Abstract
Background:
Presently the necessity of fasting time for coagulation tests is not standardized. Our hypothesis is that this can harm patient safety. This study is aimed at evaluating whether a light meal (i.e. breakfast) can jeopardize laboratory coagulation tests.
Materials and methods:
A blood sample was firstly collected from 17 fasting volunteers (12 h). Immediately after blood collection, the volunteers consumed a light meal. Then samples were collected at 1, 2 and 4 h after the meal. Coagulation tests included: activated partial thromboplastin time (APTT), prothrombin time (PT), fibrinogen (Fbg), antithrombin III (AT), protein C (PC) and protein S (PS). Differences between samples were assessed by Wilcoxon ranked-pairs test. The level of statistical significance was set at P < 0.05. Mean % differences were determined and differences between and baseline and 1, 2 and 4h samples were compared with reference change value (RCV).
Results:
A significantly higher % activity of AT was observed at 1 h and 4 h after meal vs. baseline specimen [113 (104–117) and 111 (107–120) vs. 109 (102–118), respectively; P = 0.029 and P = 0.016]. APTT at 2 h was found significantly lower than baseline samples [32.0 (29.9–34.8) vs. 34.1 (32.2–35.2), respectively; P = 0.041]. The results of both Fbg and PS tests were not influenced by a light meal. Furthermore, no coagulation tests had significant variation after comparison with RCV.
Conclusion:
A light meal does not influence the laboratory coagulation tests we assessed, but we suggest that the laboratory quality managers standardize the fasting time for all blood tests at 12 hours, to completely metabolize the lipids intake.
Keywords: diagnostic errors, fasting, postprandial period, reference values, reproducibility of results
Introduction
In the laboratory diagnostic setting, the preanalytical phase is likened to the dark side of the moon (i.e. as regards patient management), since many problems are reported, that are due to inappropriate test ordering, to errors in patient identification and preparation, such as fasting time before sample collection, to issues in sample collection, transport and delivery to the laboratory, as well as in sample handling and storage (1). Moreover, errors in preanalytical phase generate further work or additional investigation able to amplify both procedures sometimes unnecessary and expenditures for healthcare systems (2). Preanalytical issues also have downstream impact on the use of laboratory resources, hospital costs and overall quality of care (3).
The accurate investigation of haemostasis disturbances, either haemorrhagic or thrombotic, develops through a discretional use of laboratory resources, which basically includes first and second line testing. First line of routine coagulation assays, conventionally represented by activated partial thromboplastin time (APTT), prothrombin time (PT) and fibrinogen (Fbg), reflects the overall function of the coagulation pathway and is represented by the tests more frequently requested to screen for qualitative or quantitative deficiencies (4). The Clinical and Laboratory Standards Institute (CLSI) – CLSI H3-A6 document recommends to verify patient diet for restrictions and/or fasting (5). Moreover, this document reports relevant information as follows: a) time and diet restrictions vary according to the test; b) restrictions are necessary to ensure accurate test results and; c) procedures for holding meals and notifying appropriate personnel that the patient’s blood specimen has been drawn should be in accordance with the institutional policy. Furthermore, the CLSI H3-A6 document does not report standardization of fasting time, instead leaving to the discretion of each laboratory the establishment of the more appropriate procedures (5). The Working Group on Preanalytical Phase from European Federation of Clinical Chemistry and Laboratory Medicine (WG-PA EFLM) has provided a suitable framework for the harmonization of definitions for fasting requirements for routine laboratory tests but not for coagulation tests (6). Lippi el al. showed that a light meal can alter the routine hematological tests (7).
Routinely outpatients go to clinical laboratories with pre-surgical tests requirements form; if the physician’s prescription contemplates lipid profile or glucose determination among the routine pre-surgical tests (e.g. partial thromboplastin time, PT and / or Fbg concentration), the laboratory staff reminds the need for appropriate fasting time before blood collection, as suggested by international and local guidelines (8). Alternatively, if an out-patient shows a test request form without associated lipid profile or glucose determinations, no need for fasting time appears strictly justified. Nevertheless, this appears more of a habit rather than of a prescription based on research findings. Our hypothesis is that these daily practices can harm patient safety. This study is aimed at evaluating whether a light meal (i.e. breakfast) can jeopardize laboratory coagulation tests.
Material and methods
Study design
This study was conducted following the methodology previously published by our work group (7,8). A brief description follows.
A group of 17 adults of both sexes, volunteers for this study, from Verona (Italy), were evaluated. The study was submitted to the Internal Review Board, approved by the Human Research Ethics Committee and partially supported by CAPES Foundation, Ministry of Education of Brazil, Brasília - DF, Brazil (process number 0175-14-5). All volunteers signed an informed consent. The study was carried out in agreement with the Declaration of Helsinki.
The inclusion criteria were based on the lack of familiarity for blood coagulation disorders, platelets dysfunction, diabetes, dyslipidemias, thalassemia syndromes and other hemoglobinopathies and, in regards to women, that they had regular menstrual cycles and were not using hormonal contraceptives. None of the subjects took any medication. Body mass indices were < 30.0 kg/m2 in all subjects. The light meal (i.e. 563 kcal) was based on commercially available meal consisting of one yogurt, two slices of bread and one slice of cheese, chocolate snack and a fruit juice as previously described by Lippi et al. (7)
Collection of diagnostic blood specimens
The collection of all diagnostic blood specimens was performed by a single, expert phlebotomist, following the international standard from CLSI (5,9) with minor modifications as previously proposed by our working group (10) and using transilluminator device (Venoscópio IV plus, Duan do Brasil, Brazil) in order to avoid the venous stasis interference (11). All subjects observed 15 minutes of rest before venipuncture as recommended (12). Blood sample collection was performed using 20 G straight needles from a single lot (Terumo Europe NV, Leuven, Belgium), directly into 3.6 mL vacuum tubes containing 0.4 buffered sodium citrate (9NC) 0.109 mol/L: 3.2 W/V%, lot 1004001 (Terumo Europe NV, Leuven, Belgium). All tubes were filled at their nominal volume; the first tube (i.e. without additive, Vacuette® lot A101004D, Greiner Bio-One GmbH, Kremsmünster, Austria) from each volunteer was draw to prevent potential bias due to contact phase or tissue factor, and then processed for triglycerides determination. First blood sample was collected between 8.00 and 8.30 a.m. from each fasting subject (12 h, fasting time necessary to completely metabolize the lipids intake). Immediately after blood collection, the volunteers consumed a light meal (i.e. 563 kcal), containing standardized amounts of carbohydrates, protein and lipids. Subsequent blood samples were collected 1, 2 and 4 hours after the end of the meal.
Processing of diagnostic blood specimens
All the sample tubes were left in upright position (i.e. 30 min for sodium citrate, and 60 min for serum-vacuum tubes) at room temperature (i.e. 20 °C) to allow complete blood stability before centrifugation (13). Serum tubes were centrifuged at 2000 × g for 10 minutes, and plasma samples were obtained by centrifugation at 1500 × g for 15 minutes at room temperature (according to the instructions of the manufacturer). Moreover, because shortening of APTT in thawed samples may ensue, due to residual platelet debris in the thawed samples, a re-centrifugation was done and the platelet poor plasma (PPP) samples were then stored in aliquots and kept frozen at −70°C until measurement. All samples did not show any sign of haemolysis or lipemia by visual inspection.
Laboratory testing
All plasma aliquots frozen at −70 °C were thawed in water bath at 37 °C at the same time; and incubated at room temperature for 1 hour. Afterwards the samples were handled as fresh ones. The routine and specialized coagulation laboratory tests were performed immediately, after this incubation period, on the instrument ACL TOP (Instrumentation Laboratory, Milan, Italy), according to the manufacturer’s specifications and using HemosIL reagent system (proprietary reagents). The panel of tests included the following: APTT (sec), PT INR, Fbg (g/L), antithrombin III (AT % activity), protein C (PC % activity) and protein S (PS % activity). The instrument was calibrated against appropriate proprietary reference standard material and verified with the use of proprietary quality controls. Plasma citrated samples were processed for Serum Index Gen. 2® on the same Cobas 6000 <c501> module (Roche Diagnostics GmbH, Mannheim, Germany) immediately after coagulation testing determination. Serum Index Gen. 2® is an in vitro test for semi-quantitative determination of the lipemia, haemolysis and icterus index in human serum and plasma on Cobas c systems (14). Because there is poor correlation between the lipemia index (i.e. corresponding to turbidity) and triglycerides concentration, triglycerides concentrations (mmol/L) were determined on the same cobas 6000 <c501> module using serum samples. All laboratory tests were performed in duplicate to minimize the analytical coefficient of variation (CVa).
Statistical analysis
The significance of differences between samples was assessed by Wilcoxon ranked-pairs test in agreement with Simundic recommendations regarding sample size (i.e. less than 30), using licensed statistical software (GraphPad Prism® version 5.01, La Jolla, CA, USA) (15). The level of statistical significance was set at P < 0.05. The clinically significant change in consecutive results from an individual, taking into consideration both analytical and biological variations, was calculated for each test using the reference change value (RCV) equation as follows:
where: Z is a constant depending on the probability, where 1.96 is most often used as significant, that is, P < 0.05; CVw is the within-subject biological variation obtained from Westgard database (16); and CVa is the analytical coefficient of variation from our internal quality control. Mean % differences were determined according to the formula:
Finally, the mean % difference from 1, 2 and 4 hours after intake of a standardised meal were compared with RCV. Only mean % differences higher than RCV were considered clinically significant. Desirable specification for imprecision (DSI) derived from biologic variation (16) was used as our criteria of acceptance in lipemia analytical interference testing.
Results
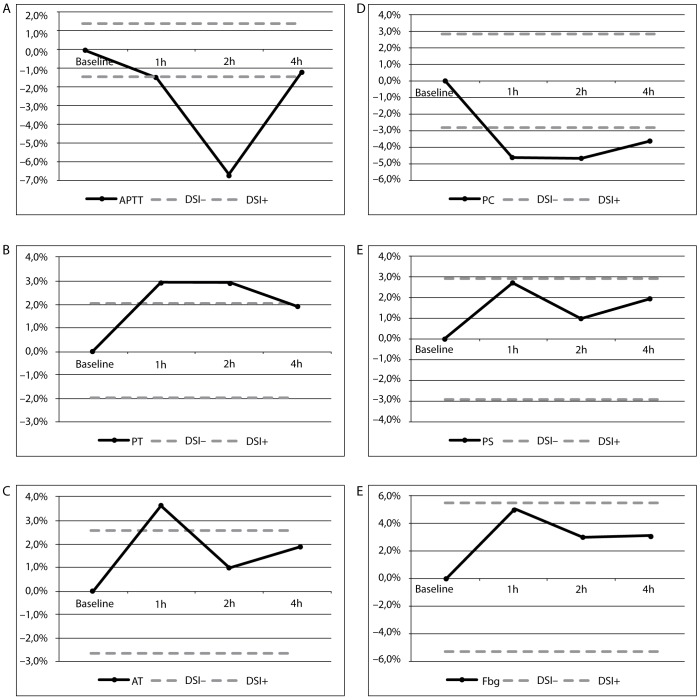
The results of this investigation are presented as median (interquartile range) in Table 1. We found a significantly higher % activity of AT in 1 h and 4 h after the ingestion of the light meal as compared to baseline specimen [113 (104–117) and 111 (107–120) vs. 109 (102–118), respectively; P = 0.029 and P = 0.016]. Two hours after the ingestion of the meal, APTT was found significantly lower than baseline samples [32.0 (29.9–34.8) vs. 34.1 (32.2–35.2), respectively; P = 0.041]. The results of both Fbg and PS tests appeared not influenced by a light meal. Furthermore, no coagulation tests had shown clinically significant variation after comparison with RCV. Lipemia analytical interference testing was observed for APTT, PT, AT and PC, after comparison with DSI derived from biologic variation (Figure 1).
Table 1.
Postprandial variation on coagulation tests after meal.
| Test | CVa (%) | CVw (%) | DSI (%) | RCV (%) | Baseline specimen | 1h after meal | Difference % mean | P | 2h after meal | Difference % mean | P | 4h after meal | Difference % mean | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APTT (sec) | 1.2 | 2.7 | 1.4 | 8.2 | 34.1 (32.2–35.2) | 33.6 (32.3–35.3) | −1.5 | 0.850 | 32.0 (29.9–34.8) | −6.6 | 0.041* | 33.7 (31.6–34.8) | −1.2 | 0.463 |
| PT (INR) | 1.3 | 4.0 | 2.0 | 11.7 | 1.05 (0.98–1.09) | 1.02 (1.00–1.11) | −2.9 | 0.379 | 1.02 (0.97–1.10) | −2.9 | 0.443 | 1.03 (0.98–1.07) | −1.9 | 0.234 |
| Fbg (g/L) | 3.6 | 10.7 | 5.4 | 31.3 | 2.89 (2.76–3.31) | 3.04 (2.70–3.33) | 4.9 | 0.236 | 2.98 (2.66–3.27) | 3.0 | 0.780 | 2.98 (2.67–3.39) | 3.0 | 0.979 |
| AT (%) | 2.8 | 5.2 | 2.6 | 16.4 | 109 (102–118) | 113 (104–117) | 3.5 | 0.029* | 110 (100–116) | 0.9 | 0.528 | 111 (107–120) | 1.8 | 0.016* |
| PC (%) | 2.0 | 5.6 | 2.9 | 16.5 | 112 (92–122) | 107 (97–122) | −4.7 | 0.442 | 107 (98–116) | −4.7 | 0.312 | 108 (96–119) | −3.7 | 0.740 |
| PS (%) | 3.0 | 5.8 | 2.9 | 18.1 | 103 (88–125) | 106 (89–128) | 2.8 | 0.376 | 104 (90–120) | 1.0 | 0.831 | 101 (86.5–119) | −2.0 | 0.154 |
| Tg (mmol/L) | 2.3 | 19.9 | 9.95 | 55.5 | 0.86 (0.74–1.03) | 1.00 (0.71–1.16) | 14.0 | 0.012* | 1.06 (0.70–1.33) | 18.9 | 0.010* | 1.18 (0.79–1.36) | 27.1 | 0.005* |
| Turbidity index | NA | NA | NA | NA | 9 (8–14) | 14 (11–20) | 35.7 | 0.001* | 16 (11–18) | 43.8 | < 0.001* | 13 (12–16) | 30.8 | 0.003* |
| Hemolysis index | NA | NA | NA | NA | 3 (2–4) | 3 (2–4) | 0 | 0.987 | 3 (2–4) | 0 | 0.992 | 3 (2–4) | 0 | 0.998 |
Results are presented as median (interquartile range).
Statistically significant difference (P < 0.05).
APTT - activated partial thromboplastin time; PT - prothrombin time; Fbg – fibrinogen; AT - antithrombin III; PC - protein C; PS - protein S; NA - not applicable; Tg – triglycerides; CVa - analytical coefficient of variation; CVw - within-subject biological variation; RCV - reference change value; DSI - desirable specification for imprecision derived from biologic variation.
Figure 1.
Lipemia interferograms for (A) APTT, (B) PT, (C) AT, (D) PC, (E) PS, (F) Fbg.
Hours after light meal (x-axis) are plotted against bias values (y-axis). Solid line – bias. Dashed lines - acceptable criteria based on desirable specification for imprecision (DSI) derived from biologic variation.
APTT - activated partial thromboplastin time; PT - prothrombin time; AT - antithrombin III; PC - protein C; PS - protein S; FbG - fibrinogen; DSI - desirable specification for imprecision derived from biologic variation.
Discussion
Our results have shown that a light meal is able to statistically modify the APTT and AT. Instrumentation Laboratory reagents manufacturer’s declaration notify that ACL TOP Family System are not affected by triglycerides up to: 11.3 mmol/L for both APTT and PT, 9.9 mmol/L for Fbg, 26.0 mmol/L for AT, 10.1 mmol/L for PC, and 17.0 mmol/L for PS. Furthermore, our results have shown that the influence of lipemia was seriously underestimated, meaning that the measured bias at declared triglycerides concentration was higher than reported by the manufacturer for APTT, PT, AT and PC. The concentration of triglycerides in our samples was lower than 2.0 mmol/L. Obviously the quality specifications derived from biological variation (17) are considered both very important and useful in the daily practice by the quality managers of the medical laboratories (16,18). Moreover, Nikolac et al. properly proposed that manufacturers should use evidence based quality specifications for assessing the allowable biases, instead of arbitrary limits (19). Because no coagulation test showed clinically significant variation after comparison with RCV, the light meal does not appear able to cause relevant changes on coagulation test results, also if the analytical bias was higher than specified. Thus, no consequences of inadequate fasting time observance are to be expected and no interference with the decisions of the caring physicians unaware of the real patient situation can ensue.
Presently many procedures are performed and/or oriented by non-laboratory professionals (e.g. nurses, non-technician personnel and administrative staff) (20). Moreover, poor control and standardization of the preanalytical phase in the whole testing process may influence the reliability of coagulation testing in the following points: i) phlebotomy procedure (21–23); ii) venous stasis (11,24): iii) vacuum tube used for blood collection by venipuncture (25). Instead, some procedures regarding the order of draw and mixing of primary blood tubes after collection by vacuum systems seem unimportant (26–29). In fact, the influence of light meal on coagulation testing was never evaluated before as reported in our protocol.
A limitation of our study design was the enrolment of a rather small number of apparently healthy volunteers. In any case, no substantial differences after a light meal were observed in this longitudinal study. Nevertheless, in order to increase the sensitivity of this finding, further research on individuals with blood clotting disorders might be needed to confirm the above outcomes.
Conclusion
It is clear that a light meal does not jeopardize the laboratory coagulation tests we assessed, but we strongly suggest that the laboratory quality managers standardize the fasting time for all blood tests at 12 hours, i.e. the fasting time necessary to completely metabolize the lipids intake. This kind of standardization could prevent possible laboratory variability that was not evaluated in our study (i.e. more caloric food intake as lunch or dinner) and unpredictable analytical bias.
Acknowledgments
This study was supported by CAPES Foundation, Ministry of Education of Brazil, Brasília - DF, Brazil, process 0175-14-5. We are grateful to Mrs. Cristina Recchi for her skilful technical support in performance all our coagulation testing.
Footnotes
Potential conflict of interest
None declared.
References
- 1.Lippi G, Guidi GC, Mattiuzzi C, Plebani M. Preanalytical variability: the dark side of the moon in laboratory testing. Clin Chem Lab Med. 2006;44:358–65. doi: 10.1515/CCLM.2006.073. http://dx.doi.org/10.1515/CCLM.2006.073. [DOI] [PubMed] [Google Scholar]
- 2.Green SF. The cost of poor blood specimen quality and errors in preanalytical processes. Clin Biochem. 2013;46:1175–9. doi: 10.1016/j.clinbiochem.2013.06.001. http://dx.doi.org/10.1016/j.clinbiochem.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Nikolac N, Supak-Smolcic V, Simundic AM, Celap I. Croatian Society of Medical Biochemistry and Laboratory Medicine: national recommendations for venous blood sampling. Biochem Med. 2013;23:242–54. doi: 10.11613/BM.2013.031. http://dx.doi.org/10.11613/BM.2013.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lippi G, Franchini M, Guidi GC. Diagnostic approach to inherited bleeding disorders. Clin Chem Lab Med. 2007;45:2–12. doi: 10.1515/CCLM.2007.006. http://dx.doi.org/10.1515/CCLM.2007.006. [DOI] [PubMed] [Google Scholar]
- 5.Clinical Laboratory Standards Institute . Procedures for the collection of diagnostic blood specimens by venipuncture. CLSI H3-A6 document. 6th ed. Wayne, PA: Clinical Laboratory Standards Institute; 2007. [Google Scholar]
- 6.Simundic AM, Cornes MP, Grankvist K, Lippi G, Nybo M. Standardization of collection requirements for fasting samples: For the Working Group on Preanalytical Phase (WGPA) of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) Clin Chim Acta. 2014;432:33–7. doi: 10.1016/j.cca.2013.11.008. http://dx.doi.org/10.1016/j.cca.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Lippi G, Lima-Oliveira G, Salvagno GL, Montagnana M, Gelati M, Picheth G, et al. Influence of a light meal on routine haematological tests. Blood Transfus. 2010;8:94–9. doi: 10.2450/2009.0142-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lima-Oliveira G, Salvagno GL, Lippi G, Gelati M, Montagnana M, Danese E, et al. Influence of a regular, standardized meal on clinical chemistry analytes. Ann Lab Med. 2012;32:250–6. doi: 10.3343/alm.2012.32.4.250. http://dx.doi.org/10.3343/alm.2012.32.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical Laboratory Standards Institute . Collection, Transport, and Processing of Blood Specimens for Testing Plasma-Based Coagulation Assays and Molecular Hemostasis Assays. CLSI H21-A5 document. 5th ed. Wayne, PA: Clinical Laboratory Standards Institute; 2008. [Google Scholar]
- 10.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. The effective reduction of tourniquet application time after minor modification of the CLSI H03-A6 blood collection procedure. Biochem Med. 2013;23:308–15. doi: 10.11613/BM.2013.037. http://dx.doi.org/10.11613/BM.2013.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lima-Oliveira G, Salvagno GL, Lippi G, Montagnana M, Scartezini M, Picheth G, et al. Elimination of the venous stasis error for routine coagulation testing by transillumination. Clin Chim Acta. 2011;412:1482–4. doi: 10.1016/j.cca.2011.04.008. http://dx.doi.org/10.1016/j.cca.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Guder WG, Narayanan S, Wisser H, Zawta B. Diagnostic samples: from the patient to the laboratory: the impact of preanalytical variables on the quality of laboratory results. 4 ed. Wiley-Blackwell; 2009. [Google Scholar]
- 13.Clinical Laboratory Standards Institute . Procedures for the handling and processing of blood specimens for common laboratory tests. CLSI H18-A4 document. 4th ed. Wayne, PA: Clinical Laboratory Standards Institute; 2010. [Google Scholar]
- 14.Roche Diagnostics . Roche Diagnostics; 2012. Cobas Link. 4.6.4.1039 ed. [Google Scholar]
- 15.Simundic AM. Practical recommendations for statistical analysis and data presentation in Biochemia Medica journal. Biochem Med. 2012;22:15–23. doi: 10.11613/bm.2012.003. http://dx.doi.org/10.11613/BM.2012.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westgard J. Desirable Biological Variation Database Specifications. 2014 Available at: http://www.westgard.com/biodatabase1.htm. Accessed February 6th, 2014.
- 17.Ricos C, Alvarez V, Cava F, Garcia-Lario JV, Hernandez A, Jimenez CV, et al. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest. 1999;59:491–500. doi: 10.1080/00365519950185229. http://dx.doi.org/10.1080/00365519950185229. [DOI] [PubMed] [Google Scholar]
- 18.Ricos C, Cava F, Garcia-Lario JV, Hernandez A, Iglesias N, Jimenez CV, et al. The reference change value: a proposal to interpret laboratory reports in serial testing based on biological variation. Scand J Clin Lab Invest. 2004;64:175–84. doi: 10.1080/00365510410004885. http://dx.doi.org/10.1080/00365510410004885. [DOI] [PubMed] [Google Scholar]
- 19.Nikolac N, Simundic AM, Miksa M, Lima-Oliveira G, Salvagno GL, Caruso B, et al. Heterogeneity of manufacturers’ declarations for lipemia interference - an urgent call for standardization. Clin Chim Acta. 2013;426:33–40. doi: 10.1016/j.cca.2013.08.015. http://dx.doi.org/10.1016/j.cca.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Lima-Oliveira G, Lippi G, Salvagno GL, Picheth G, Guidi GC. Laboratory diagnostics and quality of blood collection. J J Med Biochem. 2014 doi: 10.2478/jomb-2014-0043. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. Impact of the phlebotomy training based on CLSI/NCCLS H03-A6 - procedures for the collection of diagnostic blood specimens by venipuncture. Biochem Med. 2012;22:342–51. doi: 10.11613/bm.2012.036. http://dx.doi.org/10.11613/BM.2012.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. Incorrect order of draw could be mitigate the patient safety: a phlebotomy management case report. Biochem Med. 2013;23:218–23. doi: 10.11613/BM.2013.026. http://dx.doi.org/10.11613/BM.2013.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lippi G, Salvagno GL, Montagnana M, Lima-Oliveira G, Guidi GC, Favaloro EJ. Quality Standards for Sample Collection in Coagulation Testing. Semin Thromb He-most. 2012;38:565–75. doi: 10.1055/s-0032-1315961. http://dx.doi.org/10.1055/s-0032-1315961. [DOI] [PubMed] [Google Scholar]
- 24.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Mangueira C, Sumita N, et al. New ways to deal with known preanalytical issues: use of transilluminator instead of tourniquet for easing vein access and eliminating stasis on clinical biochemistry. Biochem Med. 2011;21:152–9. doi: 10.11613/bm.2011.024. http://dx.doi.org/10.11613/BM.2011.024. [DOI] [PubMed] [Google Scholar]
- 25.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. Sodium citrate vacuum tubes validation: preventing preanalytical variability in routine coagulation testing. Blood Coagul Fibrinolysis. 2013;24:252–5. doi: 10.1097/MBC.0b013e32835b72ea. http://dx.doi.org/10.1097/MBC.0b013e32835b72ea. [DOI] [PubMed] [Google Scholar]
- 26.Salvagno GL, Lima-Oliveira G, Brocco G, Danese E, Guidi GC, Lippi G. The order of draw: myth or science? Clin Chem Lab Med. 2013;51:2281–5. doi: 10.1515/cclm-2013-0412. http://dx.doi.org/10.1515/cclm-2013-0412. [DOI] [PubMed] [Google Scholar]
- 27.Parenmark A, Landberg E. To mix or not to mix venous blood samples collected in vacuum tubes? Clin Chem Lab Med. 2011;49:2061–3. doi: 10.1515/CCLM.2011.705. http://dx.doi.org/10.1515/CCLM.2011.705. [DOI] [PubMed] [Google Scholar]
- 28.Lima-Oliveira G, Lippi G, Salvagno GL, Brocco G, Gaino S, Dima F, et al. Processing of diagnostic blood specimens: Is it really necessary to mix primary blood tubes after collection with evacuated tube system? Biopreserv Biobank. 2014;12:53–9. doi: 10.1089/bio.2013.0043. http://dx.doi.org/10.1089/bio.2013.0043. [DOI] [PubMed] [Google Scholar]
- 29.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Gelati M, Volanski W, et al. Effects of vigoros mixing of blood vacuum tubes on laboratory test results. Clin Biochem. 2013;46:250–4. doi: 10.1016/j.clinbiochem.2012.10.033. http://dx.doi.org/10.1016/j.clinbiochem.2012.10.033. [DOI] [PubMed] [Google Scholar]