SUMMARY
For most viruses, including HIV, there is a need for antimicrobials that target unique viral molecular properties. Acyclovir (ACV) is such a drug for human herpesviruses (HHVs): it is activated into a HHV-DNA polymerase inhibitor exclusively by HHV-kinases and thus does not suppress other viruses. Here, we report on the direct inhibition of HIV-1 reverse transcriptase (RT) by phosphorylated ACV, which terminates DNA chain elongation and can trap RT at the site of termination. Consequently, ACV suppresses HIV-1 in HHV-coinfected human tissues but not in a HHV-free tissue or cell lines. However, addition of HHV-6-infected cells or administration of ACV monophosphorylated prodrugs (not requiring activation by HHV-kinases) renders HIV sensitive to ACV in these cells.
These data suggest that ACV is active against HIV-1 in tissues coinfected with various HHVs, provide new insights in ACV activity into HIV/HSV-2-coinfected patients, and provide strategies for the development of new HIV-1 RT inhibitors.
INTRODUCTION
HIV-1 infection is usually accompanied by infection with other microbes, HIV-1 copathogens, which either pre-exist in the human body or invade it de novo. The replication of HIV-1 copathogens is frequently promoted in HIV-1-infected hosts, and their suppression is often beneficial for the clinical course of HIV disease (Corey, 2007; Jacobson and Mills, 1988). Human herpesvirus (HHV) infections are commonly associated with HIV-1. In particular, herpes simplex virus 2 (HSV-2) infection is associated with an increase in HIV-1 transmission (Cohen, 2004; Freeman et al., 2006) and worsens the clinical course of HIV disease. HSV-2 reactivation may lead to increased plasma HIV-1 levels, thereby adversely affecting survival rates (Corey et al., 2004; Schacker et al., 2002).
ACV is a guanosine nucleoside analogue particularly active against HSV-2 as well as against the other α-HHVs (HSV-1 and VZV) (Elion, 1983). It also inhibits, although with lower potency, the replication of the β- HHVs (CMV, HHV-6, and HHV-7) and of the γ- HHVs (EBV and HHV-8) (De Clercq et al., 2001).
The mechanism of HHV suppression by ACV is well understood: ACV is phosphorylated in HHV -infected cells by viral-encoded kinases. The resulting monophosphate derivative (ACV-MP) is then converted into ACV triphosphate (ACV-TP) by cellular enzymes and is subsequently incorporated by the viral DNA polymerase into the nascent viral DNA chain, causing its obligate termination (Reardon and Spector, 1989). Generally, the sensitivity of different HHVs to ACV is determined by the extent of its phosphorylation by HHV kinase and by the rate of incorporation of ACV-TP into the viral DNA chain (De Clercq et al., 2001). The above-described mechanism explains why ACV does not directly affect viruses other than HHVs, including HIV-1. Nevertheless early clinical trials indicated that ACV treatment may result in survival benefits in cohorts of HIV-1 infected patients with a high incidence of clinically identifiable HHV (Ioannidis et al., 1998). In agreement with these data, recent trials showed that in HSV-2 co-infected individuals the suppression of HSV-2 by ACV is accompanied by reduction of the plasmatic, genital and rectal HIV-1 load (Baeten JM., 2007; Nagot et al., 2007; Zuckerman et al., 2007). This decrease was attributed to the suppression of HSV-2-mediated inflammation indirectly reducing HIV-1 load.
Here, we report on a newly found direct activity of ACV on HIV-1 and specifically on the inhibition of HIV-1 reverse transcriptase (RT) by ACV-TP. We demonstrate that, consequently, ACV is an anti-HIV-1 agent in human lymphoid, rectal, and genital tissues, which are widely infected with one or more HHVs that are able to phosphorylate ACV.
RESULTS
ACV suppresses HIV replication in human lymphoid tissue co-infected ex vivo with HSV-2
In this study, we used an ex vivo system that preserves the cytoarchitecture of human tissues and supports replication of various viruses without exogenous stimulation (Glushakova et al., 1995).
Initially we inoculated tissues with HIV-1LAI.04 and HSV-2 (strain G). Both viruses readily replicated in tissues. As expected, ACV at concentration of 30 μM suppressed HSV-2 replication in both HSV-2 singly infected and HIV-1 coinfected tissues (by 97 ± 1.8% and 93. ± 5.8% respectively, p = 3 × 10−5, p = 5 × 10−4, n = 4). However, in these HSV-2-coinoculated tissues ACV also suppressed HIV-1 replication by 62 ± 4.2% (p = 2 × 10−3, n = 4) (Fig. 1a).
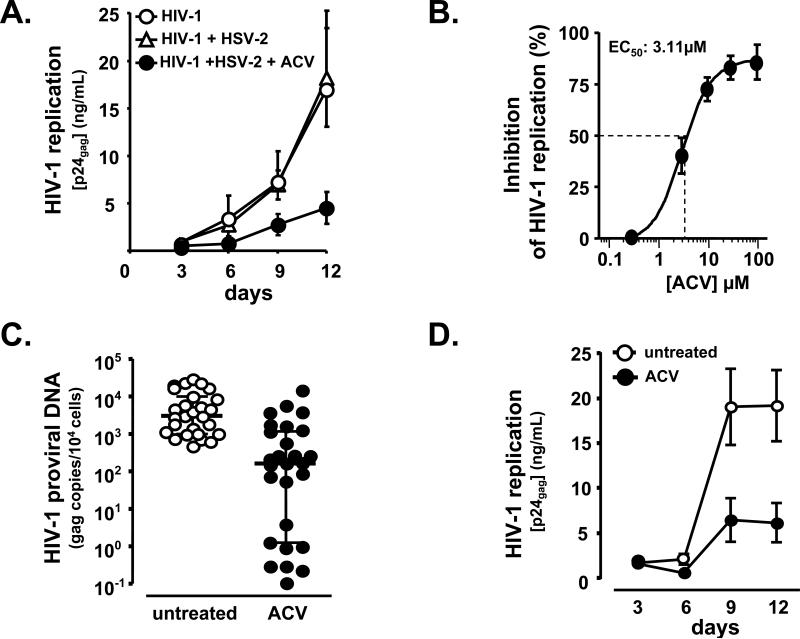
Figure 1. ACV suppresses HIV infection in human tonsillar tissues.
a. Blocks of human tonsillar tissue were co-inoculated ex vivo with HSV-2 strain G and X4LAI.04, and treated or not with ACV (30 μM). HIV-1 replication was monitored by measuring p24gag accumulated in culture media over 3 day periods. Presented are means ± SEM of the results with tissues from 4 to 17 donors. For each donor, each data point represents pooled viral release from 27 tissue blocks. Note that ACV suppresses HIV-1 in HSV-2 coinfected tissues.
b. HIV-1 replication was measured in tonsillar blocks infected with X4LAI.04 as in Fig.1a. ACV was added at the concentrations of 0.3, 3, 10, 30, and 100 μM, and its anti-HIV activity was evaluated from the suppression of viral replication compared with donor-matched HIV-infected tissues not treated with ACV. The 50% effective concentration (EC50) was estimated by fitting the data to 4 parameter logistic regression and was estimated to be 3.1 μM (95% confidence interval: 1.85-5.24). Presented are means ± SEM of the results with tissues from 3 to 7 donors Note that ACV suppresses HIV-1 replication in the tonsillar tissues in a dose-dependent manner.
c. HIV-1 proviral DNA load in tissue blocks at day 12 post-infection was measured by real time PCR. Presented are medians and interquartile ranges of the results, (n = 27). Note that ACV efficiently reduces HIV-1 proviral DNA load in blocks of tonsillar tissue.
d. HIV-1 replication was monitored as in Fig. 1a. Presented are means ± SEM of the results, (n = 38). Note that ACV efficiently suppresses HIV-1 replication in the tonsillar tissues tested.
ACV suppresses HIV replication in singly infected human lymphoid tissue ex vivo
Surprisingly, ACV was also found to suppress HIV-1 replication in tonsillar tissues from 38 donors that were not coinoculated with HSV-2 (Fig. 1b-d)
In HIV-1 singly infected tissues, the suppression of HIV-1 replication, measured as p24gag accumulation in culture medium, was dose-dependent with a 50% effective concentration (EC50) of 3.1 μM (95% confidence interval: 1.85-5.24) (Fig. 1b). HIV-1 replication, evaluated by the measurement of HIV-1 proviral DNA in tissue blocks at day 12 post-infection, was reduced by 94.8% (interquartile range [IQR] 65.9%–99.8%) in blocks of tonsillar tissues treated with ACV at the concentration of 30 μM compared with donor-matched untreated blocks. The median viral load was reduced by approximately 1.3 log10, from 3,108 gag DNA copies per 104 cells in untreated tissues (3.49 log10; IQR 2.99–4.01) to 162 gag DNA copies per 104 cells in ACV-treated tissues (2.27 log10; IQR 0.09–3.07, p < 10−4, n = 27) (Fig 1c). Consistently, ACV significantly decreased the amount of p24gag released into the culture medium on average by 80 ± 4% (p < 7 × 10−7, n = 38) in human tonsillar tissues (Fig. 1d). The average cumulative production of p24gag in the culture medium of tonsillar tissues was 46.4 ± 8 ng/mL and 16.6 ± 4.6 ng/mL, respectively in tissues untreated and treated with 30 μM ACV.
To avoid the possible confounding effects of contaminating products in commercial ACV, we used ACV from three sources and found that all of them similarly suppressed HIV (p ≥ 0.45, n = 4). Suppression of HIV-1 replication by ACV was not associated with T-cell depletion, as evidenced from the similar numbers of total T cells (CD4+CD3+ and CD8+CD3+, p ≥ 0.5, n = 3), their subsets of activated (CD25+, HLA-DR+, or CD38), naïve (CD45RA+CD62L+) or non-naive (CD45RA−CD62L+/-) (p ≥ 0.16, n = 3) cells. Moreover, ACV antiviral activity was restricted to HSV-2 and HIV-1, since as expected, ACV did not reduce vaccinia virus replication in tonsillar tissue ex vivo (data not shown).
ACV suppresses replication of CCR5- and CXCR4-tropic HIV-1 variants in various human tissues
To test whether ACV suppressed HIV-1 replication in tissues other than tonsils, we used explants of tissues that also play an important role in HIV transmission and pathogenesis in vivo: lymph nodes, cervicovaginal and colorectal tissues. ACV suppressed HIV-1 replication, measured by p24gag accumulation in culture medium, in lymph nodes by 70 ± 13.9%, (p = 2 × 10−3, n = 7), in colorectal tissues by 72.6 ± 9% (p = 2 × 10−2, n = 3) and in cervical tissues by 60.1 ± 3.5% (p = 7 × 10−3, n = 3). ACV treatment of ex vivo human lymph nodes reduced the average cumulative production of p24gag in culture medium from 67.3 ± 28.1 ng/mL to 15.4 ± 6.6 ng/mL, in ex vivo human colorectal tissues from 7.2 ± 6.8 ng/mL to 1.3 ± 1.2 ng/mL (n = 3), and in ex vivo human cervicovaginal tissues from 3.8 ± 1.7 ng/mL to 1.4 ± 0.5 ng/mL (n = 3) (Fig. 2a).
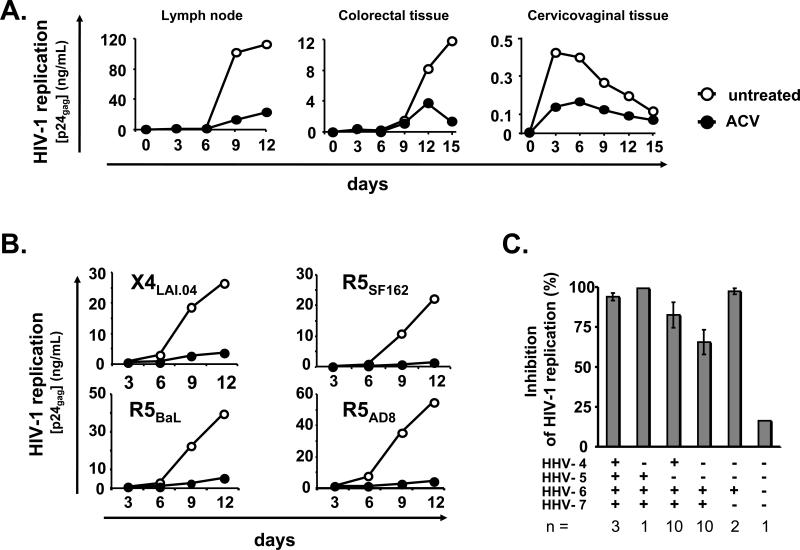
Figure 2. ACV suppresses replication of different HIV-1 variants in human tissues coinfected with various HHVs.
a. HIV-1 replication in blocks of human lymph nodes, colorectal and cervicovaginal tissues was monitored as in Fig.1a. For each type of tissue, the graph represents a typical result of 3 to 7 experiments performed with tissues from different donors. Note that ACV efficiently suppresses replication of HIV-1 in lymph node, colorectal and cervicovaginal tissues.
b. Examples of ACV suppression of different HIV-1 variants (X4LAI.04, R5BaL, R5SF162, and R5AD8). Each data point represents pooled viral release from 27 tissue tonsillar blocks. Note that ACV efficiently suppresses replication of all four HIV-1 variants.
c. Presence of HHV-1, -2, -3, -4, -5, -6, -7 and -8 by real-time PCR in blocks of tonsillar tissue. All tissues were negative for HHV-1, -2, -3, and -8. Presented are means ± SEM of the results with tonsils from n donors. Note that there are no significant differences in the level of ACV suppression of HIV-1 replication in tissues infected with various HHVs.
Furthermore, we evaluated the efficiency of ACV in suppressing different HIV-1 variants. We inoculated ex vivo lymphoid tissues with CCR5- and CXCR4-tropic HIV-1 variants X4LAI.04, R5SF162, R5BaL, and R5AD8. As shown in Figure 2b, ACV suppressed replication of all these isolates in tonsillar tissues with similar efficiency (by 88%, 95%, 89%, and 94%, respectively, as assessed from p24gag production).
Coinfection with different endogenous human herpesviruses is associated with the anti-HIV effect of ACV
To test whether suppression of HIV by ACV is related to the anti-herpetic activity of this drug, we measured the presence of various HHVs in the tonsillar tissues used in the present work (Fig. 2c). Real-time PCR analysis revealed that all 27 tonsillar tissues tested for the presence of HHVs were negative for HSV-1, HSV-2, HHV-3 and HHV-8, but were infected with HHV-4, -5, -6 and -7 in various combinations (Fig 2c). CMV was present in 15% of tissues, EBV in 52%, HHV-7 in 89%, and HHV-6 was found in all but one tissue (96%).
Similar to what was observed in immunocompromised patients (Lusso and Gallo, 1994), HHV load was increased in tissues ex vivo. There was a five-fold increase in the median HHV-6 load at day 12 in culture, from 22.4 DNA copies per 104 cells at the time of surgery (IQR 1.8–57.7) to 116.6 DNA copies per 104 cells (IQR 33.7–808, p = 3 × 10−4, n = 26). After ACV treatment, the median HHV-6 load at day 12 was reduced to 55.1 DNA copies per 104 cells (IQR 21.3–334.6, p =10−2, n = 26), demonstrating that ACV significantly suppressed HHV-6 replication. The sensitivity of HHV-6 to ACV was further evaluated in separate experiments in which ex vivo tonsillar tissues were inoculated with HHV-6B (PL-1 strain). In these experiments, ACV suppressed HHV-6B replication in a dose-dependent manner with an EC50 of approximately 27 μM. We found that ACV reduced the total production of HHV-6B in culture medium on average by 56.2 ± 11.4 % (52.2 ± 12.4 × 108 DNA copies per mL versus 22.5 ± 9.4 × 108 DNA copies per mL in control and ACV treated tissues respectively; p = 3 × 10−3, n = 3).
HHV-7, HHV-4, and HHV-5 median loads in tonsillar tissues were respectively 9.23 (IQR 0.51–56.81), 4.71 (IQR 0.06–448.3), and 0.1 (IQR 0.06–0.1) DNA copies per 104 cells at the time of surgery and 21.87 (IQR 0.9–219.7, n = 24), 31.16 (IQR 3.63–260.9, n = 14), and 388 (IQR 175.9–2416, n = 4) copies per 104 cells after 12 days of culture.
In all tissues infected with different combinations of HHVs, ACV suppressed HIV-1 replication with a similar efficiency (Fig. 2c). HHV-6 was the only HHV that was present in all combinations. Moreover, it seems that the suppression of HIV-1 by ACV is related to the amount of HHV-6. In tissue blocks in which ACV inhibited HIV-1 replication by more than 50%, the median HHV-6 load on day 12 in culture was significantly higher than in the tissues in which ACV suppressed HIV-1 replication by less than 50%: 131.1 DNA copies per 104 cells (IQR 36.1–1,154, n = 23) versus 21.6 DNA copies per 104 cells (IQR 0.6–77.4, n = 4; p = 4 × 10−2) respectively. Furthermore, we documented a correlation between the ACV mediated suppression of HIV-1 replication as measured by p24gag release and the level of HHV-6, in tissues where the HIV-1 inhibition was suboptimal (between 0 and 99%) (r = 0.43, p = 0.03, n = 25).
Although several HHVs, such as HHV-6, are ubiquitous and are transmitted early in childhood, we identified a tissue in which there was no detectable HHV. In agreement with our hypothesis, in this tissue ACV suppression of HIV-1 (at the concentration of 30 μM) was negligible (16%) (Fig. 2c).
Coinfection with HHVs is necessary and sufficient for the anti-HIV effect of ACV
To prove this claim we performed experiments with MT4 cells, an HHV-uninfected T cell line that efficiently supports the replication of HIV-1LAI.04. We found that ACV did not suppress HIV-1LAI.04 (EC50 > 250 μM) in these cultures in agreement with earlier observations (Barral et al., 2003). To test whether HHV infection is sufficient for the anti HIV-1 effect of ACV, we added various amounts of HHV-6B-infected MT4 cells to HHV-free MT4 cultures infected with HIV-1LAI.04. As expected, ACV suppressed HHV-6: after 3 days of culture the fraction of HHV-6B infected cells was reduced in a dose-dependent manner, as measured by flow cytometry (Fig. 3a). Importantly, in these cultures, ACV suppressed HIV replication as well, as evaluated by the number of p24gag+ T cells and by p24gag release into the culture medium (EC50 of ~ 50 μM) (Fig. 3a,b). In these experiments, 9% of HHV-6B infected cells were sufficient to suppress HIV-1LAI.04 replication by approximately 70% (Fig. 3a). We found that in agreement with earlier publications (De Clercq et al., 2001), the concentration that corresponds to the EC50 in these experiments did not affect cell viability (50% cytotoxic concentration > 250 μM).
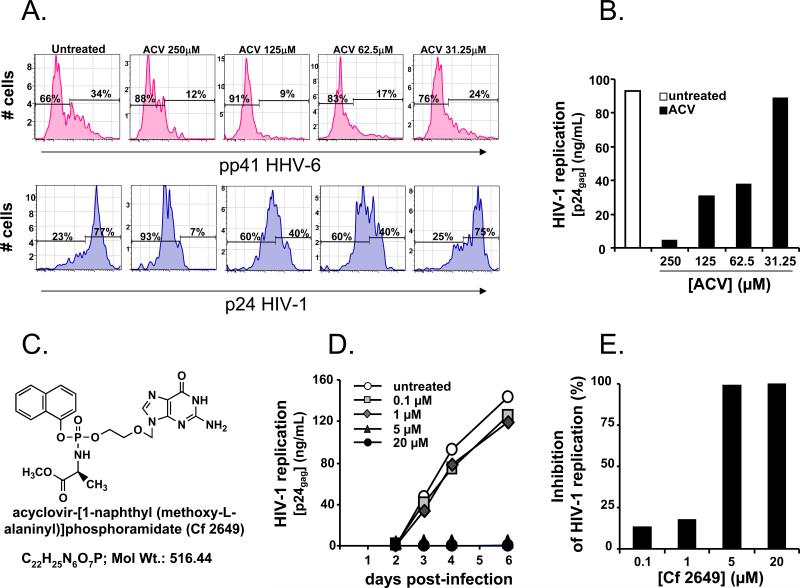
Figure 3. ACV suppresses HIV-1 in HHV-free MT-4 cell cultures either in the presence of HHV-6 infected cells or as monophosphorylated prodrug.
a. HIV-1LAI.04 -infected MT4 cells were co-cultured with HHV-6B-infected MT4 cells in a ratio 10:1. Presented are the distributions of HHV-6B (upper panel) and HIV-1 LAI.04-infected cells (lower panel) as measured by flow cytometry at day 3 post-HIV-1 infection, in cultures untreated or treated with various concentrations of ACV. Note that the fraction of both HHV-6B- and HIV-1-infected cells is reduced in a dose dependent manner by ACV treatment.
b. HIV-1LAI.04 -infected MT4 cells were co-cultured with HHV-6B infected MT4 cells. HIV-1 replication was monitored as in Fig.1a. Note that the replication of HIV-1 in HHV-6B/HIV-1-infected co-cultures is suppressed by ACV treatment in a dose dependent manner.
c. The compound acyclovir-[1-naphthyl (methoxy-L-alaninyl)]phosphoramidate (Cf2649) has been synthesized as described in Supplemental data.
d. HIV-1 LAI.04 -infected HHV-free MT4 were treated with various concentrations of the ACV monophosphorylated prodrug Cf2649. Presented are the kinetics of HIV-1LAI.04 replication, monitored by measuring p24gag accumulated in culture media at day 2, 3, 4 and 6 post-infection. Presented data are representative of two experiments. Note that compound Cf2649 suppresses the replication of HIV-1LAI.04 in a dose dependent manner.
e. HIV-1LAI.04 -infected MT4 cells not infected with any HHVs were treated with various concentrations of the compound Cf2649. The cumulative release of p24gag into culture media over 6 days of culture treated with various concentrations of the compound Cf2649 is presented as fraction of the p24gag production in the untreated cultures. Presented data are representative of two independent experiments. Note that compound Cf2649 suppresses the replication of HIV-1LAI.04 in a dose-dependent manner.
To further prove that ACV phosphorylation is required for HIV-1 inhibition, we synthesized monophosphorylated ACV prodrug acyclovir-[1-naphthyl (methoxy-L-alaninyl)]phosphoramidate (Cf2649) (Fig. 3c). This compound bypasses the requirement of HHVs for the activation of ACV since it is already monophosphorylated. Indeed, when applied to (HHV-free) MT-4 cells, Cf2649 in contrast to non-phosphorylated ACV (EC50 > 250 μM), suppressed HIV-1 replication with an EC50 of ~3 μM (Fig. 3d, e). Similar results were obtained with another HHV-free T cell line (CEM) (data not shown).
In conclusion, we demonstrated that neither in HHV-free tissue nor in HHV-free cell lines does ACV suppress HIV-1 infection. Reconstitution of the cell line system with HHV-6-infected cells or bypassing the HHV-kinases requirement by applying an ACV monophosphate derivative makes HHV-free systems susceptible to HIV suppression by ACV.
ACV-TP inhibits HIV-1 RT
Since we demonstrated that the anti-HIV-1 activity of ACV requires HHV-mediated activation and that phosphorylated derivatives of ACV are produced in HHV-infected tissue (see supplemental data, Fig. S1), we hypothesized that ACV-TP, into which ACV is ultimately converted (Elion, 1983), interferes with the activity of the HIV-1 RT and directly suppresses HIV-1 replication.
We tested whether ACV-TP suppresses RT by measuring the polymerizing activity of RT from lysed HIV-1 using an exogenously added HIV-1 template. We observed a dose-dependent inhibition of HIV-1 RT activity by ACV-TP, while we noted no suppression of HIV-1 RT activity by ACV itself, even at high concentrations (Fig. 4a).
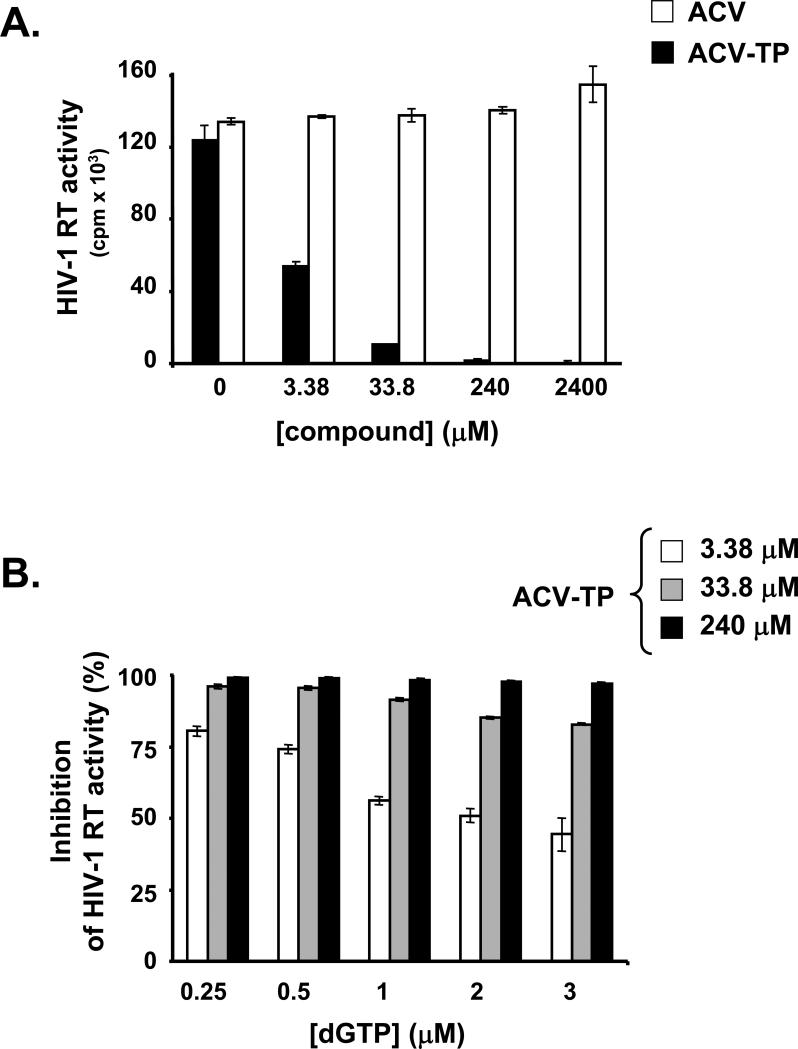
Figure 4. ACV-TP inhibits HIV-1 RT in exogenous template reverse transcriptase assays.
a. Exogenous template reverse transcriptase assays were performed in presence of various concentrations of ACV or ACV-TP as described in Experimental Procedures. Presented are means ± SEM of the results of two experiments performed in duplicates. Note that ACV-TP inhibits HIV-1 RT in a dose-dependent manner.
b. The dependence of the RT inhibition by ACV-TP on the concentration of dGTP was evaluated using an exogenous template reverse transcriptase assay. dGTP was used at the indicated concentrations. The reactions were performed in the presence of the indicated concentrations of ACV-TP. Presented are means ± SEM of the results of two experiments performed in duplicate. Note that inhibition of HIV-1 RT activity by ACV-TP is inversely dependent on the concentration of dGTP.
Since ACV-TP is a guanosine-5’-triphosphate analogue, we investigated whether it acts as a nucleotide RT inhibitor by testing if dGTP prevents ACV-TP inhibition of RT. We found that at ACV-TP concentrations of 3.38 μM or 33.8 μM, HIV-1 RT inhibition was inversely dependent on the dGTP concentrations. At the highest concentration of ACV-TP tested, no competition with dGTP was observed (Fig. 4b). Since ACV-TP lacks an additional hydroxyl group (present in dGTP and essential for DNA chain polymerization), we further hypothesized that the above-described suppression of HIV-1 RT by ACV-TP is similar to its suppression of HSV DNA polymerase, namely by incorporation into the nascent HIV DNA resulting in its chain-termination (Reardon and Spector, 1989).
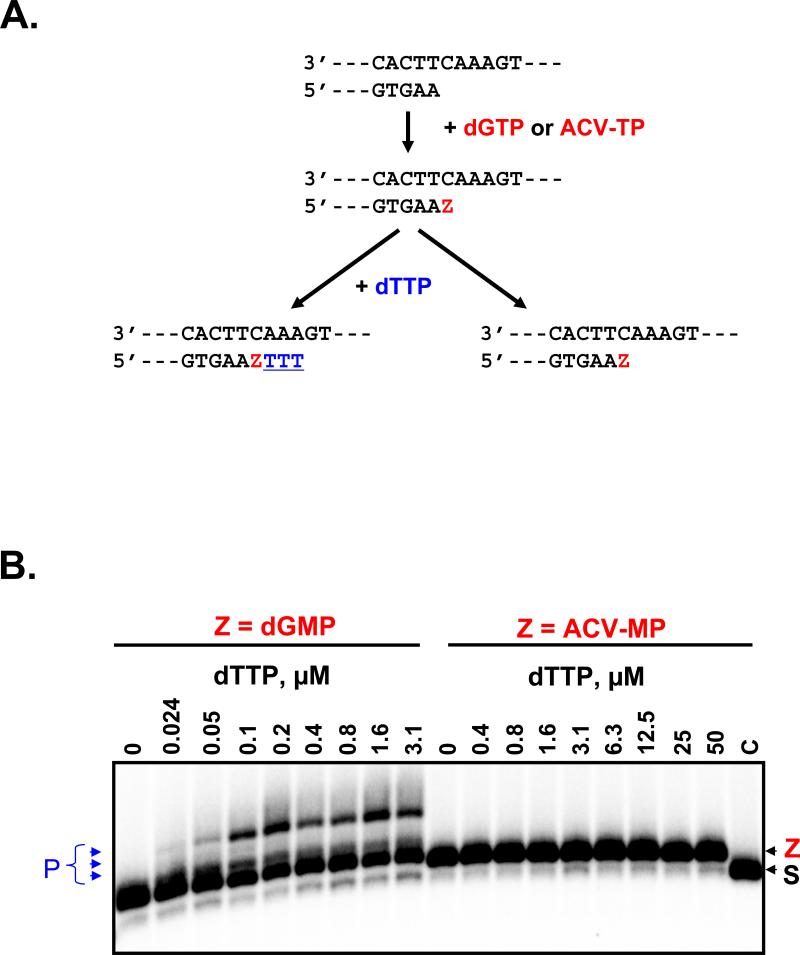
To prove this hypothesis we used a gel-based assay (Marchand and Gotte, 2003; Marchand et al., 2007) and found that HIV-1 RT incorporated ACV-MP into the DNA primer as efficiently as the natural substrate dGTP. The efficiency of single nucleotide incorporation events (kcat/Km) under steady-state conditions for dGTP and ACV-TP was 14.7 μM−1min−1 and 14.0 μM−1min−1, respectively (Supplemental Table 1). Furthermore, ACV-MP incorporation resulted in complete DNA chain-termination, as shown in Figures 5a and 5b. A primer that contained the natural dGMP at its 3’-end was successfully extended in the presence of dTTP, which is the substrate for the following three consecutive template positions (Fig. 5b left panel). In contrast, DNA synthesis was effectively blocked when the primer was terminated with ACV-MP. Even the relatively high concentration of 50 μM of dTTP did not permit its incorporation (Fig. 5b right panel). These data suggest that the mechanism of action of ACV is similar to the currently approved anti-HIV nucleoside reverse transcriptase inhibitors (NRTIs).
Figure 5. Chain-termination with ACV-MP.
a. The reaction scheme shows the relevant region of the primer and template and two possible outcomes of the primer elongation. Elongation of the primer in the presence of dGTP or ACV-TP is indicated by the “Z” in red, which refers to the incorporated monophosphate dGMP or ACV-MP, respectively. Incorporation events following position “Z” with dTTP are in blue underlined.
b. The primer (S, lane C) was initially elongated to incorporate dGMP or ACV-MP, respectively at the 3’-end referred to as “Z”. Incubation with increasing concentrations of the next nucleotide dTTP resulted in three nucleotide incorporation events with the dGMP terminated primer, that are labeled as products (P). Note that the ACV-MP terminated primer is not extended, which shows that the inhibitor acts as a chain-terminator.
DNA chain-termination by NRTIs is not irreversible, but can be subjected to phosphorolytic excision of the incorporated drug by HIV RT (Meyer et al., 1998). Binding of the next complementary nucleotide following the DNA chain-terminator can lead to the formation of a dead-end complex. In this complex, HIV RT is trapped in a conformation that blocks the excision reaction. Thus, to test whether ACV-MP-terminated DNA is accompanied with the formation of a dead-end complex, we evaluated the inhibition of ACV-MP phosphorolytic excision from the 3’-end of the primer by HIV-1 RT. The excision of a DNA chain-terminator requires the presence of pyrophosphate (PPi) or pyrophosphate donor molecules (such as ATP). We found that HIV-1 RT is capable of excising the incorporated ACV-MP in the presence of physiologically relevant concentrations of ATP (data not shown) as demonstrated for certain NRTIs. However, to analyze whether the ACV-terminated primer permits formation of a dead-end complex, the ATP-dependent excision reaction was assayed in the presence of increasing concentrations of the next complementary nucleotide. We found that the next complementary nucleotide caused 50% inhibition of ACV-MP excision (IC50) at a concentration of approximately 26 μM (Fig. 6a, b). These data strongly suggest the formation of a dead-end complex in ACV-MP-incorporated templates. The higher IC50 (26 μM versus 3.1 μM) in comparison to reactions conducted with the control DNA chain-terminator ddGMP was probably due to the acyclic nature of ACV-MP. In general, the excision reaction was inefficient; only 10 to 15% of the terminated primer strands were rescued for continuation of DNA synthesis at low concentrations of the next complementary nucleotide.
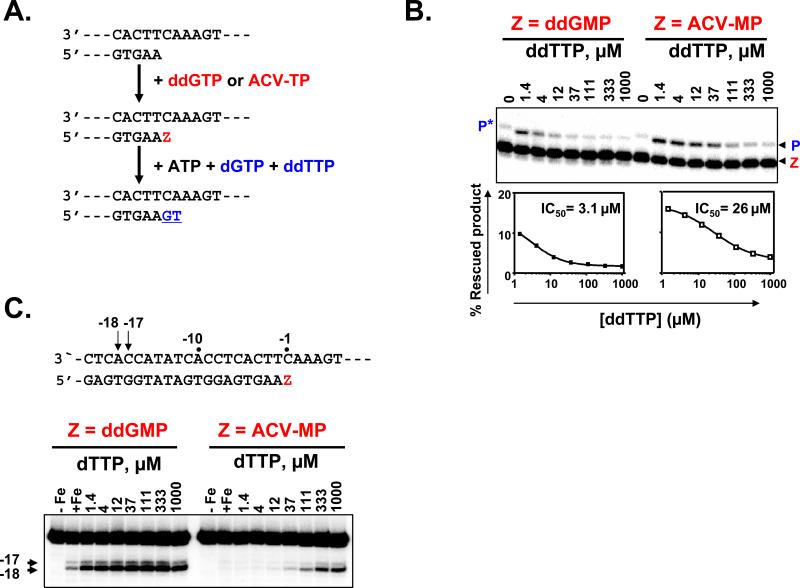
Figure 6. Inhibition of ATP-dependent excision in the presence of the next nucleotide substrate.
ATP-dependent excision was monitored in a combined excision/rescue assay as previously described in Supplemental Experimental Procedures.
a. The reaction scheme shows the relevant region of the primer and template. Extension of the primer in the presence of ddGTP or ACV-TP is indicated by the “Z” in red. The excision of a DNA chain-terminator requires the presence of ATP as pyrophosphate donor. The 3’-ultimate nucleotide (Z) was excised with ATP, and the simultaneous presence of dGTP and ddTTP (in blue) allowed the rescue of DNA synthesis.
b. The combined excision/rescue reaction was studied with ddGMP and ACV-MP terminated primers. “Z” refers to the terminated primer and “P” to the rescued product. The asterisk indicates dGMP misincorporation in the absence of the correct ddTTP substrate.
Quantification of excision/rescue reactions for ddGMP and ACV-MP are plotted. The concentration of the next complementary nucleotide required to inhibit 50% of the reaction is calculated on the basis of these two curves. Note that ACV-MP-terminated primer excision/rescue reaction is inhibited due to dead end complex formation.
c. Incorporation of ACV-TP produces a dead-end complex with HIV-1 RT. Presented is site-specific footprinting of HIV-1 RT with ddGMP and ACV-MP terminated primers in the presence of increasing concentrations of the next complementary nucleotide. Lanes “–Fe” and “+Fe” show control reactions in the absence and presence of divalent Fe2+ ions that cause site-specific cleavage on the labeled template. The arrows and the sequence underneath the gel show the position of the oxidative cleavage on the template strand at positions –17 and –18, which are indicative for post- and pre-translocated complexes, respectively. Note that for ACV-MP terminated primer, at low next complementary nucleotide concentrations no cleavage occurs indicating that the complex between RT and terminated primer is fragile whereas at higher concentrations of the next complementary nucleotide RT is blocked in a dead-end complex.
To provide additional direct evidence of dead-end complex formation, we employed site-specific footprinting that allowed us to determine the position of HIV-1 RT on its primer/template at single nucleotide resolution (Marchand and Gotte, 2003; Marchand et al., 2007). Nucleotide binding and formation of a dead-end complex can only occur in the post-translocated conformation in which the nucleotide binding site of HIV-1 RT has been cleared. In this conformation, the RT has moved a single nucleotide further downstream relative to the pre-translocated state. Our footprints are based on oxidative cleavage on the template strand at positions −17 and −18, which are indicative of post- and pre-translocated complexes, respectively. We compared footprints of complexes with ddGMP and ACV-MP terminated primers, in the presence of increasing concentrations of the next complementary nucleotide (Fig. 6c). Low concentrations of the next complementary nucleotide are sufficient to stabilize the post-translocated complex when the primer was terminated with ddGMP, proving the formation of a dead-end complex. A similar pattern is seen with ACV-MP, although the concentration of the next nucleotide required to stabilize the complex is higher. Oxidative cleavage in case of ACV-MP did not occur in the absence of or at low concentrations of the next complementary nucleotide. These findings suggest that at the low next complementary nucleotide concentration, the complex between RT and terminated primer formed at the site of the ACV-MP termination is relatively fragile, perhaps prone to dissociation. In contrast, at high concentration of the next complementary nucleotide RT can be trapped in a dead-end complex (DEC): the dissociation constant for ACV-terminated primers in the presence of a high concentration of the next nucleotide (111μM) is increased approximately three-fold (Fig. S2).
Thus, these data explain why the excision of ACV-MP is generally inefficient at both low and high concentrations of the next complementary nucleotide: At low concentrations the complex between RT and ACV-MP is relatively unstable, while at higher concentrations a DEC is formed.
To prove that ACV targets HIV-1 RT in human tissues like in cell-free assays, we tested two pairs of HIV isogenic strains that are resistant to some of the currently approved NRTIs. The isolate AZT 4x containing the mutations at D67N, K70R, T215Y and K219Q express complete resistance to AZT and resulted to be as sensitive to ACV in infected tissues as its parental HIV-1 variant (Fig S3a). However, an HIV-1 isolate carrying the M184V mutation (that confers resistance to Lamivudine) was less sensitive to ACV than its parental strain (Fig S3b). The EC50 for the M184V isolate was approximately 4 times higher than that for the parental HIV-1 isolate. Thus, the evidence that a specific isolated mutation of one amino acid in RT can reduce the sensitivity of an HIV-1 isolate to ACV further proves that HIV-1 RT is targeted and suppressed by ACV in human tissues, in agreement with the data on ACV suppression of HIV-1 RT in cell-free systems.
DISCUSSION
Growing epidemics of HIV-1 infection, especially in countries with limited resources, and the emergence of drug-resistant viruses make it necessary to find ever new safe, efficient, and inexpensive strategies against this virus. Strategies as effective and safe as those developed against several other human pathogens such as HSV, which can be efficiently treated with ACV, have yet to be developed against HIV. The anti-herpetic specificity of ACV is primarily based on the unique ability of HHV-encoded kinases to phosphorylate ACV to its monophosphate derivative, which is subsequently converted into the antivirally active ACV-TP (Reardon and Spector, 1989). Consistent with its highly restricted anti-herpetic activity, ACV is not currently used as a direct HIV-1 inhibitor.
Our findings demonstrate (i) the direct inhibitory effect of ACV-TP, but not of ACV itself, on isolated HIV-1 RT in a cell-free system; (ii) the suppression of HIV-1 replication by ACV in human tissues and in T-cell lines if and only if they carry HHV that phosphorylate ACV; (iii) the ability of a phosphorylated ACV prodrug to bypass the requirement of HHV to suppress HIV.
To our knowledge, this is the first definitive experimental evidence of inhibition of HIV-1 RT activity by phosphorylated ACV and the first demonstration that ACV phosphorylation occurring in HHV-infected human tissues transforms this widely-used anti-herpetic drug into an HIV-1 inhibitor.
The direct suppression of HIV-1 RT activity by ACV-TP was demonstrated in two different cell-free assays, one using recombinant HIV-1 RT and the other using RT extracted from lysed HIV-1 virions. We showed that ACV-TP is incorporated into the nascent viral DNA chain with a level of efficiency similar to that of its natural equivalent dGTP. Incorporation of ACV-TP results in the termination of reverse transcription, while the excision of the incorporated ACV-MP from the DNA chain is partially inhibited because of the fragility of the complex between HIV-1 RT and the terminated DNA chain and because of the formation of a dead-end complex. Although these properties of ACV resemble those of certain approved anti-HIV NRTIs (Marchand and Gotte, 2003; Meyer et al., 1999), ACV-TP is the first non-phosphonate acyclic nucleoside analogue that inhibits HIV-1 RT.
To further confirm that in HIV-1-infected cells ACV-triggered HIV-1 suppression is mediated by RT, we identified a pair of isogenic HIV-1 strains differing only by one amino acid (M184V) in RT but having different sensitivities to ACV in ex vivo human tissues. Future studies on selection of ACV-resistant HIV-1 isolates and testing of various multi-drug-resistant HIV-1 isolates for ACV sensitivity will reveal the exact set of RT mutations associated with reduced sensitivity to ACV and the rate of their evolution. Identification of ACV-resistant mutants does not in general exclude the use of ACV against HIV-1 variants resistant to other NRTI: for instance, ACV efficiently suppresses the replication of AZT-resistant HIV-1.
As demonstrated in a cell-free system, to inhibit HIV-1 RT, ACV has to be converted into ACV-TP. In human tissues, such conversion requires HHV infection. Accordingly, we found that in the presence of HHVs that are capable of phosphorylating ACV, HIV-1 was inhibited in ACV-treated tonsils, lymph nodes, cervico-vaginal and colorectal tissues, where the critical events of HIV-1 pathogenesis and transmission occur in vivo.
Various HHVs, including the ubiquitous HHV-6 detected in all but one tissue ex vivo, may mediate HIV-1 suppression by ACV. The level of HHV-6 replication may be essential: in tonsillar tissues in which ACV inhibited HIV replication by more than 50%, the median HHV-6 load was significantly higher than in the tissues in which ACV suppressed HIV replication by less than 50%. Moreover, in tissues where the HIV-1 inhibition was suboptimal there was a correlation between the ACV-mediated suppression of HIV-1 replication and the level of HHV-6. These results demonstrate again the critical role of HHVs in ACV-mediated suppression of HIV-1 and indicate the importance of HHV-6 in mediating HIV-1 suppression by ACV in our ex vivo tissue system.
HHV-6 and HHV-7 are ubiquitous viruses, and therefore the probability of finding an HHV-free tissue is very low. Nevertheless, by testing tissues from multiple donors we identified one tonsillar tissue that was not infected by any HHV. ACV did not inhibit HIV-1 replication in this tissue.
However, to further demonstrate that HHVs are necessary and sufficient for the anti-HIV effect of ACV, we used the HHV-uninfected MT4 cell line. Consistently with the proposed mechanism based on HHV-mediated activation, ACV did not inhibit HIV replication in this HHV-free cell line. However, when HHV-6-infected cells were added to the HIV-1-infected cultures, ACV became an HIV-1 suppressor. This effect was dependent on both the concentration of ACV and the fraction of HHV-6-infected cells. Apparently, in reconstituted MT4 cultures ACV that is phosphorylated in HHV-6-infected cells is transferred to HIV-1-infected cells, since the majority of these cells were not coinfected with HHV-6. These results are in full agreement with the published data on the transfer of phosphorylated ACV between cells (Burrows et al., 2002; Degreve et al., 1999). In tissues, transfer of phosphorylated ACV between cells is facilitated by specialized contacts (Nicholas et al., 2003). These and probably other factors (e.g., rapid proliferation and large endogenous dNTP pools, as well as a high level of HIV-1 replication) may contribute to a lower sensitivity of HIV-infected cell lines to ACV compared with integral tissues.
To further demonstrate the necessity of ACV activation for HIV suppression, we synthesized an already monophoshorylated (activated) ACV in which the phosphate is masked by liphophilic groups. In contrast to non-phosphorylated ACV, this prodrug suppressed HIV-1 in HHV-free cultures of MT4 or CEM cells. Although enzymatic reactions mediating ACV prodrug conversion into its active form have been described (Congiatu et al., 2007), the entire process of this conversion remains to be elucidated. Nevertheless, our results provide strong evidence that upon phosphorylation ACV suppresses HIV-1 replication in cells. Importantly, these experiments also demonstrate the feasibility of designing a new class of anti-HIV compounds. However, unlike ACV (with a proven safety record and exhaustively studied pharmacokinetics), ACV prodrug effects in various systems have to be evaluated in order to form conclusions on their potential clinical use.
In summary, the following mechanism seems to be responsible for ACV suppression of HIV-1 in human tissues ex vivo, the majority of which carry one or several HHVs, including HHV-6: ACV is monophosphorylated by herpesviral enzymes in HHV-infected cells and then further converted to ACV-TP, which suppresses HIV by inhibiting HIV-1 RT, similarly to other NRTIs.
Our results suggest that ACV may be therapeutically beneficial for various HIV-1-infected patients, since the majority of humans are already infected with HHV-6, often together with other HHVs that activate ACV at least during reactivation. In particular, in immunocompromised patients for whom HHV replication is frequent, those HHVs that are not completely suppressed by ACV (e.g. HHV-6) can continuously generate phosphorylated ACV derivatives. The incomplete inhibition of HHV-6 by ACV confirmed in our experiments is consistent with the much higher Ki of ACV-TP for HHV-6 kinase (UL69) compared with that of HSV-2 or HSV-1 TK (Bapat et al., 1989).
However, clinical trials are needed to test whether replication of HHVs, in particular of HHV-6, which is typically maintained in various organs including the intestines and the vagina, even in immunocompetent individuals (De Bolle et al., 2005), would be sufficient to suppress HIV-1 in ACV-treated individuals. Also, clinical trials should reveal whether the range of ACV concentrations used in our study to suppress HIV-1 is clinically relevant. Although ACV penetration efficiency and drug clearance were unknown for ex vivo tissues, the calculated EC50 of 3.1 μM was in the range of what was reported in vivo: A dose of 1 g of oral valacyclovir per day results in a plasma peak concentration of 29.5 μM, a minimum concentration in serum of 3 μM and a plasma concentration time curve of 89 μM/h ACV (Lycke et al., 2003; Soul-Lawton et al., 1995). Moreover, the therapeutic dose of the orally administered ACV prodrug valacyclovir, depending on clinical indications, can be increased to as much as 3 g per day.
Four recent clinical trials performed so far are in agreement with our ex vivo results and demonstrated that ACV is efficient in suppressing HIV in HSV-2-coinfected individuals (Baeten JM., 2007; Nagot et al., 2007; Zuckerman et al., 2007): Valacyclovir treatment, at the dose of 1g per day, reduced the HIV-1 plasma load in these individuals by 50% to 70%, an effect comparable to that reported here for human tissues infected with other HHVs. This HIV-1 viral load reduction was clinically beneficial (Corey, 2007) and similar to that reported for AZT or stavudine monotherapy (approximately 70%) (Delta Coordinating Committee 1999; Katzenstein et al., 2000; Rey et al., 1998). In contrast to the established ACV activity in reducing HIV load in patients coinfected with HSV-2, recent trials failed to demonstrate that acquisition of HIV-1 is affected by suppression of HSV-2 by ACV were marred by the low HSV-2 suppression (Celum et al., 2008; Cohen, 2007; Lisco and Vanpouille, 2008; Watson-Jones et al., 2008). However, none of the approved NTRIs widely used for therapy was yet developed into an efficient preventive drug when used alone.
Our results provide new insights into the effect of ACV in HIV-1-infected patients: in all previous trials, the effect of ACV on HIV-1 was considered to be indirect and due to the suppression of HSV-2 mediated inflammation. Here, we demonstrate that ACV directly suppresses HIV-1 RT in HHV-coinfected tissue. This effect depends on the levels of HHVs, which were not evaluated in these clinical trials or in previous in vitro studies (Resnick et al., 1986). Obviously, the results obtained in tissues ex vivo should be extrapolated to the in vivo situation with caution. However, the reliance on lymphotropic HHVs to create the active HIV suppressor at the site of HIV replication may in principle solve the critical pharmacological problem of drug delivery.
In conclusion, our data on HIV-infected tissues coinfected with various HHVs suggest that ACV may be used to decrease the HIV load in both the peripheral blood and the genital compartments of patients infected with one or several HHVs, including the highly prevalent HHV-6 (Campadelli-Fiume et al., 1999). Although the magnitude of HIV-1 suppression by ACV as well as by the currently approved NRTIs is too low to be used in monotherapy, it is sufficient to be an important part of drug cocktails.
In general, the combination of ACV with an endogenous HHV infection to suppress HIV may constitute a new principle of anti-HIV therapy; a “binary weapon” in which one inert component is converted by another, endogenous component, into an active therapeutic compound. In case of ACV, its exceptionally low toxicity and the low cost of ACV and related drugs that have been safely used in humans for more than 30 years, as well as their existing formulations as pills and creams, make them potentially applicable for HIV treatment, possibly in combination with other drugs. New targeted clinical trails will test whether ACV and its derivatives can be used for this new purpose in line with a popular trend to identify new uses for old drugs (Chong and Sullivan, 2007).
EXPERIMENTAL PROCEDURES
Tissue and cell culture
Tonsillar tissues from routine surgery was obtained from the Children's National Medical Center,Washington DC. Lymph nodes and colorectal and cervicovaginal tissues were obtained either from routine surgery or from cadavers through the National Disease Research Interchange (Philadelphia, USA). All tissues were obtained according to IRB-approved protocols. Tissues were dissected into 2-mm3 blocks and cultured as described earlier (Fletcher et al., 2006; Glushakova et al., 1995; Grivel et al., 2007). MT4 cells were obtained from the American Type Culture Collection (Manassas, VA) and cultured in RPMI 1640 medium with 10% heat-inactivated FCS.
Viral infections
Tissue inoculation with HIV-1 and HHV-6B was performed as described earlier (Grivel et al., 2007). For further details see the Supplemental Experimental Procedures.
ACV and ACV monophosphorylated prodrug treatment
ACV pharmaceutical formulations for intravenous infusion (Bedford Laboratories, Bedford, OH; NDC 55390-612-10) were diluted in distilled water and used at the indicated concentrations.. A different pharmaceutical formulation for intravenous infusion (American Pharmaceutical Partners, Schaumburg, IL; NDC 63323-325-10) and a commercial preparation of ACV (Sigma-Aldrich, St. Louis, MO) were tested for HIV-1 suppression in matched tonsillar tissues from four donors, with similar results. Synthesis of the monophosphorylated ACV prodrug - acyclovir-[1-naphthyl (methoxy-L-alaninyl)]phosphoramidate (Cf2649) is described in Supplemental Experimental Procedures. Compounds were added to the culture medium 12 hr prior to HIV-1 infection and again at each culture medium change.
Real-time PCR
The HHV load and the HIV-1 proviral load in tissues were determined by measurement of the number of viral DNA copies. DNA from two blocks of tissue was extracted with the QIAamp kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer's instructions. For further details on real-time PCR TaqMan assay, primers and probes sequences see the Supplemental Experimental Procedures
Exogenous template RT assay
Exogenous template HIV-1 RT assays were performed as described previously (Gorelick et al., 1990), with modifications specified in Supplementary Experimental Procedures.
Steady-state kinetics, ATP-dependent excision, site-specific footprinting
These assays were performed as described previously (Gotte et al., 1998; Marchand and Gotte, 2003; Marchand et al., 2007) with modifications specified in Supplemental Experimental Procedures.
Statistical analysis
Each datum point is the result of analysis of sets of 9 to 27 tissue blocks derived from each of n donors, where n is indicated in the text. Since the absolute level of HIV-1 replication varied from donor to donor, for every experiment we compared these levels using data from matched control blocks as the basis of normalization. This allowed us to pool results obtained from tissues from different donors. We analyzed these data using a two-tailed paired Student's t test. Because the distribution of the numbers of HHV-6 and HIV-1 (proviral) DNA equivalents failed the Kolmogorov-Smirnov normality test, we used distribution-free non-parametric methods (Wilcoxon Match-Pairs Signed-Ranks Test and Mann-Whitney U test) to evaluate the significance of the differences between various experimental groups. However, when these data were log10-transformed, the normality was achieved and we applied parametric methods (paired or unpaired Student's t test). The statistical significance of differences between data from various experimental groups evaluated after transformation was similar to that evaluated with non-parametric methods applied to non-transformed results. Depending on the type of statistical analysis, the pooled data are presented either as means ± standard error of the mean (SEM) or as median and interquartile range (IQR). All the hypothesis tests were two-tailed, and a p value of ≤ 0.05 defined statistical significance.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. M. Santi and the entire staff of the Department of Pathology of Children's National Medical Center for their generous assistance in obtaining human tonsillar tissues.
This research was supported, in part, by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, and by federal funds from the National Cancer Institute, NIH, under contract NO1-CO-12400. RFS is supported in part by NIH grants 5P30-AI-50409 (CFAR), 5R37-AI-041980 and by the Department of Veterans Affairs. JB is supported by the Geconcerteerde Onderzoeksacties (GOA No. 05/15). MG is the recipient of a national career award and a research grant from the Canadian Institutes of Health Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Delta Coordinating Committee and Delta Virology Committee HIV-1 RNA response to antiretroviral treatment in 1280 participants in the Delta Trial: an extended virology study. Aids. 1999;13:57–65. doi: 10.1097/00002030-199901140-00008. [DOI] [PubMed] [Google Scholar]
- Baeten JM, S. L., Lucchetti A, Whittington WLH, Sanchez J, Coombs RW, Margaret A, Wald A, Corey L, Celum C. Herpes simplex virus suppressive treatment decreases plasma HIV-1 load in HSV-2/HIV-1 co-infected: a randomised, placebo-controlled, cross-over trial. 17th International Society fpr Sexually Transmitted Diseases Research Meeting; Seattle, WA. 2007. [Google Scholar]
- Bapat AR, Bodner AJ, Ting RC, Cheng YC. Identification and some properties of a unique DNA polymerase from cells infected with human B-lymphotropic virus. J Virol. 1989;63:1400–1403. doi: 10.1128/jvi.63.3.1400-1403.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral K, Hider RC, Balzarini J, Neyts J, De Clercq E, Camplo M. Synthesis and antiviral evaluation of 3-hydroxy-2-methylpyridin-4-one dideoxynucleoside derivatives. Bioorg Med Chem Lett. 2003;13:4371–4374. doi: 10.1016/j.bmcl.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Burrows FJ, Gore M, Smiley WR, Kanemitsu MY, Jolly DJ, Read SB, Nicholas T, Kruse CA. Purified herpes simplex virus thymidine kinase retroviral particles: III. Characterization of bystander killing mechanisms in transfected tumor cells. Cancer Gene Ther. 2002;9:87–95. doi: 10.1038/sj.cgt.7700401. [DOI] [PubMed] [Google Scholar]
- Campadelli-Fiume G, Mirandola P, Menotti L. Human herpesvirus 6: An emerging pathogen. Emerg Infect Dis. 1999;5:353–366. doi: 10.3201/eid0503.990306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celum C, Wald A, Hughes J, Sanchez J, Reid S, Delany-Moretlwe S, Cowan F, Casapia M, Ortiz A, Fuchs J, et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:2109–2119. doi: 10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong CR, Sullivan DJ., Jr. New uses for old drugs. Nature. 2007;448:645–646. doi: 10.1038/448645a. [DOI] [PubMed] [Google Scholar]
- Cohen J. AIDS research. Promising prevention interventions perform poorly in trials. Science. 2007;317:440. doi: 10.1126/science.317.5837.440. [DOI] [PubMed] [Google Scholar]
- Cohen MS. HIV and sexually transmitted diseases: lethal synergy. Top HIV Med. 2004;12:104–107. [PubMed] [Google Scholar]
- Congiatu C, Brancale A, McGuigan C. Molecular modelling studies on the binding of some protides to the putative human phosphoramidase Hint1. Nucleosides Nucleotides Nucleic Acids. 2007;26:1121–1124. doi: 10.1080/15257770701521656. [DOI] [PubMed] [Google Scholar]
- Corey L. Synergistic copathogens--HIV-1 and HSV-2. N Engl J Med. 2007;356:854–856. doi: 10.1056/NEJMe068302. [DOI] [PubMed] [Google Scholar]
- Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004;35:435–445. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18:217–245. doi: 10.1128/CMR.18.1.217-245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E, Naesens L, De Bolle L, Schols D, Zhang Y, Neyts J. Antiviral agents active against human herpesviruses HHV-6, HHV-7 and HHV-8. Rev Med Virol. 2001;11:381–395. doi: 10.1002/rmv.336. [DOI] [PubMed] [Google Scholar]
- Degreve B, De Clercq E, Balzarini J. Bystander effect of purine nucleoside analogues in HSV-1 tk suicide gene therapy is superior to that of pyrimidine nucleoside analogues. Gene Ther. 1999;6:162–170. doi: 10.1038/sj.gt.3300806. [DOI] [PubMed] [Google Scholar]
- Elion GB. The biochemistry and mechanism of action of acyclovir. J Antimicrob Chemother. 1983;12(Suppl B):9–17. doi: 10.1093/jac/12.suppl_b.9. [DOI] [PubMed] [Google Scholar]
- Fletcher PS, Elliott J, Grivel JC, Margolis L, Anton P, McGowan I, Shattock RJ. Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides. Aids. 2006;20:1237–1245. doi: 10.1097/01.aids.0000232230.96134.80. [DOI] [PubMed] [Google Scholar]
- Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. Aids. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- Glushakova S, Baibakov B, Margolis LB, Zimmerberg J. Infection of human tonsil histocultures: a model for HIV pathogenesis. Nat Med. 1995;1:1320–1322. doi: 10.1038/nm1295-1320. [DOI] [PubMed] [Google Scholar]
- Gorelick RJ, Nigida SM, Jr., Bess JW, Jr., Arthur LO, Henderson LE, Rein A. Noninfectious human immunodeficiency virus type 1 mutants deficient in genomic RNA. J Virol. 1990;64:3207–3211. doi: 10.1128/jvi.64.7.3207-3211.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotte M, Maier G, Gross HJ, Heumann H. Localization of the active site of HIV-1 reverse transcriptase-associated RNase H domain on a DNA template using site-specific generated hydroxyl radicals. J Biol Chem. 1998;273:10139–10146. doi: 10.1074/jbc.273.17.10139. [DOI] [PubMed] [Google Scholar]
- Grivel JC, Elliott J, Lisco A, Biancotto A, Condack C, Shattock RJ, McGowan I, Margolis L, Anton P. HIV-1 pathogenesis differs in rectosigmoid and tonsillar tissues infected ex vivo with CCR5- and CXCR4-tropic HIV-1. Aids. 2007;21:1263–1272. doi: 10.1097/QAD.0b013e3281864667. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP, Collier AC, Cooper DA, Corey L, Fiddian AP, Gazzard BG, Griffiths PD, Contopoulos-Ioannidis DG, Lau J, Pavia AT, et al. Clinical efficacy of high-dose acyclovir in patients with human immunodeficiency virus infection: a meta-analysis of randomized individual patient data. J Infect Dis. 1998;178:349–359. doi: 10.1086/515621. [DOI] [PubMed] [Google Scholar]
- Jacobson MA, Mills J. Serious cytomegalovirus disease in the acquired immunodeficiency syndrome (AIDS). Clinical findings, diagnosis, and treatment. Ann Intern Med. 1988;108:585–594. doi: 10.7326/0003-4819-108-4-585. [DOI] [PubMed] [Google Scholar]
- Katzenstein DA, Hughes M, Albrecht M, Hammer S, Para M, Murphy R, Valdez H, Haubrich R, Liou S. Virologic and CD4+ cell responses to new nucleoside regimens: switching to stavudine or adding lamivudine after prolonged zidovudine treatment of human immunodeficiency virus infection. ACTG 302 Study Team. AIDS Clinical Trials Group. AIDS Res Hum Retroviruses. 2000;16:1031–1037. doi: 10.1089/08892220050075282. [DOI] [PubMed] [Google Scholar]
- Lisco A, Vanpouille C. Effect of HSV-2 suppression on HIV-1 incidence. NEJM. 2008 doi: 10.1056/NEJMc081075. in press. [DOI] [PubMed] [Google Scholar]
- Lusso P, Gallo RC. Human herpesvirus 6 in AIDS. Lancet. 1994;343:555–556. doi: 10.1016/s0140-6736(94)91515-6. [DOI] [PubMed] [Google Scholar]
- Lycke J, Malmestrom C, Stahle L. Acyclovir levels in serum and cerebrospinal fluid after oral administration of valacyclovir. Antimicrob Agents Chemother. 2003;47:2438–2441. doi: 10.1128/AAC.47.8.2438-2441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand B, Gotte M. Site-specific footprinting reveals differences in the translocation status of HIV-1 reverse transcriptase. Implications for polymerase translocation and drug resistance. J Biol Chem. 2003;278:35362–35372. doi: 10.1074/jbc.M304262200. [DOI] [PubMed] [Google Scholar]
- Marchand B, Tchesnokov EP, Gotte M. The pyrophosphate analogue foscarnet traps the pre-translocational state of HIV-1 reverse transcriptase in a Brownian ratchet model of polymerase translocation. J Biol Chem. 2007;282:3337–3346. doi: 10.1074/jbc.M607710200. [DOI] [PubMed] [Google Scholar]
- Meyer PR, Matsuura SE, Mian AM, So AG, Scott WA. A mechanism of AZT resistance: an increase in nucleotide-dependent primer unblocking by mutant HIV-1 reverse transcriptase. Mol Cell. 1999;4:35–43. doi: 10.1016/s1097-2765(00)80185-9. [DOI] [PubMed] [Google Scholar]
- Meyer PR, Matsuura SE, So AG, Scott WA. Unblocking of chain-terminated primer by HIV-1 reverse transcriptase through a nucleotide-dependent mechanism. Proc Natl Acad Sci U S A. 1998;95:13471–13476. doi: 10.1073/pnas.95.23.13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagot N, Ouedraogo A, Foulongne V, Konate I, Weiss HA, Vergne L, Defer MC, Djagbare D, Sanon A, Andonaba JB, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–799. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- Nicholas TW, Read SB, Burrows FJ, Kruse CA. Suicide gene therapy with Herpes simplex virus thymidine kinase and ganciclovir is enhanced with connexins to improve gap junctions and bystander effects. Histol Histopathol. 2003;18:495–507. doi: 10.14670/HH-18.495. [DOI] [PubMed] [Google Scholar]
- Reardon JE, Spector T. Herpes simplex virus type 1 DNA polymerase. Mechanism of inhibition by acyclovir triphosphate. J Biol Chem. 1989;264:7405–7411. [PubMed] [Google Scholar]
- Resnick L, Markham PD, Veren K, Salahuddin SZ, Gallo RC. In vitro suppression of HTLV-III/LAV infectivity by a combination of acyclovir and suramin. J Infect Dis. 1986;154:1027–1030. doi: 10.1093/infdis/154.6.1027. [DOI] [PubMed] [Google Scholar]
- Rey D, Hughes M, Pi JT, Winters M, Merigan TC, Katzenstein DA. HIV-1 reverse transcriptase codon 215 mutation in plasma RNA: immunologic and virologic responses to zidovudine. J Acquir Immune Defic Syndr Hum Retrovirol. The AIDS Clinical Trials Group Study 175 Virology Team. 1998;17:203–208. doi: 10.1097/00042560-199803010-00003. [DOI] [PubMed] [Google Scholar]
- Schacker T, Zeh J, Hu H, Shaughnessy M, Corey L. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J Infect Dis. 2002;186:1718–1725. doi: 10.1086/345771. [DOI] [PubMed] [Google Scholar]
- Soul-Lawton J, Seaber E, On N, Wootton R, Rolan P, Posner J. Absolute bioavailability and metabolic disposition of valaciclovir, the L-valyl ester of acyclovir, following oral administration to humans. Antimicrob Agents Chemother. 1995;39:2759–2764. doi: 10.1128/aac.39.12.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson-Jones D, Weiss HA, Rusizoka M, Changalucha J, Baisley K, Mugeye K, Tanton C, Ross D, Everett D, Clayton T, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med. 2008;358:1560–1571. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman RA, Lucchetti A, Whittington WL, Sanchez J, Coombs RW, Zuniga R, Magaret AS, Wald A, Corey L, Celum C. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007;196:1500–1508. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.