [Google Scholar]
- 25.Osnes T, Sandstad O, Skar V, Osnes M. Lipopolysaccharides and beta-glucuronidase activity in choledochal bile in relation to choledocholithiasis. Digestion. 1997;58:437–443. doi: 10.1159/000201480. [DOI] [PubMed] [Google Scholar]
- 26.Chen XM, O’Hara SP, LaRusso NF. The immunobiology of cholangiocytes. Immunol Cell Biol. 2008;86:497–505. doi: 10.1038/icb.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichtman SN, Keku J, Clark RL, Schwab JH, Sartor RB. Biliary tract disease in rats with experimental small bowel bacterial overgrowth. Hepatology. 1991;13:766–772. [PubMed] [Google Scholar]
- 28.Lichtman SN, Okoruwa EE, Keku J, Schwab JH, Sartor RB. Degradation of endogenous bacterial cell wall polymers by the muralytic enzyme mutanolysin prevents hepatobiliary injury in genetically susceptible rats with experimental intestinal bacterial overgrowth. J Clin Invest. 1992;90:1313–1322. doi: 10.1172/JCI115996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lichtman SN, Wang J, Clark RL. A microcholangiographic study of liver disease models in rats. Acad Radiol. 1995;2:515–521. doi: 10.1016/s1076-6332(05)80410-6. [DOI] [PubMed] [Google Scholar]
- 30.Yamada S, Ishii M, Liang LS, Yamamoto T, Toyota T. Small duct cholangitis induced by N-formyl L-methionine L-leucine L-tyrosine in rats. J Gastroenterol. 1994;29:631–636. doi: 10.1007/BF02365447. [DOI] [PubMed] [Google Scholar]
- 31.Haruta I, Kikuchi K, Hashimoto E, Nakamura M, Miyakawa H, Hirota K, et al. Long-term bacterial exposure can trigger nonsuppurative destructive cholangitis associated with multifocal epithelial inflammation. Lab Invest. 2010;90:577–588. doi: 10.1038/labinvest.2010.40. [DOI] [PubMed] [Google Scholar]
- 32.Karrar A, Broome U, Sodergren T, Jaksch M, Bergquist A, Bjornstedt M, et al. Biliary epithelial cell antibodies link adaptive and innate immune responses in primary sclerosing cholangitis. Gastroenterology. 2007;132:1504–1514. doi: 10.1053/j.gastro.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 33.Pollheimer MJ, Trauner M, Fickert P. Will we ever model PSC? -"it’s hard to be a PSC model!". Clin Res Hepatol Gastroenterol. 2011;35:792–804. doi: 10.1016/j.clinre.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 34.Pohl J, Ring A, Stremmel W, Stiehl A. The role of dominant stenoses in bacterial infections of bile ducts in primary sclerosing cholangitis. Eur J Gastroenterol Hepatol. 2006;18:69–74. doi: 10.1097/00042737-200601000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Hiramatsu K, Harada K, Tsuneyama K, Sasaki M, Fujita S, Hashimoto T, et al. Amplification and sequence analysis of partial bacterial 16S ribosomal RNA gene in gallbladder bile from patients with primary biliary cirrhosis. J Hepatol. 2000;33:9–18. doi: 10.1016/s0168-8278(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 36.Olsson R, Bjornsson E, Backman L, Friman S, Hockerstedt K, Kaijser B, et al. Bile duct bacterial isolates in primary sclerosing cholangitis: a study of explanted livers. J Hepatol. 1998;28:426–432. doi: 10.1016/s0168-8278(98)80316-4. [DOI] [PubMed] [Google Scholar]
- 37.Sasatomi K, Noguchi K, Sakisaka S, Sata M, Tanikawa K. Abnormal accumulation of endotoxin in biliary epithelial cells in primary biliary cirrhosis and primary sclerosing cholangitis. J Hepatol. 1998;29:409–416. doi: 10.1016/s0168-8278(98)80058-5. [DOI] [PubMed] [Google Scholar]
- 38.Folseraas T, Melum E, Rausch P, Juran BD, Ellinghaus E, Shiryaev A, et al. Extended analysis of a genome-wide association study in primary sclerosing cholangitis detects multiple novel risk loci. J Hepatol. 2012;57:366–375. doi: 10.1016/j.jhep.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu JZ, Hov JR, Folseraas T, Ellinghaus E, Rushbrook SM, Doncheva NT, et al. Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat Genet. 2013;45:670–675. doi: 10.1038/ng.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGovern DP, Jones MR, Taylor KD, Marciante K, Yan X, Dubinsky M, et al. Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn’s disease. Hum Mol Genet. 2010;19:3468–3476. doi: 10.1093/hmg/ddq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eksteen B, Mora JR, Haughton EL, Henderson NC, Lee--Turner L, Villablanca EJ, et al. Gut homing receptors on CD8 T cells are retinoic acid dependent and not maintained by liver dendritic or stellate cells. Gastroenterology. 2009;137:320–329. doi: 10.1053/j.gastro.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eksteen B, Grant AJ, Miles A, Curbishley SM, Lalor PF, Hubscher SG, et al. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J Exp Med. 2004;200:1511–1517. doi: 10.1084/jem.20041035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borchers AT, Shimoda S, Bowlus C, Keen CL, Gershwin ME. Lymphocyte recruitment and homing to the liver in primary biliary cirrhosis and primary sclerosing cholangitis. Semin Immunopathol. 2009;31:309–322. doi: 10.1007/s00281-009-0167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabibian JH, O’Hara SP, Splinter PL, Trussoni CE, Larusso NF. Cholangiocyte senescence via N-Ras activation is a characteristic of primary sclerosing cholangitis. Hepatology. 2014 doi: 10.1002/hep.26993. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeyapalan JC, Sedivy JM. Cellular senescence and organismal aging. Mech Ageing Dev. 2008;129:467–474. doi: 10.1016/j.mad.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 48.Burton DG. Cellular senescence, ageing and disease. Age (Omaha) 2009;31:1–9. doi: 10.1007/s11357-008-9075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trougakos IP, Saridaki A, Panayotou G, Gonos ES. Identification of differentially expressed proteins in senescent human embryonic fibroblasts. Mech Ageing Dev. 2006;127:88–92. doi: 10.1016/j.mad.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 52.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 53.Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 54.Tabibian JH, Macura SI, O’Hara SP, Fidler JL, Glockner JF, Takahashi N, et al. Micro-computed tomography and nuclear magnetic resonance imaging for noninvasive, live-mouse cholangiography. Lab Invest. 2013;93:733–743. doi: 10.1038/labinvest.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melum E, Franke A, Schramm C, Weismuller TJ, Gotthardt DN, Offner FA, et al. Genome-wide association analysis in primary sclerosing cholangitis identifies two non-HLA susceptibility loci. Nat Genet. 2011;43:17–19. doi: 10.1038/ng.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rankin JG, Boden RW, Goulston SJ, Morrow W. The liver in ulcerative colitis; treatment of pericholangitis with tetracycline. Lancet. 1959;2:1110–1112. doi: 10.1016/s0140-6736(59)90098-4. [DOI] [PubMed] [Google Scholar]
- 57.Elfaki DA, Lindor KD. Antibiotics for the treatment of primary sclerosing cholangitis. Am J Ther. 2011;18:261–265. doi: 10.1097/MJT.0b013e3181b7b8c0. [DOI] [PubMed] [Google Scholar]
- 58.Farkkila M, Karvonen AL, Nurmi H, Nuutinen H, Taavitsainen M, Pikkarainen P, et al. Metronidazole and ursodeoxycholic acid for primary sclerosing cholangitis: a randomized placebo-controlled trial. Hepatology. 2004;40:1379–1386. doi: 10.1002/hep.20457. [DOI] [PubMed] [Google Scholar]
- 59.Silveira MG, Torok NJ, Gossard AA, Keach JC, Jorgensen RA, Petz JL, et al. Minocycline in the treatment of patients with primary sclerosing cholangitis: results of a pilot study. Am J Gastroenterol. 2009;104:83–88. doi: 10.1038/ajg.2008.14. [DOI] [PubMed] [Google Scholar]
- 60.Mistilis SP, Skyring AP, Goulston SJ. Effect of long-term tetracycline therapy, steroid therapy and colectomy in pericholangitis associated with ulcerative colitis. Australas Ann Med. 1965;14:286–294. doi: 10.1111/imj.1965.14.4.286. [DOI] [PubMed] [Google Scholar]
- 61.Kozaiwa K, Tajiri H, Sawada A, Tada K, Etani Y, Miki K, et al. Three paediatric cases of primary sclerosing cholangitis treated with ursodeoxycholic acid and sulphasalazine. J Gastroenterol Hepatol. 1998;13:825–829. doi: 10.1111/j.1440-1746.1998.tb00740.x. [DOI] [PubMed] [Google Scholar]
- 62.Cox KL, Cox KM. Oral vancomycin: treatment of primary sclerosing cholangitis in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1998;27:580–583. doi: 10.1097/00005176-199811000-00015. [DOI] [PubMed] [Google Scholar]
- 63.Broccoletti TCE, Spaziano M, Capuano G, Petrone E, Mandato C, Migliaro F, et al. Refractory primary sclerosing cholangitis becoming responsive after sulphasalazine treatment of an underlying silent colitis. Ital J Pediatr. 2002;28:515–517. [Google Scholar]
- 64.Tada S, Ebinuma H, Saito H, Hibi T. Therapeutic benefit of sulfasalazine for patients with primary sclerosing cholangitis. J Gastroenterol. 2006;41:388–389. doi: 10.1007/s00535-005-1758-x. [DOI] [PubMed] [Google Scholar]
- 65.Boner AL, Peroni D, Bodini A, Delaini G, Piacentini G. Azithromycin may reduce cholestasis in primary sclerosing cholangitis: a case report and serendipitous observation. Int J Immunopathol Pharmacol. 2007;20:847–849. doi: 10.1177/039463200702000423. [DOI] [PubMed] [Google Scholar]
- 66.Davies YK, Cox KM, Abdullah BA, Safta A, Terry AB, Cox KL. Long-term treatment of primary sclerosing cholangitis in children with oral vancomycin: an immunomodulating antibiotic. J Pediatr Gastroenterol Nutr. 2008;47:61–67. doi: 10.1097/MPG.0b013e31816fee95. [DOI] [PubMed] [Google Scholar]
- 67.Mathew KK. Metronidazole in primary cholangitis. J Indian Med Assoc. 1983;80:31, 33. [PubMed] [Google Scholar]
- 68.Davies YK, Tsay CJ, Caccamo DV, Cox KM, Castillo RO, Cox KL. Successful treatment of recurrent primary sclerosing cholangitis after orthotopic liver transplantation with oral vancomycin. Case Rep Transpl. 2013;2013:314292. doi: 10.1155/2013/314292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vleggaar FP, Monkelbaan JF, van Erpecum KJ. Probiotics in primary sclerosing cholangitis: a randomized placebo-controlled crossover pilot study. Eur J Gastroenterol Hepatol. 2008;20:688–692. doi: 10.1097/MEG.0b013e3282f5197e. [DOI] [PubMed] [Google Scholar]
- 70.Abarbanel DN, Seki SM, Davies Y, Marlen N, Benavides JA, Cox K, et al. Immunomodulatory effect of vancomycin on Treg in pediatric inflammatory bowel disease and primary sclerosing cholangitis. J Clin Immunol. 2013;33:397–406. doi: 10.1007/s10875-012-9801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]