Abstract
Complementary structural and functional neuroimaging techniques used to examine the Default Mode Network (DMN) could potentially improve assessments of psychiatric illness severity and provide added validity to the clinical diagnostic process. Recent neuroimaging research suggests that DMN processes may be disrupted in a number of stress-related psychiatric illnesses, such as posttraumatic stress disorder (PTSD).
Although specific DMN functions remain under investigation, it is generally thought to be involved in introspection and self-processing. In healthy individuals it exhibits greatest activity during periods of rest, with less activity, observed as deactivation, during cognitive tasks, e.g., working memory. This network consists of the medial prefrontal cortex, posterior cingulate cortex/precuneus, lateral parietal cortices and medial temporal regions.
Multiple functional and structural imaging approaches have been developed to study the DMN. These have unprecedented potential to further the understanding of the function and dysfunction of this network. Functional approaches, such as the evaluation of resting state connectivity and task-induced deactivation, have excellent potential to identify targeted neurocognitive and neuroaffective (functional) diagnostic markers and may indicate illness severity and prognosis with increased accuracy or specificity. Structural approaches, such as evaluation of morphometry and connectivity, may provide unique markers of etiology and long-term outcomes. Combined, functional and structural methods provide strong multimodal, complementary and synergistic approaches to develop valid DMN-based imaging phenotypes in stress-related psychiatric conditions. This protocol aims to integrate these methods to investigate DMN structure and function in PTSD, relating findings to illness severity and relevant clinical factors.
Keywords: Medicine, Issue 89, default mode network, neuroimaging, functional magnetic resonance imaging, diffusion tensor imaging, structural connectivity, functional connectivity, posttraumatic stress disorder
Introduction
Neuroimaging represents a tool with unprecedented potential to examine diagnostic validity, severity of illness, prognostics and treatment response in neuropsychiatry. A wide range of complementary neuroimaging techniques is now available to characterize the structure and function of key brain systems, and to aid in the identification of neuroimaging phenotypes in psychiatric populations. Of these systems, the Default Mode Network (DMN) has received a great deal of attention in the cognitive and clinical neuroscience literature over the last decade.
The DMN is a so-called “resting state network” that includes the medial prefrontal cortex (MPFC) as the main anterior node, posterior cingulate cortex/precuneus (PCC) as the principle posterior node, along with the inferior-lateral parietal cortices and medial temporal regions. They key feature of this network is that it exhibits its highest activity during periods of rest, which occurs while subjects are awake and alert but not involved in a specific task; this resting state activity was coined the “Default Mode” of brain function1. Resting state activity in the DMN is also highly synchronized, which is described as resting state functional connectivity. The other key feature of the DMN is that it demonstrates diminished activity during periods of increased external cognitive demands,which is observed as task-induced deactivation during functional neuroimaging paradigms2,3. It is hypothesized that the balance between the internal (i.e. the resting state) and external (i.e. task-related activity) demands are needed to maintain healthy brain functioning3-5.
The following sections provide a brief overview of three methods to study the DMN: functional connectivity and task-associated deactivation, followed by structural connectivity. These three methods are described as complementary ways to characterize this network in clinical samples, such as patients with post-traumatic stress disorder and related psychiatric conditions.
Resting State DMN Functional Connectivity
Resting state functional connectivity has recently become a common approach used to evaluate patterns of baseline brain function in the absence of task demands. Functional connectivity is an analytic method that quantifies coherence, or the degree of synchrony in blood oxygen level dependent (BOLD) signal over time, across different brain regions. A growing body of research literature suggests that the typical patterns of DMN connectivity may be altered in clinical and at-risk populations, and particularly those with previous exposure to significant stress or trauma. The most common finding has been decreased DMN resting state functional connectivity associated with PTSD6. This diminished connectivity may have direct clinical applications, as decreased DMN connectivity may be predictive of those who may develop PTSD after an acute stressor7. Diminished DMN functional connectivity can be interpreted in several ways, most commonly that it reflects poor communication between crucial brain regions involved in self-processing, which may lead to an inability to reallocate internal resources from baseline DMN processing to external demands. This network disruption may explain core clinical symptoms of psychiatric disorders such as PTSD and other stress-related psychiatric conditions8. Further investigation into the etiology of these disruptions is an important area for future research.
From a more general perspective, advantages of examining the functional connectivity of the DMN include relatively easy implementation and a robust pattern of resting state functional connectivity in healthy controls that allows for a reliable comparison9,10. Therefore, this method has the potential to be developed into an easily implemented and robust neuroimaging biomarker of stress related psychiatric disorders that informs how the brain functions in the absence of specific task demands in individuals with PTSD and other stress-related psychiatric conditions.
Task-Associated DMN Deactivations
Examining DMN response during working memory (WM) offers another approach to investigate the function and dysfunction of this network beyond resting state synchrony. This approach, which reflects a more standard functional magnetic resonance imaging (FMRI) method, provides different information about response to task demands that may have clinical significance11. Previous research has documented that participants with PTSD demonstrate impaired WM functioning and a greater degree of DMN deactivations during WM tasks, perhaps reflecting increased cognitive effort12-15. Using WM as an FMRI challenge has several advantages. For instance, it reliably disengages several key DMN regions, from resting to an active state. Most relevant to PTSD and other stress related psychiatric conditions, WM tasks reliably disengage the MPFC, the major anterior DMN node that is involved in critical pathways dysregulated in PTSD. It has been well established that the MPFC modulates ascending amygdala activity, and likely plays a crucial role in fear conditioning16. Assessments of MPFC activity may also be a useful metric in future clinical care. For instance, in one previous study of traumatized police officers, exposure psychotherapy increased MPFC activity and decreased amygdala activity during traumatic memory retrieval. These neuroimaging changes were associated with decreased PSTD symptoms17. This instance of WM-induced MPFC deactivations is but one example of how neuroimaging metrics can be applied to clinical populations, and further exploration of other DMN components is likely to be a fruitful area of future research.
In this protocol, the n-back task of verbal working memory is used. The n-back task is widely used in FMRI research, and provides reliable activation of executive activation and default mode network deactivation regions18,19. This task includes three components, a 0-back letter vigilance task, the 2-back task of working memory and resting baseline for comparison. During the 0-back vigilance task, Participants respond “yes” when a predetermined target consonant (“H” or “h”) appeared and “no” for other consonants using a two-button response box while inside the scanner. Six 0-back control blocks of 9 consonants are presented during this task. During the 2-back, a series of consonants are presented visually for 500 msec each, with an interstimulus interval of 2,500 msec. Participants make a “yes” or “no” response, after each consonant presented, to indicate whether it is the same or different from the consonant presented two previously in a series (e.g., w, N, r, N, R, Q, r, q, N, W etc., with correct answers indicated in bold). During the 2-back, six 45 sec series of 15 consonants are presented. To perform successfully the participant must maintain a demanding cognitive set that includes constant phonemic buffering (i.e. holding consonants in short term memory), subvocal phonemic rehearsal (i.e. repeating consonants without articulating out loud), and executive coordination. In both 0- and 2-back blocks, the rate of presentation is the same, 33% of targets are presented in random locations, and capitalization is randomized to encourage verbal encoding. A 30 sec resting baseline with a crosshair fixation point is presented prior to each 0-back block; this baseline is used for subsequent comparisons of task-associated activity compared to baseline during data analyses.
Taken together, the extant data suggests that characterization of task-associated DMN activity during a variety of tasks may play an important role in the clinical use of functional DMN analysis. There are other advantages to using WM as an FMRI challenge in stress related psychiatric conditions. Similar to resting state connectivity, there is a clear pattern of DMN deactivations during WM in healthy individuals, which facilitates comparisons with clinical samples. WM is also trauma neutral, which may avoid triggering clinical PTSD symptoms during scanning. Therefore this method also has the potential to be developed into a neuroimaging biomarker that reflects how the brain responds to external demands in stress related psychiatric disorders.
DMN Structural Connectivity
While functional imaging is able to describe changes in brain connectivity or activity associated with stress exposure, functional approaches do not describe the etiology behind observed brain changes. Structural imaging methods, such as diffusion tensor imaging (DTI), are able to measure and quantify the integrity of the white matter tracts connecting brain regions. DTI is the most common structural neuroimaging approach and measures white matter integrity based on the anisotropic (i.e. directional) flow of water molecules along white matter tracts, as water flows predominantly along white matter tracts (compared to across them). This difference in directional flow is expressed as fractional anisotropy (FA). Lower degrees of FA are thought to reflect microstructural changes in white matter tracts, which may be manifestations of neuronal injury from a variety of causes, including the consequences of stress exposure4. From a network perspective, coordinated brain activity (i.e. resting state activity or coordinated task-related activity) must rely upon structural connections. In the case of previous DMN findings, structural injury impairs the communication between DMN nodes, leading to decreased DMN functional connectivity. Similarly, increased patterns of deactivation may reflect microstructural damage that necessitates the recruitment of greater areas of cortex during task response. Relevant to PTSD and the DMN, several studies have shown decreased FA in the cingulum bundle20,21, which is the white matter tract that connects major limbic structures of the brain22. It is likely that more precise measures utilizing tractography (i.e. that directly trace white matter tracts at the neuronal level) will be able to elucidate specifically which white matter fibers are involved in network disruption. The advantages to DTI imaging is that it is relatively easy to acquire as there are no required tasks to perform in the scanner.
In the following protocol, the functional approaches of resting state functional connectivity and quantification of task-induced deactivations are combined with an examination of structural connectivity using DTI, in order to map DMN structure and function and relate these findings to illness severity and relevant clinical factors in PTSD. We have previously implemented this approach in trauma-exposed healthy adults18,23 and found that this protocol provides a cogent method to characterize the DMN that lends itself to adaptation to the study of PTSD and other stress related psychiatric illnesses.
Protocol
Eligible participants sign written, informed consent to participate in the research project. Research is performed in compliance with institutional, national and international guidelines for human welfare.
1. Participant Screening and Diagnostic Interviews
After informed consent, perform diagnostic interviews to verify the diagnosis of PTSD and illness severity. NOTE: These measures include the Structured Clinical Interview for DSM-IV-TR (SCID)24 and the Clinician Administered PTSD Scale (CAPS)25, as well as the Folstein Mini-Mental Status Exam (MMSE)26 to evaluate cognitive status.
Ask participants to fill out self-report scales relevant to stress and mood. NOTE: These include the Life Stressor Checklist–Revised (LSC-R)27, Childhood Trauma Questionnaire (CTQ)28, Perceived Stress Scale (PSS)29 and Quick Inventory of Depressive Symptoms (QIDS-SR)30.
Schedule eligible participants for MRI, where participants arrive approximately 1 hr before the scheduled scan session, to review components needed for scanning, such as MRI safety and study procedures.
Obtain urine, pregnancy (when appropriate), and toxicology tests prior to scanning.
2. Training Participants to Perform the N-back Task
- Begin the first run with the 0-back letter vigilance test.
- Instruct participants to indicate “Yes” to a target consonant (“h” or “H”) via a two-button response box and “No” to all other consonants.
- Show the participant 9 consonants for 500 msec each, with an interstimulus time of 2,500 ms, for a total of 27 sec, and ask them to respond as directed above. NOTE: The target consonant will be shown 4 times within each 0-back block.
- Next, have participants practice the 2-back test.
- Instruct participants to make a “Yes” or “No” response on the two-button response box, after each consonant presented, to indicate whether it is the same or different from the consonant presented two previously in a series.
- Show the participant a series of 15 consonants, for 500 msec each, with an interstimulus interval of 2,500 msec, for a total of 45 sec. NOTE: A target stimulus is shown 5 times.
Train participants to perform the n-back task outside the scanner, until their performance reaches >75% correct on the 2-back component. NOTE: The above parameters can be automated using stimulus presentation software (see Table of Materials/Equipment).
3. MRI Acquisition
- Have the participant change into MRI-compatible clothing, and bring them inside a 3 Tesla MRI scanner room. Have them wear earplugs for hearing protection, and then lie down in a gurney that will ultimately move them into the middle of the MRI machine.
- Place cushions around their head to minimize head motion. Provide them with the MRI-compatible response box for the n-back working memory task, squeeze bulb to stop the scan in case of an emergency, and place a pulse oximeter on their finger for physiologic monitoring and recording.
- Place the 32-channel head coil and presentation screen over the participant’s head, and move them into the middle of the scanner.
Ensure the participant is comfortable and can see the screen, and then begin the MRI scan session. Start with acquisition of high-resolution (1 mm3) anatomic brain scans. Enter high resolution MRI parameters on the scanner console at Echo Time (TE) = 2.98 msec, Repetition Time (TR) = 1,900 msec, Field of View (FOV) = 256 mm2 and matrix size 642 in 1 mm slices. Start the MRI acquisition by pressing the “run” button on the scanner console.
Set FMRI BOLD image acquisition parameters on the scanner console as TR = 2,500 msec, TE = 28 msec, FOV = 192 mm2, and matrix size 642 in 3 mm axial slices.
- Next, acquire FMRI images on working memory, using the n-back test (see section 2) with the following parameters:
- Present a 30 sec baseline fixation cross, to the patient, prior to each of the 0-back blocks using stimulus presentation software. NOTE: This will provide a baseline for comparison for the other 0- and 2-back blocks during data analysis.
- Project the instructions to the patient for 3 sec prior to each 0 or 2-back task using the stimulus presentation software.
- In total, include three 0-back and 2-back portions along with two baseline blocks, in two imaging runs, presented in counter-balanced order.
Press “run” on the MRI scanner console to start.
After completion of the n-back, ensure the participant is comfortable and ready to move on. Instruct them that the rest block is next, and tell them not to fall asleep. Use stimulus presentation software to display a fixation cross on the screen.
Acquire resting state images for the next 4 min, using the same FMRI settings as were used to acquire n-back images (see 3.3), by pressing “run” on the MRI scanner console.
Repeat steps 3.4. and 3.5. Prior to each new section, ask the participant if they are comfortable and if they are able to continue. If they are able, continue the protocol. If they are not, pause the MRI scanner and make adjustments for comfort as needed.
Next, tell the participant that the scanner may be shaking during the next sequences, and instruct them to close their eyes and relax as best they can in the scanner. Then acquire a DTI sequence by pressing the “run” button on the scanner console.
Set DTI image acquisition parameters in the scanner console to double spin echo-planar diffusion weighted images (DWI), with diffusion gradients applied in 64 non-collinear directions (b = 1,000), one DWI for each gradient direction and 10 non-weighted (b = 0) normalization images, TR = 10,060 msec, TE = 103 msec, FOV = 226 mm, 1282 matrix, slice thickness = 1.8 mm, with partial echoes and interpolation on.
Remove the participant from the scanner, and inquire about how the session went. Answer any questions they may have, and thank them for their participation. Have the MRI scanner computer write a DVD with participant images and physiologic recording for subsequent data analyses.
4. Data Analysis
- Data Preprocessing
- Using FMRI processing software, reconstruct raw data into 3D + time datasets, concatenate and register to the fifth volume of the first series, to minimize movement artifact and yield motion correction parameters. Apply bandpass filtering (0.009-0.08 Hz) to isolate the DMN frequency domain and reduce effects of low frequency drift and high frequency noise. NOTE: Nuisance variables for each voxel should include average ventricle and white matter time series as well as 6 parameter estimates of head motion; these estimates should include both demeaned and derivative values. The predicted time course of nuisance variables should be removed from the full voxel time series to yield a “residual” time series data to be used for later correlation analyses31.
- Scale data to normalize within-run intensity, and smooth data up to a 4 mm full width half maximum (FWHM) Gaussian kernel. Censor images with greater than 1.5 mm displacement from the dataset32. Do not perform global signal regression (GSR) since GSR can influence correlations in resting state data33,34.
- Resting State Connectivity Analyses
- Use seed-region connectivity analyses to evaluate the relationship between a priori defined regions to evaluate functional connectivity11. NOTE: Seeds included are the major anterior and posterior nodes of the DMN, the MPFC and PCC, respectively. Functional coordinates of these locations are generally superior to atlas-defined locations35.
- Extract the average BOLD time series from these seeds and conduct a whole-brain correlation analysis. Transform correlational R values to Z scores36 for subsequent hypothesis testing.
- Compare Z values between groups on a voxel by voxel basis to evaluate significant differences in functional connectivity between PTSD and controls as the primary outcome measure. Threshold these results at a two-tailed significance at p < 0.05, using family-wise (i.e. cluster) error correction. NOTE: Cluster correction is generated using Monte Carlo simulations to estimate the probability of false positive clusters. Use statistical algorithms to calculate cluster correction as a function of FOV, resolution, smoothness, and signal intensity at the individual voxel level37.
- To evaluate the relationship between clinical symptoms and imaging results, conduct follow-up analyses that include correlations between rating scale scores and average Z scores of connectivity of DMN regions. Include correlation analyses that account for relevant demographic information, such as depression severity, traumatic brain injury, as well as education and other associated variables.
- Working Memory Analyses
- Use FMRI processing software to pre-process the data and voxel-based GLM to quantify task-specific activity in each brain voxel of individual datasets11,31. NOTE: Independent variables in the GLM are the temporal course of rest and the 0- and 2-back tasks (including hemodynamic transitions modeled as a gamma function) and covariates (linear drift and observed movement), with the BOLD signal over time as the dependent variable.
- Average resulting GLM beta weights across specified DMN regions. NOTE: These averaged n-back responses from individual-level datasets serve as the basic measure of brain activity in subsequent group-level statistical analyses.
- Use analyses of covariance to examine group-level differences between PTSD and non-PTSD groups and to estimate effects of task difficulty (i.e. comparisons of activity during 0- vs. 2-back tasks) in each DMN region; Also include analyses of any relevant statistical control steps as required during resting state analyses in 4.2.
- Structural Connectivity Using DTI
- Preprocessing
- Using DTI processing software, co-register non-diffusion (i.e. b = o) images to correct for motion artifacts, and use as a normalization image for subsequent diffusion-weighted images. Use a 12 parameter affine transformation to register the diffusion-weighted images to account for motion and eddy current artifacts.
- Ensure that the gradient vector for each diffusion direction is rotated to account for transformations prior to model fitting. Calculate a second-order diffusion tensor per voxel from the diffusion weighted signal attenuations using a nonlinear constrained fitting procedure38.
- Use the diffusion-weighted images to calculate eigenvalue, eigenvector and fractional anisotropy maps of diffusion.
- Use tractography software to quantify the integrity of the of the cingulum bundle. Utilize standard atlases for seed region selection, such as those by Mori et al.39 and Catani and De Schotten40. Filter resultant tractography through a midline exclusion region to remove fibers crossing between hemispheres. Calculate mean FA, trace, axial and radial diffusivity for all voxels through which the cingulum bundle passes.
- Use mixed model ANOVA for each diffusion measure, with hemisphere as a within-subject variable, to compare group differences between PTSD and non-PTSD participants, statistically controlling for other factors, such as depression severity, substance abuse, mild TBI and education and demographic variables using ANCOVA.
Representative Results
Representative results are based on data collected using the same imaging approach in two different samples of individuals with a history of childhood trauma and maltreatment, but without PTSD21,22. Results from resting state functional connectivity analyses revealed a spatial pattern consistent with major nodes of the DMN (Figure 1)1-3,8 including the MPFC, PCC, angular gyrus/inferior parietal lobule and middle temporal regions. Confirmation of this spatial distribution serves as an initial validity check, and allows subsequent hypothesis testing.
Patterns of brain activity during working memory are displayed in Figure 2. Images from the 2-back component (Figure 2a) show increased activation in the executive network that co-occurs with deactivation within the DMN. Activation in executive regions, such as the middle frontal gyrus, supplementary motor area and inferior parietal lobule are depicted in orange and red, juxtaposed with deactivation in DMN regions (i.e. MPFC, PCC and medial temporal regions) shown in blue. This pattern is consistent with prior n-back literature11,41 and serves as a validity check before proceeding to hypothesis testing. Figure 2b shows results from the 0-back component of the n-back, which demonstrates modest deactivation, particularly in the PCC, but without strong MPFC deactivation. Moderate activation is also seen in the medial frontal cortex.
Last, the extent of the cingulum bundle, as revealed by probabilistic tractography, is displayed in Figure 3. Three-dimensional images display the overall shape and distribution of the cingulum fibers, which roughly trace the overall shape of DMN regions (Figure 3a). To verify the accuracy of the displayed fibers, it is recommended that these results be overlaid with an individuals’ cortical map (e.g., generated by programs that differentiate specific cortical regions). Figure 3b shows the white matter tract passing through the MPFC and PCC, and Figure 3c shows tracts reaching the medial temporal regions. This ensures that subsequent group analyses include fibers connecting relevant brain regions.
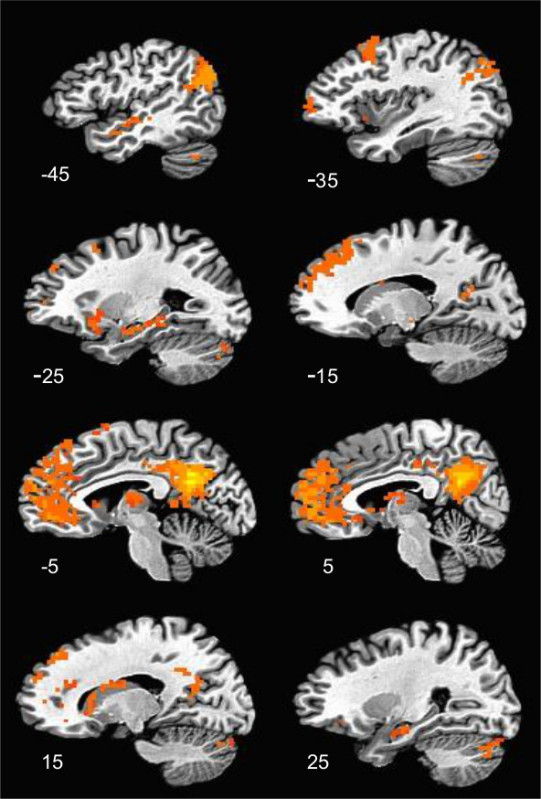 Figure 1. Resting State Functional Connectivity Map of the Default Mode Network. These images show a sagittal display of DMN areas exhibiting significant positive functional connectivity with the PCC. Images are thresholded at a p < 0.05, corrected for multiple comparisons. X coordinates of each slice are shown in the bottom left of the corresponding image.
Figure 1. Resting State Functional Connectivity Map of the Default Mode Network. These images show a sagittal display of DMN areas exhibiting significant positive functional connectivity with the PCC. Images are thresholded at a p < 0.05, corrected for multiple comparisons. X coordinates of each slice are shown in the bottom left of the corresponding image.
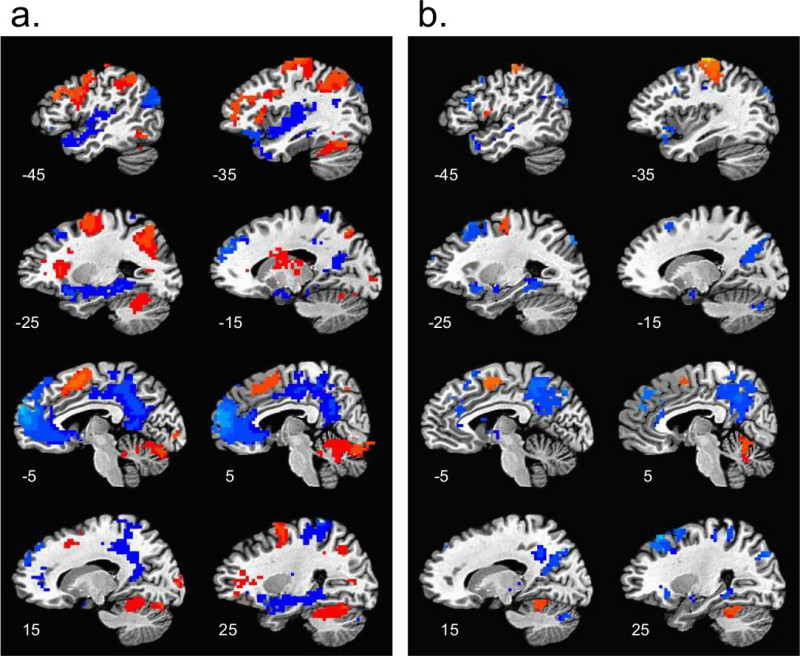 Figure 2. Spatial Pattern of Activation During Working Memory. a) sagittal section of the brain to illustrate patterns associated with the 2-back working memory task. Activation patterns within the executive network are illustrated in orange/red and DMN deactivation is displayed in blue. Images are thresholded at p < 0.05 and corrected for multiple comparisons. b) demonstrates 0-back activity, which is typically combined with working memory to control for attention. Activation patterns are in orange/red and deactivation in blue; evident here is some DMN deactivation with little executive activation. Images are thresholded at p < 0.05, corrected for multiple comparisons.
Figure 2. Spatial Pattern of Activation During Working Memory. a) sagittal section of the brain to illustrate patterns associated with the 2-back working memory task. Activation patterns within the executive network are illustrated in orange/red and DMN deactivation is displayed in blue. Images are thresholded at p < 0.05 and corrected for multiple comparisons. b) demonstrates 0-back activity, which is typically combined with working memory to control for attention. Activation patterns are in orange/red and deactivation in blue; evident here is some DMN deactivation with little executive activation. Images are thresholded at p < 0.05, corrected for multiple comparisons.
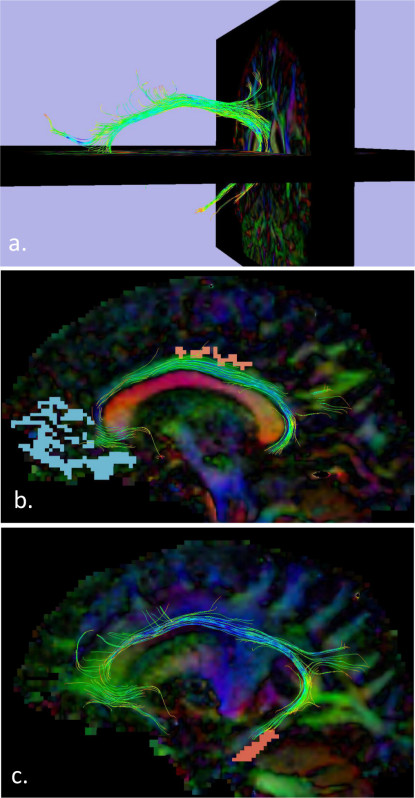 Figure 3. Probabilistic Tractography/Structural Connectivity of the Cingulum Bundle. a) shows the three-dimensional shape and pattern of these fibers, with cross-sections of the brain included for visual reference; b) illustrates how these fibers travel through the MPFC and PCC (red and blue, respectively), and c) demonstrates how these fibers travel through the medial temporal component of the DMN.
Figure 3. Probabilistic Tractography/Structural Connectivity of the Cingulum Bundle. a) shows the three-dimensional shape and pattern of these fibers, with cross-sections of the brain included for visual reference; b) illustrates how these fibers travel through the MPFC and PCC (red and blue, respectively), and c) demonstrates how these fibers travel through the medial temporal component of the DMN.
Discussion
The two most critical steps for successful implementation of the neuroimaging protocol are accurately capturing resting state and working memory effects.
Conceptually, the acquisition of resting state images is straightforward. Since there is no task to perform, experimenters often describe brain activity during these epochs as “rest.” However, as this field is relatively new compared to other areas of the neuroimaging1, there is no explicit consensus of how to precisely define “rest” in the scanner. Most protocols, including this one, ask participants to view a fixation cross on a screen. The duration of the individual resting state scan is also highly variable in the literature, generally ranging from 4 to 12 min, and with either eyes open or eyes closed42. In this protocol, two 4 min scans were implemented for a total of 8 min, with eyes open, viewing a simple white fixation cross against a black background. Future research would benefit greatly from the acceptance of a standardized approach to resting state data acquisition to facilitate generalization across studies.
Another critical issue during resting state acquisition is the impact of head motion. Recent research has clearly demonstrated that motion during resting state scans leads to false correlations in subsequent functional connectivity analyses43-45. Therefore, participants must remain as motionless as possible throughout the resting state scan session. During protocol development, highly anxious participants were not able to remain still for very long, often in the order of 4-5 min. Reflecting this experience, several procedures can minimize the impact of participant movement, including acquisition of two 4 min resting state scans and censoring any images with motion greater than 1.5 mm (corresponding to 1/2 voxel)32. Censoring anything smaller than 1 mm movement (e.g., 0.5 mm) in clinical participants may lead to data reduction that compromises further dataset analyses.
Another critical component in image acquisition is the importance of practicing working memory tasks prior to imaging. Since the principle interest of this protocol is in the deactivation of the DMN in response to difficult task demands, the executive network must be sufficiently challenged. This requires striking a careful balance between overwhelming a clinical participant (who may have significant anxiety) and capturing images during the cognitive challenge. This balance can be struck by having the participant practice the working memory task outside the scanner. This is typically done while seated in a separate room, using an identical input device (if possible) as is used in the scanner. A quick scoring of the n-back behavioral results reveals whether or not participants are performing adequately. It is also important to remind participants that the experiment is designed to induce cognitive effort and perfect scores are not expected. In previous studies, DMN deactivations occurred similarly with both correct and incorrect answers18,23. This might be expected given the nature of the n-back paradigm, which elicits a cognitive set that requires consistent cognitive functions throughout the task, regardless of the accuracy of any given response.
This approach has several limitations, which are inherent to a field that is rapidly progressing. For example, the term DMN was coined in 2001, so it is reasonable to assume that the imaging methods to characterize its structure and function remain, if not in infancy, in early adolescence. New imaging protocols and parameters are constantly being developed relevant to stress related psychiatric conditions23,46, raising the question whether previous results can be replicated using different approaches. Another excellent example of this is the impact of motion on resting state scans, which gained widespread recognition in 201243-45. While current researchers implement motion correction procedures, the lack of this correction complicates the interpretation of previously published data. Another important example is the controversy over the removal of global signal, which is a common preprocessing technique used to reduce noise, but may induce false correlations into resting state data33,34.
In summary, this protocol uses complementary resting state, working memory and structural neuroimaging methods to visualize the DMN. The principal advantage of this approach is its multimodal evaluation of a single brain network; each of these neuroimaging approaches provides unique and complementary information regarding the function of this important network. While the protocol described here was used to characterize correlates of stress exposure, combinations of any or all of these approaches lend themselves to further development as neuroimaging biomarkers of mood and anxiety disorders.
Disclosures
The authors have no conflicts of interest to disclose relevant to the content of this manuscript. Both Dr. Philip and Ms. Carpenter are US Government Employees.
Acknowledgments
Generation of representative data was supported by NIH Grant R01HL084178, 5R01MH068767-08, and grants from the Brown MRI Research Facility and Rhode Island Foundation. VA CSR&D Grant 1 IK2 CX000724-01A2 supported protocol development and further work. We thank all of our participants.
References
- Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Conrad CD, et al. Chronic glucocorticoids increase hippocampal vulnerability to neurotoxicity under conditions that produce CA3 dendritic retraction but fail to impair spatial recognition memory. J Neurosci. 2007;27:8278–8285. doi: 10.1523/JNEUROSCI.2121-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, et al. Disruptive effects of glucocorticoids on glutathione peroxidase biochemistry in hippocampal cultures. J Neurochem. 2002;82:118–125. doi: 10.1046/j.1471-4159.2002.00948.x. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, et al. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci. 2009;34:187–194. [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, et al. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand. 2010;121:33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- Sripada RK, et al. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med. 2012;74:904–911. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, et al. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet LH, et al. Effects of nicotine withdrawal on verbal working memory and associated brain response. Psychiatry Res. 2010;183:69–74. doi: 10.1016/j.pscychresns.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson KW, et al. Neuropsychological functioning in posttraumatic stress disorder and alcohol abuse. Neuropsychology. 2006;20:716–726. doi: 10.1037/0894-4105.20.6.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasterling JJ, et al. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12:125–133. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- Yehuda R, et al. Learning and memory in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1995;152:137–139. doi: 10.1176/ajp.152.1.137. [DOI] [PubMed] [Google Scholar]
- Moores KA, et al. Abnormal recruitment of working memory updating networks during maintenance of trauma-neutral information in post-traumatic stress disorder. Psychiatry Res. 2008;163:156–170. doi: 10.1016/j.pscychresns.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Rougemont-Bucking A, et al. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neurosci Ther. 2011;17:227–236. doi: 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres JF, et al. Police officers under attack: resilience implications of an fMRI study. J Psychiatr Res. 2011;45:727–734. doi: 10.1016/j.jpsychires.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Philip NS, et al. Early life stress is associated with greater default network deactivation during working memory in healthy controls: a preliminary report. Brain Imaging Behav. 2013;7:204–212. doi: 10.1007/s11682-012-9216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet LH, et al. Imaging phonological similarity effects on verbal working memory. Neuropsychologia. 2008;46:1114–1123. doi: 10.1016/j.neuropsychologia.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Abe O, et al. Voxel-based diffusion tensor analysis reveals aberrant anterior cingulum integrity in posttraumatic stress disorder due to terrorism. Psychiatry Res. 2006;146:231–242. doi: 10.1016/j.pscychresns.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Kim SJ, et al. Asymmetrically altered integrity of cingulum bundle in posttraumatic stress disorder. Neuropsychobiology. 2006;54:120–125. doi: 10.1159/000098262. [DOI] [PubMed] [Google Scholar]
- Vogt BA, et al. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Philip NS, et al. Decreased default network connectivity is associated with early life stress in medication-free healthy adults. Eur Neuropsychopharmacol. 2013;23:24–32. doi: 10.1016/j.euroneuro.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders. 1994.
- Blake DD, et al. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Folstein MF, et al. Mini-mental state'. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Wolfe JW, Kimerling R, Brown PJ, Chrestman KR, Levin K. Psychometric review of The Life Stressor Checklist-Revised. Sidran Press; 1996. [Google Scholar]
- Bernstein DP, Fink L. Childhood trauma questionnaire: a retrospective self-report. Pearson Education, Inc; 1998. [Google Scholar]
- Cohen S, et al. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Rush AJ, et al. The 16-item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Reynolds R. AFNI program: afni_proc.py. http://afni.nimh.nih.gov/pub/dist/doc/program_help/afni_proc.py.html. 2006.
- Posner J, et al. Antidepressants normalize the default mode network in patients with dysthymia. JAMA Psychiatry. 2013;70:373–382. doi: 10.1001/jamapsychiatry.2013.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, et al. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced. Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, et al. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, et al. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22:158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. Frequency distribution of the values of the correlation coefficient in samples of an indefinitely large population. Biometrika. 1915;10:507–521. [Google Scholar]
- Cox RW. AFNI program: 3dClustSim. http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html. 2010.
- Smith SM, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PCM. MRI Atlas of Human White Matter. 2005. [DOI] [PubMed]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Sweet LH, et al. Default network response to a working memory challenge after withdrawal of continuous positive airway pressure treatment for obstructive sleep apnea. Brain Imaging Behav. 2010;4:155–163. doi: 10.1007/s11682-010-9095-y. [DOI] [PubMed] [Google Scholar]
- Cole DM, et al. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci. 2012;4 doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, et al. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, et al. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, et al. Regional homogeneity and resting state functional connectivity: associations with exposure to early life stress. Psychiatry Res. 2013;214:247–2453. doi: 10.1016/j.pscychresns.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


