Despite improvements in screening, diagnosis, treatment and surveillance follow-up of colorectal cancer patients over the past 20 years, a significant proportion of individuals who undergo curative-intent treatment experience recurrence within five years. However, due to existing guideline differences and limited supporting data, postoperative surveillance is variably performed. This retrospective cohort study, performed in Toronto, Ontario, used information from several databases and provides insight to the care patients receive in the presence of conflicting guidelines and the mixed results reported in similar studies.
Keywords: Colorectal adenocarcinoma, Curative-intent surgery, Postoperative imaging, Surgery, Surveillance
Abstract
BACKGROUND:
Postoperative surveillance following curative-intent resection of colorectal cancer (CRC) is variably performed due to existing guideline differences and to the limited data supporting different strategies.
OBJECTIVES:
To examine population-based rates of surveillance imaging and endoscopy in patients in Ontario following curative-intent resection of CRC with no evidence of recurrence, as well as patient or disease factors that may predispose certain groups to more frequent versus less frequent surveillance; to provide insight to the care patients receive in the presence of conflicting guidelines, in efforts to help improve care of CRC survivors by identifying any potential underuse or overuse of particular surveillance modalities, or inequalities in access to surveillance.
METHOD:
A retrospective cohort study was conducted using data from the Ontario Cancer Registry and several linked databases. Ontario patients undergoing curative-intent CRC resection from 2003 to 2007 were identified, excluding patients with probable disease relapse. In the five-year period following surgery, the number of imaging and endoscopic examinations was determined.
RESULTS:
There were 4960 patients included in the study. Over the five-year postoperative period, the highest proportion of patients who underwent postoperative surveillance received the following number of tests for each modality examined: one to three abdominopelvic computed tomography (CT) scans (n=2073 [41.8%]); one to three abdominal ultrasounds (n=2443 [49.3%]); no chest CTs, one to three chest x-rays (n=2385 [48.1%]); and two endoscopies (n=1845 [37.2%]). Odds of not receiving any abdominopelvic imaging (CT or abdominal ultrasound) were higher in those who did not receive adjuvant chemo-therapy (OR 6.99 [95% CI 5.26 to 9.35]) or those living in certain geographical areas, but were independent of age, sex and income. Nearly all patients (n=4473 [90.2%]) underwent ≥1 endoscopy at some point during the follow-up period.
CONCLUSION:
In contrast to findings from similar studies in other jurisdictions, most Ontario CRC survivors receive postoperative surveillance with imaging and endoscopy, and care is equitable across sociodemographic groups, although unexplained geographical variation in practice exists and warrants further investigation.
Abstract
HISTORIQUE :
La surveillance postopératoire après une résection de cancer colorectal (CCR) à visée curative est variable, en raison de différences dans les lignes directrices et de données limitées en appui à diverses stratégies.
OBJECTIFS :
Examiner les taux de surveillance en population de l’imagerie et de l’endoscopie chez des patients de l’Ontario après une résection de CCR à visée curative sans preuve de récurrence, de même que les facteurs liés aux patients ou à la maladie susceptibles de prédisposer certains groupes à une surveillance plus fréquente. Donner un aperçu des soins reçus en présence de directives contradictoires. Ce faisant, chercher à améliorer les soins aux survivants du CCR en déterminant toute sous-utilisation ou surutilisation potentielle de certaines modalités de surveillance toute d’inégalité d’accès à la surveillance.
MÉTHODOLOGIE :
Les chercheurs ont mené une étude de cohorte rétrospective au moyen des données du Registre des cas de cancer de l’Ontario et de plusieurs bases de données qui y sont liées. Ils ont repéré les patients de l’Ontario qui avaient subi une résection de CCR à visée curative entre 2003 à 2007, à l’exception des récurrences éventuelles. Dans les cinq ans suivant l’opération, ils ont répertorié le nombre d’examens d’imagerie et d’endoscopie.
RÉSULTATS :
Un total de 4 960 patients ont participé à l’étude. Pendant la période postopératoire de cinq ans, la plus forte proportion de patients qui s’étaient soumis à une surveillance postopératoire a subi le nombre suivant de tests par modalité examinée : de une à trois tomodensitométries abdominopelviennes (n=2 073 [41,8 %]); de une à trois échographies abdominales (n=2 443 [49,3 %]); aucune tomodensitométrie du thorax, de une à trois radiographies pulmonaires (n=2 385 [48,1 %]) et deux endoscopies (n=1 845 [37,2 %]). Le risque de ne pas subir d’imagerie abdomino-pelvienne (par tomodensitométrie ou échographie abdominale) était plus élevé chez les patients qui ne recevaient pas de chimiothérapie adjuvante (RR 6,99 [95 % IC 5,26 à 9,35]) ou qui vivaient dans certaines régions géographiques, mais ne dépendaient pas de l’âge, du sexe ou du revenu. Presque tous les patients (n=4 473 [90,2 %]) ont subi au moins une endoscopie pendant la période de suivi.
CONCLUSION :
Contrairement aux observations découlant d’études similaires menées dans d’autres territoires, la plupart des survivants du CCR de l’Ontario ont reçu une surveillance postopératoire par imagerie et endoscopie, et les soins sont équitables entre les divers groupes sociodémographiques. Cependant, il existe des variations géographiques inexpliquées en pratique, qui justifient un examen plus approfondi.
In Canada, colorectal cancer (CRC) is the third most common cancer in men and women (13.8% and 11.6%, respectively), but is the second most common cause of cancer death in men and third most common cause of cancer death in women (12.7% and 11.6%, respectively) (1). The Canadian Cancer Society estimated that 23,900 Canadians were diagnosed with CRC in 2013 and 9200 died from the disease (1). Specifically in Ontario, it was estimated that were 8700 new cases of CRC in 2013 and that 3350 died from the disease, representing some of the highest rates in the world (1,2). While annual age-adjusted incidence has remained relatively stable (59 to 65 cases per 100,000 Canadians) since 1985, a decrease in age-adjusted CRC mortality from 33 deaths per 100,000 Canadians in 1985 to 24 in 2007 may reflect improvements in screening, diagnosis, treatment and surveillance of CRC patients (1). Nevertheless, approximately 30% to 50% of patients receiving curative-intent treatment experience a recurrence (3), and 90% of these recurrences occur in the first five years after treatment (4). More intensive follow-up of curatively treated cancers may improve survival if there are additional treatment options available (5); however, this surveillance may be costly and may pose problems such as increased exposure to radiation and increased patient distress (6).
Several studies have investigated the means and effectiveness of surveillance following primary CRC resection (7–13); a 2008 Cochrane review examining many of these studies (14) has concluded that there has been a significant improvement in five-year survival with more-intensive versus less-intensive follow-up strategies. Substantial variation among the follow-up strategies used in the included studies precluded any determination of optimal method and frequency of follow-up. Furthermore, recent conference proceedings from Mant et al (15) comparing four different surveillance strategies following curative resection of CRC revealed that performing a computed tomography (CT) scan and obtaining a carcinoembryonic antigen level within the first one to two years after surgery was equivalent to more intensive follow-up strategies. Their results suggest that a single evaluation at 12 to 18 months identifies a majority of patients who would benefit from subsequent surgical intervention, which would likely be more cost effective (15). Given the variation in surveillance regimens noted in the literature, it is not surprising that guidelines regarding CRC surveillance also vary considerably in their recommendations (16–19), although the American Society of Clinical Oncology (ASCO) recently adopted guidelines similar to Cancer Care Ontario (CCO), with addition of several qualifying statements (20) (Table 1).
TABLE 1.
Variation in surveillance guidelines for patients after a curative-intent colorectal cancer resection
| Guideline, year (reference) | Abdominopelvic imaging | Chest imaging | Endoscopy |
|---|---|---|---|
| ASCO, 2005 (16) | CT abdo/pelvis every 12 months for 3 years in high-risk patients* | CT chest every 12 months for 3 years in high-risk patients* | Colonoscopy at 3 years after resection for colon cancer survivors; sigmoidoscopy every 6 months for 5 years for rectal cancer survivors |
| ASCO, 2013 (20) | CT abdo/pelvis every 12 months for 3 years in high-risk patients | CT chest every 12 months for 3 years in high-risk patients | Colonoscopy 1 year after resection; if normal, repeat at 5 years. If not performed before diagnosis, colonoscopy should be performed after completion of adjuvant chemotherapy (before 1 year) |
| CCO, 2003 (19) | May undergo liver imaging at time of clinical assessment† | May have chest imaging at time of clinical assessment* | Colonoscopy before or within 6 months of resection; if normal, repeat at 3 to 5 years; if tubular or villous adenoma >1 cm, repeat at 1 year |
| CCO, 2012 (17) | CT abdo/pelvis every 12 months for 3 years. OR liver U/S every 6 to 12 months for 3 years then every 12 months for 2 years | CT chest every 12 months for 3 years. OR chest x-ray every 6 to 12 months for 3 years then every 12 months for 2 years | Colonoscopy 1 year after resection; if normal, repeat at 5 years |
| NCCN, 2003 (18) | CT abdo/pelvis if symptoms develop | CT chest if symptoms develop | Colonoscopy at 1 year after resection |
Did not define high risk;
Called for clinical assessment every six months for three years, then every 12 months for five years for high-risk (stage IIb-III) patients, and every 12 months for low-risk (stage I-IIa) patients. abdo Abdominal; ASCO American Society of Clinical Oncology; CCO Cancer Care Ontario; CT Computed tomography; NCCN National Comprehensive Cancer Network; U/S Ultrasound
Accordingly, we sought to examine population-based rates of surveillance imaging and endoscopy in patients in Ontario, as well as patient or disease factors that may predispose certain groups to more frequent versus less frequent surveillance. We limited our population of interest to individuals who underwent curative-intent resection for CRC and who remained free of recurrence for at least five years of follow-up after operation. The present study provides insight to the care patients receive in the presence of conflicting guidelines, and may help to improve care of CRC survivors by identifying any potential underuse or overuse of particular surveillance modalities or inequalities in access to surveillance.
METHODS
A retrospective cohort study was performed at the Institute for Clinical and Evaluative Sciences (ICES, Toronto, Ontario). Source information included the Ontario Cancer Registry (OCR) and the following linked administrative databases: the Canadian Institutes of Health Information Discharge Abstract Database (CIHI-DAD), the Ontario Health Insurance Plan (OHIP), the ICES physician database, and the Registered Persons database. Research Ethics Board approval for the present study was obtained from the Sunnybrook Health Sciences Centre and the University of Toronto, Toronto, Ontario.
Patient selection criteria
Patients 18 to 80 years of age, with an International Classification of Diseases, Ninth Revision (ICD-9) diagnosis of colon or rectal cancer and an ICD-0-2 histology code of adenocarcinoma were identified from the OCR from January 1, 2003 through December 31, 2007. Patients with a diagnosis of any other neoplasm at any time based on OCR records were excluded to remove the confounding effect of receiving imaging or follow-up care for tumours other than CRC. Patients who underwent a CRC resection were identified by searching for linked OHIP billings and CIHI-DAD procedures, starting from 14 days before the diagnosis date to ensure inclusion of patients who did not have a preoperative diagnosis of CRC (eg, those who presented emergently with an obstruction or perforation). Either one OHIP claim or one CIHI code for CRC resection was considered to be sufficient for classifying a patient as having undergone surgery. Patients who did not undergo a colorectal resection or who underwent a CRC resection >120 days after diagnosis were excluded because the population of interest was limited to those who underwent a potentially curative CRC resection, and resections performed >120 days from diagnosis were not likely for curative intent.
Once the cohort of patients who underwent curative-intent resection had been identified, additional series of exclusion criteria were applied to eliminate patients who likely developed a recurrence. These patients were excluded to avoid contamination of results with tests performed for diagnosis or treatment planning rather than surveillance. Thus, patients experiencing the following within the entire five-year follow-up period after primary CRC resection were excluded: death; a diagnosis of advanced (secondary) disease, based on CIHI diagnosis codes for hospital admissions; evidence of early relapse of disease via lung or liver procedures (resection, destruction, or biopsy); a palliative care consult, determined by OHIP fee codes; the first claim for chemotherapy >120 days following primary resection; and the number of days between first and last chemotherapy claim exceeding 270 days, between days 0 and 395 after CRC resection. The fifth exclusion is based on data from the Cancer Quality Council of Ontario indicating that approximately 85% of patients will initiate a course of adjuvant chemotherapy within four months of primary CRC resection (21). Initiation of chemotherapy later than this suggests treatment of early disease relapse. The final exclusion was applied because the usual duration of a course of adjuvant chemotherapy is 180 days. Allowing time for breaks in treatment due to side effects or delays, or duration of chemotherapy >270 days, likely represents ongoing treatment for metastatic disease or relapse.
Finally, patients were excluded if they lacked five years of follow-up data, residence within one of the 14 health regions in Ontario referred to as Local Health Integration Network (LHIN) of residence, or income information. Patients residing in the southeast LHIN were also excluded due to the alternative funding plan used by this LHIN, which affects the completeness of OHIP billings.
Patient follow-up
The follow-up period was defined as commencing on the date of primary surgery and patients were followed for five full years thereafter. Modalities of follow-up care that were measured included: abdominopelvic imaging, defined as CT of the abdomen/pelvis (CT A/P) or abdominal ultrasound (AUS); chest imaging, defined as chest x-ray (CXR) or CT of the chest; and endoscopy, defined as flexible sigmoidoscopy or colonoscopy.
Patient-related variables
Demographic variables collected included sex, age at diagnosis of CRC, LHIN of residence, mean neighbourhood income and rural versus urban residence. Mean neighbourhood income quintiles were derived from the Registered Persons database, postal codes and census tract information (census year 2001). Rural status was based on the Statistics Canada definition, namely, residence in a community population <10,000 based on the 2001 census.
Receipt of chemotherapy was used as a proxy for stage to classify relapse risk because stage information was not available in the OCR for the time period of the present study. Patients were labelled as high risk for recurrence if they received chemotherapy in their immediate postoperative period (within four months of operation); otherwise, they were considered to be low risk for recurrence.
Statistical analysis
Descriptive statistics were performed on variables of interest with categorical variables summarized using counts and percentages. For patients with complete five-year follow-up data with no evidence of recurrence, descriptive tables were generated by counts of each type of surveillance modality for the follow-up period, stratifying the cohorts according to low-risk and high-risk patients. To explore factors associated with receiving no abdominal imaging (CT or ultrasound) in the entire follow-up period, univariate and multivariable logistic regression analyses were performed with the outcome of zero abdominal imaging modalities versus one or more. The predictor variables of interest were patient age category, sex, LHIN of residence, rural status, income quintile and risk category of the primary tumour. The estimates from the models were presented as ORs and their associated 95% CIs. All tests were two-sided; P<0.05 was considered to be statistically significant. All analyses were performed using SAS version 9.1.3 for Unix (SAS Institute, USA).
RESULTS
In total, 12,109 patients who underwent curative CRC resection for colorectal adenocarcinoma, with no other primary malignancy, were identified from the OCR from January 1, 2003 through December 31, 2007 (Figure 1). These patients underwent a CRC resection from 14 days before to 120 days after the OCR diagnosis date and were disease free at the beginning of the follow-up period. After application of exclusion criteria, of these 12,109 patients, 4960 (41%) had complete five-year data to examine frequencies of follow-up modalities, no out-migration and no disease recurrence or death between years 1 and 5 after resection. In total, 3101 (62.5%) patients were considered to be low risk and 1859 (37.5%) high risk. Overall patient characteristics are summarized in Table 2.
Figure 1).
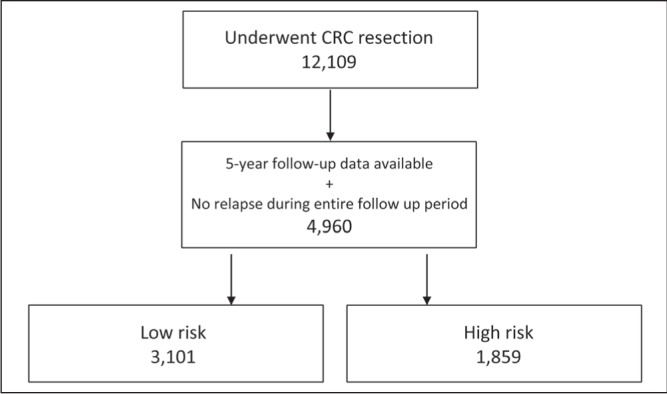
Patient selection. CRC Colorectal cancer
TABLE 2.
Multivariable logistic regression analysis for patients receiving neither abdominopelvic computed tomography scan nor abdominal ultrasound during the five-year follow-up period
| OR (95% CI) | P | |
|---|---|---|
| Age, years | ||
| <50 | Reference | |
| 50–64 | 0.97 (0.68–1.39) | 0.88 |
| 65–74 | 1.07 (0.75–1.52) | 0.73 |
| >75 | 1.21 (0.83–1.74) | 0.32 |
| Sex | ||
| Male | Reference | |
| Female | 0.85 (0.71–1.02) | 0.08 |
| Risk category | ||
| High | Reference | |
| Low | 6.99 (5.26–9.35) | <0.01 |
| Rural | ||
| No | Reference | |
| Yes | 1.00 (0.77–1.31) | 0.99 |
| Income quintile | ||
| 1 (low) | Reference | |
| 2 | 1.08 (0.81–1.43) | 0.62 |
| 3 | 0.94 (0.70–1.26) | 0.67 |
| 4 | 1.07 (0.80–1.44) | 0.63 |
| 5 (high) | 1.07 (0.80–1.43) | 0.64 |
| Local Health Integration Network | ||
| 1 | Reference | |
| 2 | 1.03 (0.70–1.52) | 0.86 |
| 3 | 1.32 (0.78–2.21) | 0.30 |
| 4 | 1.11 (0.75–1.63) | 0.60 |
| 5 | 1.90 (1.26–2.86) | <0.01 |
| 6 | 1.06 (0.74–1.52) | 0.76 |
| 7 | 1.03 (0.66–1.60) | 0.90 |
| 8 | 1.43 (0.93–2.18) | 0.10 |
| 9 | 1.14 (0.68–1.92) | 0.62 |
| 10 | 0.41 (0.16–1.07) | 0.07 |
| 11 | 2.03 (1.38–2.98) | <0.01 |
| 12 | 0.61 (0.37–0.99) | 0.05 |
| 13 | 1.20 (0.75–1.92) | 0.44 |
Bolded values indicate statistical significance
Abdominopelvic imaging
Over the five-year follow-up period, 1354 (27.3%) patients did not undergo any CT A/P (Figure 2). The largest proportion of patients (n=2073 [41.8%]) underwent between one and three CT A/P in the follow-up period, with progressively smaller numbers of patients undergoing four to six, seven to 10 and ≥11 scans, respectively. This analysis was repeated by stratifying the cohort according to low- and high-risk primary tumours. While 1166 (37.6%) low-risk patients did not receive any CT A/P in the entire follow-up period, only 188 (10.1%) high-risk patients did not receive a single CT A/P.
Figure 2).
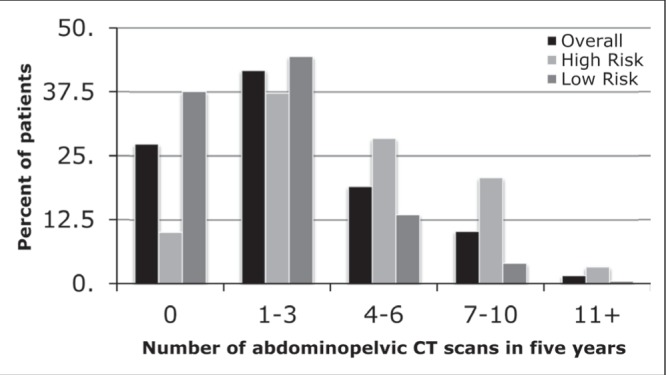
The number of abdominopelvic computed tomography (CT) scans received in the five-year follow-up period, stratified according to high and low risk for recurrence
Frequency of abdominal ultrasound (AUS) was also examined throughout the follow-up period. Overall, 1177 (34.6%) did not receive any AUS, while almost one-half (2443 [49.3%]) underwent one to three AUS (Figure 3). Results were similar when stratified according to high- and low-risk patients.
Figure 3).
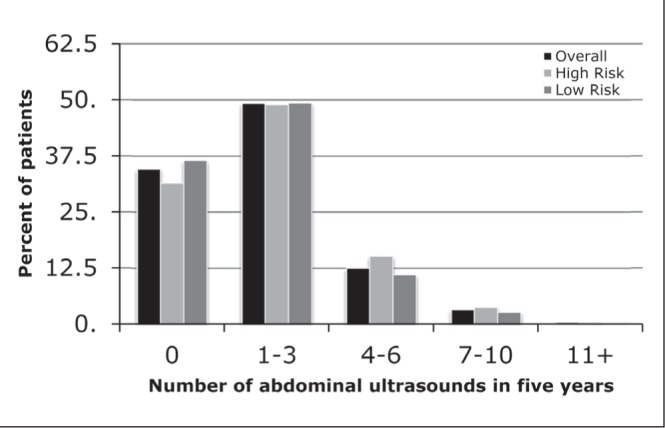
The number of abdominal ultrasounds received in the five-year follow-up period, stratified according to high and low risk for recurrence
To understand the proportion of patients that did not undergo any abdominopelvic imaging whatsoever, the subgroup of patients who did not receive any CT A/P was analyzed for the frequency of AUS performed. Of the 1354 patients who did not receive CT A/P during the follow-up period, 592 (43.7%) also did not receive AUS (11.9% of cohort). This group was comprised primarily of patients at low risk for recurrence (538 [91.9%]). No differences in the proportion of patients with no abdominopelvic imaging were noted according to age, sex, income or rural residence (Table 2). However, compared with the referent LHIN (LHIN 1), there was a significant variation in the proportion of patients without abdominopelvic imaging depending on LHIN (Table 2). The strongest association was observed for patients with low-risk primary cancers to have increased odds of not receiving any abdominal imaging (OR 6.99 [95% CI 5.26 to 9.35]).
Chest imaging
The majority of patients did not receive any CT scans of the chest (2863 [57.7%]) (Figure 4). High-risk patients were slightly more likely to receive CT chest in each frequency category. The number of CXRs patients received during the five-year follow-up period was also examined (Figure 5). Patients most frequently underwent one to three CXRs in each of the overall (2385 [48.1%]), high-risk (885 [47.6%]) and low-risk (1500 [48.4%]) categories.
Figure 4).
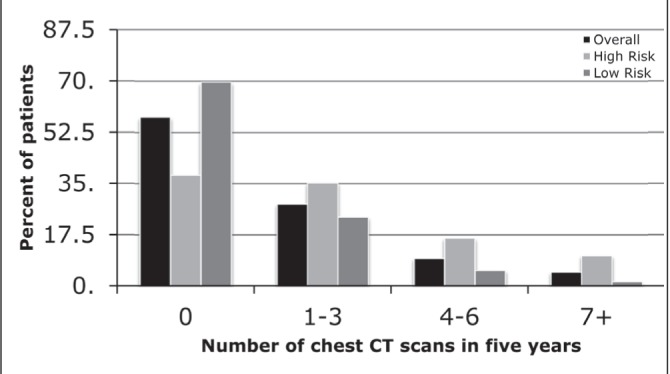
The number of chest computed tomography (CT) scans received in the five-year follow-up period, stratified according to high and low risk for recurrence
Figure 5).
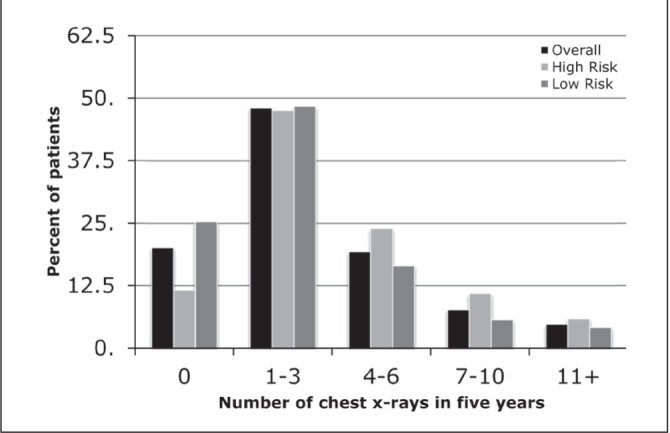
The number of chest x-rays received in the five-year follow-up period, stratified according to high- and low-risk for recurrence
Endoscopy
Most patients (4473 [90.2%]) underwent endoscopy at some point during the follow-up period, with the highest proportion of patients undergoing two endoscopies in each of the overall (1845 [37.2%]), low-risk (1133 [36.5%]) and high-risk (712 [38.3%]) groups (Figure 6).
Figure 6).
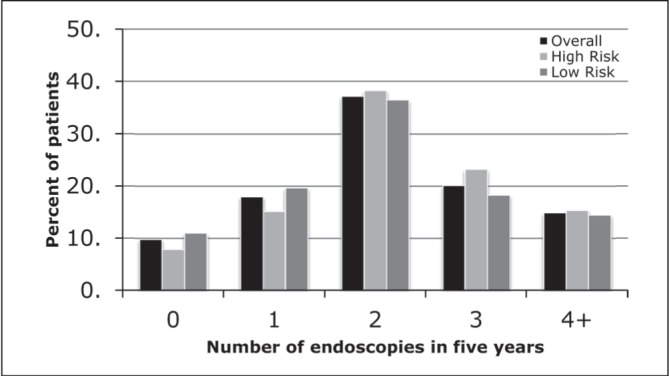
The number of endoscopies received in the five-year follow-up period, stratified according to high and low risk for recurrence
Univariate and multivariate analysis was performed to test for associations of surveillance endoscopies with age, sex, LHIN of residence, income and risk category. As income quintile increased, there was a trend toward more patients undergoing ≥4 endoscopies and fewer patients undergoing zero endoscopies (Figure 7).
Figure 7).
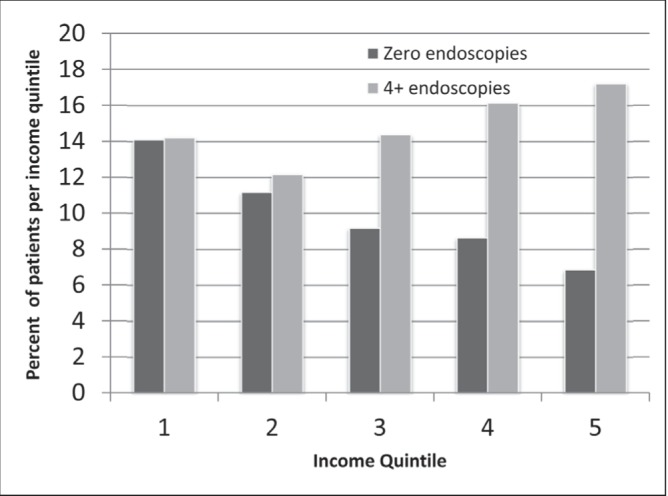
Comparison of percentage of patients undergoing zero versus four or more endoscopies, stratified according to income quintile
DISCUSSION
In the present population-based cohort study, the actual imaging and endoscopic surveillance received by Ontario patients post curative-intent CRC resection was examined. For both imaging studies and endoscopy, patients were noted to have undergone a variable number of tests during their five-year follow-up period. This is not unexpected given the heterogeneity of guidelines at the time of our study, but it is interesting to note the wide variation that existed in the number of tests. For example, while some patients did not receive a single CT A/P or AUS during their follow-up, some patients received ≥11. While those receiving many scans may have undergone imaging for reasons other than CRC surveillance, it is also possible that different patients and physicians have different attitudes regarding the benefits and necessity of imaging surveillance after curative-intent CRC resection. This would be consistent with the finding that, among the subgroup of patients who received no abdominopelvic surveillance imaging, most had low-risk primary tumours. Both patients and physicians may believe there to be a lesser need for surveillance imaging in patients judged to have a lower risk for recurrence.
Variations among LHINs in the ORs for receiving no abdominopelvic surveillance imaging during the five-year follow-up period is concerning for potential health care inequity. However, such variations are not unexpected given that Cooper et al (22) previously demonstrated similar geographical variations in surveillance patterns in the United States. However, our results must be interpreted cautiously given that other potential confounders, such as patient preference, number of medical comorbidities or patient distance from nearest imaging facility, were not taken into account.
Income quintile was not an independent predictor for receiving no abdominopelvic surveillance imaging in the present study. This is in contrast to findings by Elston Lafata et al (23) in the United States showing that lower income patients had a decreased chance of receiving surveillance metastatic disease testing in the form of CT, ultra-sound, magnetic resonance imaging, CXR or serum transaminases. While no other Ontario study has examined patient income as a predictor for CRC imaging surveillance, Booth et al (24) did demonstrate decreased overall and cancer-specific survivals for lower-income Ontario patients. However, Grunfeld et al (25) noted small differences (<2%) between income quintiles when examined as a predictor of imaging surveillance for breast cancer patients. It is possible that surveillance imaging patterns are not influenced by income in Ontario because of our universal health care system, and that Booth et al’s results were, thus, not related to surveillance imaging practices.
However, the income equality noted in our study for surveillance imaging did not persist when surveillance endoscopy was considered. On the contrary, more affluent Ontarians are more likely to receive four or more endoscopies and less likely to receive zero endoscopies compared with less affluent individuals. The lack of association between income and imaging surveillance in Ontario, but the presence of this association for endoscopic surveillance may be a result of endoscopy being a relatively scarce resource compared with imaging modalities such as CT scans and ultrasounds. Further study involving individual level data and actual household income may be useful in further defining the relationship between receipt of surveillance endoscopy and socioeconomic status. It is not until these potential explanatory mechanisms are elucidated that further intervention, such as knowledge translation initiatives or deployment of resources, can be used to address socioeconomic status as a potential barrier to receiving surveillance endoscopy.
The finding that most patients receive at least one endoscopic evaluation postoperatively is consistent with both the 2003 CCO guideline, which called for colonoscopy before or within six months of surgery, and the 2009 NCCN guideline, which recommended colonoscopy at three years following resection (26). However, the American Society of Clinical Oncology guideline, while suggesting colonoscopy at one year for colon cancer survivors, also suggested sigmoidoscopy every six months for rectal cancer patients. The latter should lead to rectal cancer survivors undergoing approximately 10 endoscopic evaluations over the course of their five-year follow-up, which clearly very few patients achieved (16). The subset of patients receiving no endoscopy postoperatively may reflect individuals too unwell to undergo the procedure itself (or any treatment that would be indicated should a recurrence be noted), those who preferred to forego the examination, or perhaps those who, in accordance with the CCO guideline, only received preoperative endoscopy.
With respect to chest imaging, it is important to note that CCO’s recommendations at the time of the present study did not address CT scanning and did not define a recommended frequency for CXR (19). As such, it is not surprising that relatively few Ontario patients received surveillance chest CT. More patients received CXR, but this result could be confounded because CXR are common tests ordered for a wide range of indications, not just CRC surveillance.
Other studies have also examined adherence to CRC surveillance guidelines, with mixed results. A study involving 409 French patients (27) showed poor adherence to French guidelines at the time, with 65% of patients undergoing fewer than the recommended number of AUS, 52% undergoing fewer than the recommended number of CXR and 20% undergoing fewer than the recommended number of colonoscopies. In the United States, a study focusing on patients >65 years of age demonstrated that 60% of patients received less than the recommended amount of postoperative surveillance (28). In contrast, a smaller single-centre Norwegian study demonstrated a 62% compliance rate with Norwegian surveillance recommendations, including 85% undergoing liver ultrasound and 55% undergoing colonoscopy within one year (29). The latter study was aimed primarily at determining patient compliance because all patients were ordered testing in accordance with guidelines by their treating physician. Relative to these observations in other nations, it would appear that Ontario physicians’ and patients’ adherence to guidelines regarding colonoscopy is quite good. It is difficult to make generalized statements about compliance with imaging in the current study because guidelines at the time were nonspecific.
It is interesting to compare the results of the present study with those of a survey conducted by Earle et al (30) in 2003 to measure Canadian physicians’ surveillance strategies following curative-intent CRC resection. Surgeons, medical oncologists and radiation oncologists all specializing in CRC were presented with a hypothetical patient – a 50-year-old man, otherwise healthy, with stage III disease after a curative CRC resection – and asked to provide their recommendations for follow-up. Of the 160 physicians who completed the survey, <10% recommended body surveillance by abdominal CT in any follow-up year, and only approximately one-third recommended AUS imaging in any follow-up year. In contrast, approximately 90% of physicians recommended bowel surveillance with colonoscopy in follow-up year 1. Opinions regarding chest imaging were not solicited in the survey. Clearly, with almost 90% of patients in the current study receiving at least one abdominopelvic CT or ultrasound within five years of follow-up, patients are receiving more body surveillance than the study by Earle et al (30) would predict. This could be a result of changing practice because the survey was conducted in 2003, differences in practice between Ontario and other Canadian provinces, or contamination of our study data with imaging obtained for an alternate indication. The high proportion of physicians recommending surveillance endoscopy in the study by Earle et al is consistent with our findings that most Ontario patients receive at least one postoperative endoscopy.
Limitations to the present study were primarily related to the use of administrative data. Although such population-level data generate a large sample size, the data at the individual level are lost. For example, unlike the Surveillance, Epidemiology, and End Results database from the National Cancer Institute, our administrative data do not identify patient race or ethnicity, which would allow us to evaluate other social elements that may affect postoperative CRC surveillance patterns in addition to income level. Also in the present study, lack of information on the indications for CT, AUS, CXR and endoscopic evaluations may have allowed confounding of surveillance data to occur if these tests were ordered for indications other than CRC surveillance. Furthermore, an additional limitation to the present study arises from the application of strict exclusion criteria, which reduced our sample size for analysis from 12,109 to 4960 patients to eliminate bias caused by additional testing around the time of detection of disease relapse. However, it is still possible that a small number of patients with disease relapse were included in the sample, which could skew results toward higher use of all surveillance modalities. An additional limitation of our study involved trying to determine the number of patients that received a colonoscopy within one year of resection, as recommended by most guidelines. In the use of administrative data, it is extremely problematic to develop rules to define and determine adherence to guidelines. For example, a one-year follow-up colonoscopy can be performed anywhere between 10 and 14 months after resection, but this represents an arbitrary range. If a specific cutoff was used at 12 months postresection, many one-year follow-up colonoscopies may be systematically excluded. However, we are able to measure whether any surveillance was performed during the five-year follow-up period, which will reflect issues of follow-up and access to care. Therefore, we reported the number of colonoscopies over the five-year follow-up period. Given these limitations, the present study provides a seminal overview of current surveillance patterns in the province of Ontario and these observations should be further studied and confirmed with individual-level data in a variety of settings throughout the province, including both academic and nonacademic centres, as well as urban and rural centres. Further study is recommended in determining the current knowledge guidelines of physicians who provide CRC follow-up, as well as access to resources in the area where they practice.
Acknowledgments
This study was conducted with the support of the Ontario Institute for Cancer Research and Cancer Care Ontario through funding provided by the Government of Ontario. This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Dr Coburn (Career Scientist Award) and Dr Law (Hanna Family Research Chair in Surgical Oncology) held support grants.
REFERENCES
- 1.Canadian Cancer Society’s Steering Committee on Cancer Statistics . Canadian cancer statistics 2013. Toronto: Canadian Cancer Society; 2013. [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2011;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Tjandra JJ, Chan MK. Follow-up after curative resection of colorectal cancer: A meta-analysis. Dis Colon Rectum. 2007;50:1783–99. doi: 10.1007/s10350-007-9030-5. [DOI] [PubMed] [Google Scholar]
- 4.Pfister DG, Benson AB, III, Somerfield MR. Clinical practice. Surveillance strategies after curative treatment of colorectal cancer. N Engl J Med. 2004;350:2375–82. doi: 10.1056/NEJMcp010529. [DOI] [PubMed] [Google Scholar]
- 5.Furman MJ, Lambert LA, Sullivan ME, Whalen GF. Rational follow-up after curative cancer resection. J Clin Oncol. 2013;31:1130–3. doi: 10.1200/JCO.2012.46.4438. [DOI] [PubMed] [Google Scholar]
- 6.Virgo KS, Wade TP, Longo WE, Coplin MA, Vernava AM, Johnson FE. Surveillance after curative colon cancer resection: Practice patterns of surgical subspecialists. Ann Surg Oncol. 1995;2:472–82. doi: 10.1007/BF02307079. [DOI] [PubMed] [Google Scholar]
- 7.Castells A, Bessa X, Daniels M, et al. Value of postoperative surveillance after radical surgery for colorectal cancer: Results of a cohort study. Dis Colon Rectum. 1998;4:714–23. doi: 10.1007/BF02236257. discussion 23–4. [DOI] [PubMed] [Google Scholar]
- 8.Kjeldsen BJ, Kronborg O, Fenger C, Jorgensen OD. A prospective randomized study of follow-up after radical surgery for colorectal cancer. Br J Surg. 1997;84:666–9. [PubMed] [Google Scholar]
- 9.Makela JT, Laitinen SO, Kairaluoma MI. Five-year follow-up after radical surgery for colorectal cancer. Results of a prospective randomized trial. Arch Surg. 1995;130:1062–7. doi: 10.1001/archsurg.1995.01430100040009. [DOI] [PubMed] [Google Scholar]
- 10.Ohlsson B, Breland U, Ekberg H, Graffner H, Tranberg KG. Follow-up after curative surgery for colorectal carcinoma. Randomized comparison with no follow-up. Dis Colon Rectum. 1995;38:619–26. doi: 10.1007/BF02054122. [DOI] [PubMed] [Google Scholar]
- 11.Pietra N, Sarli L, Costi R, Ouchemi C, Grattarola M, Peracchia A. Role of follow-up in management of local recurrences of colorectal cancer: A prospective, randomized study. Dis Colon Rectum. 1998;41:1127–33. doi: 10.1007/BF02239434. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Moranta F, Salo J, Arcusa A, et al. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: A prospective, multicenter, randomized, controlled trial. J Clin Oncol. 2006;24:386–93. doi: 10.1200/JCO.2005.02.0826. [DOI] [PubMed] [Google Scholar]
- 13.Schoemaker D, Black R, Giles L, Toouli J. Yearly colonoscopy, liver CT, and chest radiography do not influence 5-year survival of colorectal cancer patients. Gastroenterology. 1998;114:7–14. doi: 10.1016/s0016-5085(98)70626-2. [DOI] [PubMed] [Google Scholar]
- 14.Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2007;(1):CD002200. doi: 10.1002/14651858.CD002200.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Mant D, Perera R, Gray A, et al. Effect of 3–5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: FACS randomized controlled trial. 2013 ASCO Annual Meeting. J Clin Oncol. 2013;(Suppl):A3500. [Google Scholar]
- 16.Desch CE, Benson AB, III, Somerfield MR, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512–9. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- 17.Earle C, Annis R, Sussman J, Haynes AE, Vafaei A. Follow up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer. 2 Vol. 26. Toronto: Cancer Care Ontario; 2012. Program in Evidence based Care Evidence Based Series. [Google Scholar]
- 18.Engstrom PF, Benson AB, III, Saltz L. Colon cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2003;1:40–53. doi: 10.6004/jnccn.2003.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Figueredo A, Rumble RB, Maroun J, et al. Follow-up of patients with curatively resected colorectal cancer: A practice guideline. BMC Cancer. 2003;3:26. doi: 10.1186/1471-2407-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyerhardt JA, Mangu PB, Flynn PJ, et al. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2013;31:4465–70. doi: 10.1200/JCO.2013.50.7442. [DOI] [PubMed] [Google Scholar]
- 21.Cancer Care Ontario Informatics Centre of Excellence Wait time between Diagnosis and Adjuvant Chemotherapy. Cancer Quality Council of Ontario. 2013 [Google Scholar]
- 22.Cooper GS, Yuan Z, Chak A, Rimm AA. Geographic and patient variation among Medicare beneficiaries in the use of follow-up testing after surgery for nonmetastatic colorectal carcinoma. Cancer. 1999;85:2124–31. [PubMed] [Google Scholar]
- 23.Elston Lafata J, Johnson CC, Ben-Menachem T, Morlock RJ. Sociodemographic differences in the receipt of colorectal cancer surveillance care following treatment with curative intent. Med Care. 2001;39:361–72. doi: 10.1097/00005650-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Booth CM, Li G, Zhang-Salomons J, Mackillop WJ. The impact of socioeconomic status on stage of cancer at diagnosis and survival: A population-based study in Ontario, Canada. Cancer. 2010;116:4160–7. doi: 10.1002/cncr.25427. [DOI] [PubMed] [Google Scholar]
- 25.Grunfeld E, Hodgson DC, Del Giudice ME, Moineddin R. Population-based longitudinal study of follow-up care for breast cancer survivors. J Oncol Pract. 2010;6:174–81. doi: 10.1200/JOP.200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engstrom PF, Arnoletti JP, Benson AB, III, et al. NCCN Clinical Practice Guidelines in Oncology: Colon cancer. J Natl Compr Canc Netw. 2009;7:778–831. doi: 10.6004/jnccn.2009.0056. [DOI] [PubMed] [Google Scholar]
- 27.Boulin M, Lejeune C, Le Teuff G, et al. Patterns of surveillance practices after curative surgery for colorectal cancer in a French population. Dis Colon Rectum. 2005;48:1890–9. doi: 10.1007/s10350-005-0096-7. [DOI] [PubMed] [Google Scholar]
- 28.Cooper GS, Kou TD, Reynolds HL., Jr Receipt of guideline-recommended follow-up in older colorectal cancer survivors: A population-based analysis. Cancer. 2008;113:2029–37. doi: 10.1002/cncr.23823. [DOI] [PubMed] [Google Scholar]
- 29.Korner H, Soreide K, Stokkeland PJ, Soreide JA. Systematic follow-up after curative surgery for colorectal cancer in Norway: A population-based audit of effectiveness, costs, and compliance. J Gastrointest Surg. 2005;9:320–8. doi: 10.1016/j.gassur.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Earle CC, Grunfeld E, Coyle D, Cripps MC, Stern HS. Cancer physicians’ attitudes toward colorectal cancer follow-up. Ann Oncol. 2003;14:400–5. doi: 10.1093/annonc/mdg101. [DOI] [PubMed] [Google Scholar]


