Abstract
Major and biologically most explored components of natural organic matter (NOM) are humic acid (HA) and fulvic acid (FA). We have explored rock shilajit as a source of NOM. On the other hand carbamazepine (CBZ) is a well known anticonvulsant drug and has a limited accessibility to brain. Bioavailability and pharmacokinetic profiles of CBZ have been improved by complexation and different techniques also.
Present study has assessed the comparative abilities of FA and HA as complexing agent for CBZ in order to enhance pharmacokinetic profile of CBZ and accessibility to the brain. These two complexing agents have been compared on various indices such as their abilities to cause complexation and enhance solubility, permeability and dissolution. The present study also compared pharmacodynamic and biochemical profiles after oral administration of complexes. With the help of various pharmaceutical techniques such as freeze drying, physical mixture, kneading and solvent evaporation, two molar ratios (1:1 and 1:2) were selected for complexation and evaluated for conformational analysis (molecular modeling). Complex formed was further characterized by differential scanning calorimetry (DSC), Fourier transform infrared spectroscopy (FT-IR), mass spectroscopy and X-ray diffraction (XRD).
Preclinical study on rodents with CBZ–HA and CBZ–FA has yielded appreciable results in terms of their anticonvulsant and antioxidants activities. However, CBZ–HA (1:2) demonstrated better result than any other complex.
Keywords: Shilajit, Fulvic acid, Humic acid, Carbamazepine and brain permeability
Graphical Abstract
Highlights
► Pharmaceutically less explored humic and fulvic acids have been investigated comparatively. ► Their effects on complexation, permeability, release and solubility are studied. ► Permeation, pharmacodynamics, biochemical estimations and pharmacokinetic studies were also evaluated. ► Results pointed out that these could be explored further as pharmaceutical excipients.
1. Introduction
Natural Organic Matters more often consist of humic substances (HS) and non-humic substances. Non-humic substances are all those materials that can be placed in one of the categories of discrete compounds such as sugars, amino acids, fats, etc. HSs are series of relatively high molecular weight, brown to black colored substances formed by secondary synthetic reactions. HS is mostly used as a generic name to describe colored material or its fractions obtained on the basis of solubility characteristics.
Shilajit is a rich source of HS extracted from rocks in many mountain ranges of the world especially the Himalayas and Hindukush of the Indian subcontinent [1]. It is a refreshing, revitalizing agent used in traditional systems of medicine of many countries including India. Intensive studies during the 1980s have highlighted its constituent components, which primarily comprised of humus (60–80% and including other components such as benzoic acid, hippuric acid, fatty acid, ichthyol, ellagic acid, resin, triterpenes, sterol, aromatic carboxylic acid, 3,4-benzocoumarins, amino acids and phenolic lipids). The presence of a bioactive compound such as dibenzo-alpha-pyrones along with humic acid (HA) and fulvic acid (FA) (Fig. 1B–C), acting as carrier molecules for the active ingredients, endows physiological properties to shilajit [2], [3]. HA (Avg. mol. wt. 6500) is dark brown to blackish in color, insoluble in water under acidic conditions but soluble at higher pH values. FA (Avg. mol. wt. 1200) is light yellowish in color, with higher percentage of' carboxylic groups than HA [4], [5], which makes it soluble in water at any pH value.
Fig. 1.
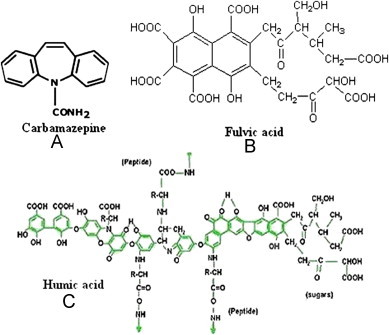
Molecular structures of (A) carbamazepine, (B) fulvic acid and (C) humic acid.
Carbamazepine (CBZ) is a well known anticonvulsant agent, used in the treatment of simple and complex seizures, trigeminal neuralgia and bipolar affective disorder [6] and is a drug of choice (Fig. 1A) for simple and complex partial seizures. CBZ is practically insoluble in water (<200 μg/ml) and lies in class II of Biopharmaceutical Classification System [7] and has a dissolution rate-limited absorption [8]. It is obtained in four polymorphic forms, which contribute to its variable absorption profile. Low aqueous solubility and poor wettability of the drug further contributes to its unpredicted absorption, which in turn gives inconsistent bioavailability [9]. CBZ is commonly available in tablet dosage forms that give peak plasma concentrations during 4–32 h. Thus there are plenty of opportunities to further improve the dissolution characteristics of CBZ, which may increase the rate and the extent of absorption and consequently enhance the therapeutic efficacy. Dissolution characteristics and oral bioavailability may be enhanced by complexing it with a suitable complexing agent. Thus, the current study attempts to assess the comparative abilities of FA and HA as complexing agent in order to enhance the pharmacokinetic profile of CBZ and to enhance the accessibility to brain.
2. Materials and methods
Rock shilajit was obtained and authenticated from Dabur Research Foundation, Ghaziabad, India. Carbamazepine was procured as a gift sample from Novartis Pharmaceuticals Ltd., India. Chemicals and reagents used for the study were of A.R. grade.
2.1. Method for obtaining fulvic and humic acids from rock shilajit
A slightly modified method that has been previously described by Ghosal [2] was used to extract FA and HA. The method consisted of successive extraction of rock and powdered shilajit with hot organic solvents of increasing polarity to remove the bioactive components. The residue (marc) was dissolved in 0.1 N NaOH [10] with intermittent shaking in the presence of nitrogen. The suspension was filtered and the filtrate was acidified to a pH of less than 3 to precipitate out the humic acids. The filtrate was further shaken with macro-porous ion exchange resin in order to absorb the FAs, which were then eluted using 0.1 N aqueous sodium hydroxide solutions.
2.2. Phase solubility behavior and development of complexes
Phase solubility studies were carried out at room temperature (25°) in triplicate according to the method reported by Higuchi and Conners [11]. Excess amount of carbamazepine was added to distilled water containing various concentrations (0.2–2% w/v) of complexing agents (FA and HA) in a series of stopper conical flasks (100 ml) and shaken for 48 h on a rotary flask shaker. The suspensions were passed through membrane filter (0.45 μm) and analyzed for carbamazepine using a UV spectroscopy (Shimadzu, UV 1601) at 285 nm against blanks prepared using the same concentration of HA/FA in distilled water.
2.3. Preparation of the inclusion complexes
Complexes of carbamazepine were prepared with fulvic acid and humic acid, extracted from shilajit using different techniques in two different molar ratios 1:1 and 1:2 (drug:complexing agent). The resulting mass was powdered in a glass mortar and pestle and passed through a 100-mesh sieve to obtain a uniformly sized fine powder [12]. Equal amount of drug also underwent the same process in order to check the process effect.
2.3.1. Physical mixture (PM)
Complexes of carbamazepine were prepared by grinding the mixture for 60 min in a clean dry glass pestle and mortar and the resulting mass was passed through a 100-mesh sieve to obtain a uniformly sized fine powder [12].
2.3.2. Freeze drying (FD)
Weighed amount of carbamazepine was dissolved in water using co-solvency (ethanol) and also aqueous solutions of complexing agents were prepared. Both the solutions were mixed and stirred (200 rpm, 60 min) and then sonicated for an hour. The solution was frozen for 24 h in a Lyph-lock apparatus and then freeze dried (Dry winner, DW-8-85 Heto Holten, Denmark) for 12 h. Sucrose solution was (2% w/v) added as a cryoprotectant. The resulting mass was then powdered in a glass mortar and pestle and passed through a 100-mesh sieve to obtain the uniformly sized fine powder [12].
2.3.3. Solvent evaporation (SE)
Calculated amount of drug was dissolved in water with the help of few drops of ethanol and poured into aqueous solution of HA/FA. The solution was then sonicated for an hour. The obtained solution was dried in a rotary evaporator under vacuum (Hahn shin science Co., Hs-2001N, South Korea) and passed through a 100-mesh sieve to obtain the uniformly size fine powder [12].
2.3.4. Kneading (KD)
Solid complexes of carbamazepine were also prepared by the kneading method [12]. Weighed amount of carbamazepine and HA/FA was triturated for 15 min in a clean dry glass pestle and mortar. During the trituration process, ethanol was added empirically to adjust the consistency of the paste. Trituration was continued until the product started drying on the walls of mortar. The products were further dried in the hot air oven at 60 °C for 30 min, powdered, passed through a 100-mesh sieve and stored in desiccators [13].
2.4. Characterization of the solid complexes
2.4.1. Differential Scanning Calorimetry (DSC)
For Differential Scanning Calorimetry (DSC) study, samples of the solid complex, pure drug and FA/HA (10 mg) were taken in a flat-bottomed aluminum pan and heated over a temperature range of 30–350 °C at a constant rate of 10 °C/min with purging of nitrogen (50 ml/min) using alumina as a reference standard in a differential scanning calorimeter (DSC-7, Perkin Elmer Pyris 6 instrument, USA).
2.4.2. Fourier Transforms Infrared Spectroscopy (FT-IR)
The FT-IR spectra of carbamazepine, FA/HA and inclusion complexes were recorded on the Perkin Elmer using the potassium bromide (KBr) disk technique. Five mg of previously dried sample was mixed with 100 mg KBr and compressed into a pellet on an IR hydraulic press. Base line was corrected and scanning was performed at 4000–400 cm−1.
2.4.3. Powder X-ray diffraction (XRD)
X-ray diffraction of carbamazepine, FA/HA and their inclusion complexes was studied using an X-ray diffractometer (PW 1830, Phillips, Japan). The samples (1000 mg) were rotated during data collection to reduce orientation effects. PXRD patterns of solid complex, pure drug and FA were recorded between 2θ=10° and 70° at 35 kV and 30 mA.
2.4.4. Mass spectroscopy
The samples (HA and FA) were dissolved in Milli-Q water to make a stock of 1 mg/mL and then further dilution was prepared in Milli-Q water:methanol (50:50). Finally a concentration of 100 ng/mL was prepared and injected into the mass (Snapt Mass Spectrometry, Q-TOF with UPLC) on electrospray ionization with positive mode. Capillary, sampling cone and extraction voltages were 2.51, 21 and 5.3 units, respectively. Source and desolvation temperatures were 80 and 250 °C, respectively. Nitrogen gas was used as cone and desolvation gas at 50 and 600 L/h, respectively. Trap collision energy was used (6.0 units). The system was from Waters bearing serial no. JAA 272 waters, USA. Software was used MassLynx V 4.1 waters.
2.5. Conformational analysis by computational method
3D molecular structures were generated and optimized with Chem 3D-Ultra 8.0 software. While all calculations used are for geometric optimization, all the energy minimizations were carried out till the RMS gradient is less than 0.08. Optimized molecular structures and partial atomic charges were used for the molecular modeling in humic and fulvic acids. H-bonding analysis was based on ORTEP III [v1.0.3].
2.6. HPLC analysis of carbamazepine in vitro
The concentration of carbamazepine in vitro samples was determined by validated method [14] using a Shimadzu LC2010 system (Kyoto, Japan) consisting of quaternary LC-10A VP pump, SPD-10AVP column oven, variable wavelength programmable UV/VIS detector (285 nm), SCL 10AVP system controller, Rheodyne injector fitted with a 20 μL loop, degasser and a data processor. Chromatographic separation was achieved using a LiChrospher®100 reversed-phase C-18 column (250×4.6 mm2), which was packed with 5 μm particles with a mobile phase consisting of water and acetonitrile (60:40::water:acetonitrile). The mobile phase was pumped at a flow rate of 1.0 ml/min at an ambient temperature (25±2 °C). The eluent was monitored by ultraviolet absorbance at a wavelength of 285 nm with retention times for CBZ being 4.8±0.35 min.
2.7. Determination of carbamazepine in complexes (at equilibrium in water)
Excess amount of complex was kept in amber colored bottles containing 10 ml of distilled water and stirred on thermostated mechanical shaker (Grower enterprises, New Delhi, India) at 25 °C for 5 days. Suspensions were filtered through a 0.22 μm “Millipore” filter, adequately diluted with distilled water and analyzed by reported HPLC at λ=285 nm [14].
2.8. Release of carbamazepine from complex
Drug release study of API (40 mg CBZ solution) and inclusion complexes (equivalent to 40 mg CBZ) was performed using USP II dissolution apparatus (Hanson Research SRS , USA) in 900 mL of distilled water at 37.5±0.5 °C (75 rpm, 60 min). The study was carried out by putting the constituted suspension (5 ml) in dialysis bag (Spectra-Por dialysis bag, Sigma Aldrich, St. Louis, MO with cutoff 12,000–14,000 Da). The concentration of the drug in solution at various time intervals was analyzed by HPLC at 285 nm. All dissolution studies were carried out in triplicate. The amount of carbamazepine was chosen as per the recommended dose of the drug [15]. The amount of humic substances present in a dose of suspension was also within the permissible limit, i.e. 512 mg kg−1 body weight [16].
2.9. In vitro everted intestinal sac permeation study
Rats were anesthetized by ether sprinkled onto a piece of cotton wool in a glass container equipped with a lid. After making a midline incision in the abdomen, the small intestine was cut at two positions: at about 18 cm distal to the stomach and at about 30 cm (being the medial jejunum). This segment was then removed and ligated with silk thread to one end of a glass rod and carefully everted on the rod, rinsed with saline solution and then cut and secured to the tip of a 1 ml disposable syringe barrel. The gut sac was filled with the modified KRPB buffer solution and was then placed inside the bath containing 100 ml of test solution continuously bubbled (95% O2 and 5% CO2) [17]. After stabilization 3 ml (equivalent to about 10 mg drug) CBZ (API), 1:2 freeze dried (HA and FA complexes) and 1:2 kneading (HA and FA complexes) complex solution were added into the sac. The tubes were maintained at 37 °C and shaken continuously at 60 rpm with bubbling oxygen supply. 100 μl of samples was withdrawn at an interval of 0, 0.25, 0.5, 1, 2, 4, 8, 12, 24 h from the dissolution medium and centrifuged at 4000 rpm for 5 min. After filtering through Millipore filter (0.45 μm) these were analyzed by HPLC [14].
2.10. Maximal Electro Seizure (MES) induced convulsion
Swiss albino mice with the average body weight (20–30 g) of either sex were used for the experiment. Animals were reared in the Central Animal House for 2 weeks in polypropylene cages and fed on standard animal feed and water. Dose of CBZ was taken as per the literature, i.e. 30 mg/kg body weight of mice, which gives 100% protection to animal in MES [18], accordingly dose of complex was chosen as a fraction of dose of CBZ (i.e. 1/3rd), as it was evident from the initial experimentations that complexes were showing 2–3 times better potency than the API alone. Amount of FA and HA present in dose was also taken to check the antiepileptic potential of the complexing agent. The animals were divided into eight groups, i.e. control, CBZ pure, HA, FA, HA–CBZ complex (1:2 freeze dried, 1:2 kneading) and FA–CBZ complex (1:2 freeze dried, 1:2 kneading) with 6 animals, each with an average group weight of 25 g. The control and the different dosages of complexes were given 30 min before the induction of MES to separate group of mice. Then, the stimulus train was applied via ear-clip electrode (50 mA, 0.2 s, average voltage 200–250 V) through electroconvulsiometer (Techno India). The incidence and the duration of extensor tonus were noted. The duration of seizures (tonic-clonic convulsions) was recorded [19]. Solutions (q.s to 5 ml) of drug and complexes were prepared in glycerin. 0.2 ml of these solutions was given orally to the mice. All animal experiments were carried out in accordance with Jamia Hamdard Animal Ethics Committee.
2.11. Ageing study
Freeze dried complexes of humic acid (1:1 and 1:2) and fulvic acid (1:1 and 1:2) were stored for 6 months in hermitically sealed containers at room temperature. They were analyzed by TLC for any ageing affect [20] and compared with fresh samples. Previously reported [21] mobile phase, ethyl acetate–toluene–methanol::7.0:2.0:1.0 (v/v) was used for the development of chromatogram. Precoated silica gel linchosphere aluminum sheets 60 F254 (20 cm×10 cm: 200 μm thickness, E. Merck, Darmstadt, Germany) were used.
2.12. Picrotoxin induced oxidative stress
2.12.1. Thiobarbituric acid reactive substances (TBARs)
1 ml of suspension medium was taken from the 10% tissue homogenate. 0.5 ml of 30% TCA was added to it, followed by 0.5 ml of 0.8% TBA reagent. The tubes were then be covered with aluminum foil and kept in shaking water bath for 30 min at 80 °C. After 30 min tubes were taken out and kept in ice-cold water for 30 min. Then these were centrifuged at 3000 rpm for 15 min.
The absorbance of the supernatant was read at 540 nm at room temperature against appropriate blank. Blank consists of 1 ml distilled water, 0.5 ml of 30% TCA and 0.5 ml of 0.8% TBA. The concentration of MDA will be read from standard curve prepared using TEP [22].
2.12.2. Tissue glutathione
A 10% tissue homogenate of brain was prepared in 0.02 M EDTA and 4 ml of cold distilled water was added to it. It was mixed well with intermittent shaking for 10 min using vortex mixer and the contents will then be transferred to centrifuge tubes (rinsed in EDTA) and centrifuged at 6000 rpm for 15 min. After that, 2 ml of supernatant was mixed well with 4 ml of tris buffer (0.4 M, pH 8.9) and 0.1 ml of 0.01 M DTNB was added to it. The absorbance was read within 5 min of the addition of DTNB at 410 nm against a reagent blank with no homogenate [23].
2.13. Pharmacokinetic study
A total of nine male wistar rats (250–300 g) were taken. Animals were reared in the Central Animal House in polypropylene cages and fed on standard animal feed and water. Before oral dosing with CBZ, rats were fasted overnight. A dose equivalent to 5 mg/kg body weight of CBZ was given to rats after dispersing the API and complexes in glycerin. 0.25 ml of blood sample was collected from retro-orbital tract at each time point and placed in a vial containing heparin and kept refrigerated until processing. Time points selected for sampling were 0.25, 0.5, 0.75,1, 2, 4, 8, 12, 16 and 24 h.
The LC/MS/MS system for blood plasma analysis of carbamazepine and its main metabolite carbamazepine 10, 11-epoxide in rat, described by [24], was used. The method consists of a liquid–liquid extraction procedure and electrospray UPLC/MS/MS analysis. The chromatographic separation was achieved within 5 min using an Acquity UPLC BEH C8(2.1×100, 1.7 μm) column with a mobile phase composed of Milli-Q water/acetonitrile/acetic acid (69.5:30:0.5, v/v/v) at a flow rate of 0.25 ml/min. d10-Carbamazepine was used as the internal standard for all compounds. Analytes were determined by electrospray ionization synapt mass spectrometry in the positive ion mode. Carbamazepine was monitored by scanning m/z 237→194, carbamazepine 10, 11-epoxide by m/z 253→210 and d10-carbamazepine by m/z 247→204. The lower limit of quantification (LLOQ) was 5 ng/ml for each analyte based on 0.1 ml aliquots of rat plasma. The extraction recovery of analytes from rat plasma was over 84.9%. Intra-day and inter-day assay coefficients of variations were in the range of 3.6–4.5 and 2.0–3.6%, respectively. Linearity was observed over the range of 5–2000 ng/ml.
3. Results and discussion
3.1. Characterization of solid complexes
The phase solubility studies revealed a nonlinear relationship (Fig. 2) for aqueous drug solubility with increasing concentration of HS. The curves were characteristic AN type (according to Higuchi and Connors) with different r2 values. It was 0.864 for HA–CBZ and 0.916 for FA–CBZ. In both the cases up to the concentration of 1% w/v of CBZ the relationship was linear but nonlinear afterwards and more HSs were consumed for the complexation of CBZ as compared to initial regions. Thus, molar ratios we opted for complexation were 1:1 and 1:2 for both the complexing agents (HA and FA).
Fig. 2.
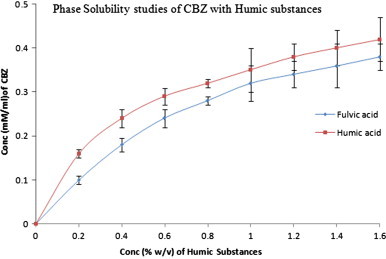
Phase solubility studies of CBZ with HA and FA at room temperature (25 °C) in triplicate mode.
Another finding we could conclude from the data is that at higher concentrations of HS (1–2% w/v), solubility of CBZ exhibits much variable solubility as the deviations are much noticeable. Comparing both the complexing agents, HA was showing better interaction as it was more inclined towards the Y-axis. Better binding capacity of HA is evident from literature also as it shows several folds higher binding [25] to model chemicals. Existence of some other mechanism other than inclusion is also evident from the data obtained. High molecular weight and basic hydrophobicity of humic acids also favor the formation of “micelle”-like structures [5], [26] with hydrophilic groups on the water side and the hydrophobic nucleus useful to give superficial adsorption and, further, inner absorption of organic moieties [27].
The phase solubility graph was also used to find out the binding constant and Gibb's free energy [28]. The binding constant was calculated according to the formula Ks=[slope/S0(1−slope)], where S0 is the solubility of carbamazepine without humic substances. To check out the spontaneity and the feasibility of the entrapment by thermodynamic approach, changes in Gibb's free energy (ΔG) were calculated (at constant temperature and pressure). It is the net energy available for useful work. ΔG=−2.303RT log[S0/Ss], where, Ss and S0 are the solubility of the drug in the presence and absence of humic substances. As the ΔG0 becomes more negative the reaction becomes more feasible. In the present case physical phenomenon (inclusion of CBZ into HS) is assumed to be a process and being evaluated. The binding constants were found to be 5503.73 (M−1) for HA–CBZ complex and 5410.44 (M−1) for FA–CBZ complex. Similarly, ΔG0s for different complexes are shown in Table 1.
Table 1.
Solubility and thermodynamic data of CBZ and complexes in water (pH 7) at room temperature (25 °C).
| Complexes | Solubility of CBZ in FA complexes (μg/ml) | % Increase in solubility of CBZ in FA complexes | −ΔG0(cal) for FA–CBZ complex formation in water | −ΔH(J/g) of CBZ lattice as it form FA complexes | −ΔS (J mol−1 K−1) of CBZ lattice as it form FA complexes | Solubility of CBZ in HA complex (μg/ml) | % Increase in solubility of CBZ in HA complexes | −ΔG0(cal) for HA–CBZ complex formation in water | ΔH(J/g) of CBZ lattice as it form HA complexes | −ΔS(J mol−1 K−1) of CBZ lattice as it form FA complexes |
|---|---|---|---|---|---|---|---|---|---|---|
| 1:1 PM | 94.66±9.63 | 648.30±16.19 | 1191.94 | 145.22 | 98.49 | 76.45±1.21 | 504.348±15.38 | 1065.40 | 148.21 | 102.65 |
| 1:2 PM | 102.47±10.21 | 710.03±2.17 | 1238.89 | 144.29 | 97.09 | 84.4±2.17 | 567.1937±16.19 | 1123.99 | 145.51 | 99.69 |
| 1:1 SE | 119.32±4.19 | 843.24±15.38 | 1329.05 | 145.95 | 97.14 | 141.36±5.21 | 1017.47±21.13 | 1429.43 | 148.83 | 97.71 |
| 1:2 SE | 137.36±19.93 | 985.84±19.73 | 1412.43 | 144 | 94.42 | 157.89±13.13 | 1148.142±21.19 | 1494.93 | 147.05 | 95.68 |
| 1:1 KD | 156.65±5.01 | 1138.34±21.13 | 1490.26 | 111.85 | 67.80 | 123.05±9.63 | 872.7273±12.59 | 1347.28 | 117.21 | 74.07 |
| 1:2 KD | 187.33±13.23 | 1380.67±21.19 | 1596.18 | 104.73 | 60.65 | 135.6±4.19 | 971.9368±18.93 | 1404.80 | 111.33 | 68.59 |
| 1:1 FD | 198.15±29.73 | 1466.43±14.2 | 1629.44 | 107.54 | 62.41 | 176.72±2.02 | 1296.996±14.2 | 1561.65 | 104.33 | 60.82 |
| 1:2 FD | 299.65±1.35 | 2268.75±29.73 | 1874.38 | 107.22 | 58.71 | 233.12±1.35 | 1742.846±29.73 | 1725.69 | 102.34 | 56.93 |
ΔG0 is the standard Gibb's free energy expressed in terms of calorie. ΔH is the change in enthalpy, expressed in terms of J/g. ΔS is the change in entropy as the complex develops. All the experiments were carried out in triplicate.
From the XRD analysis and negative tendencies of ΔG, it was evident that the disorder of the system was found to decrease as the CBZ got entrapped into humic substances. To find out the values of change in disorder, entropy is calculated according to the formula (ΔG=ΔH−TΔS). Here, ΔH is the change in enthalpy as the CBZ gets into the macromolecule (HA and FA). It was calculated using van't Hoff equation. All the thermodynamic parameters are given in Table 1. From the negative values of ΔG, ΔH and ΔS we can say that this physical phenomenon (inclusion of CBZ into humic substances) is spontaneous at low temperature but nonspontaneous at high temperature.
3.1.1. Differential Scanning Calorimetry (DSC)
DSC of pure carbamazepine shows a sharp exothermic peak at 189 °C (Fig. 3) and ΔH of about 156.45 J/g, which is in accordance with the melting point reported in literature [29]. Fulvic acid shows blunt endotherm and exotherm in the region of 100–200 °C while humic acid shows blunt endotherm and exotherm in the region of 100–340 °C [30].
Fig. 3.
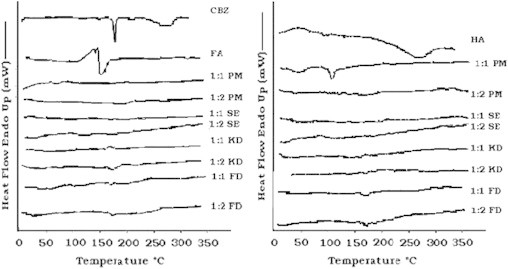
Representative differential scanning calorimetry profiles of CBZ, HA, FA and different complexes.
In DSC analysis all the methods employed showed the development of complexes, except physical mixture (1:1 HA–CBZ complex). Disappearance or shifting of peaks of drug was a strong indication of the formation of complex. In the method of physical mixture there was not any solution medium that could help drug molecule get complexed with HS. But it was rigorous trituration that would have helped in complexation since interaction with different functional groups to develop complexes has been established [31]. So, an exotherm was observed in the 1:1 HA–CBZ complex. Comparing the ratios, 1:2 was more promising as the thermogram was more linear. This observation was in accordance with the data obtained from the phase solubility studies (existence of different mechanisms at higher concentration of complexing agent). Between different complexing agents, there were no significant differences observed by DSC analysis.
3.1.2. Fourier Transforms Infrared Spectroscopy
The FT-IR spectrum shows characteristics peaks of carbamazepine (Fig. 4A) peaks at 1752 cm−1 (C O stretching), 3460 cm−1 (N—H vibration) and 1550 cm−1 (C C stretching of phenyl) [29]. FT-IR absorption bands of fulvic acid and humic acid (4A) extracted from shilajit were found in accordance with those reported in literature [32]. Interactions of carbonyl peak of carbamazepine with the carboxylic group of HS, stretching vibration of N—H (3460 cm−1) of CBZ with O—H vibration, were observed. Olefinic and carbonyl peaks of the drug are widespread and dispersed indicating weak interaction with similar bands in the complexing agent (Fig. 4B and C). Peaks of the fingerprint regions (1300–400 cm−1) are more diminished, indicating greater interaction between the drug and the complexing agent.
Fig. 4.
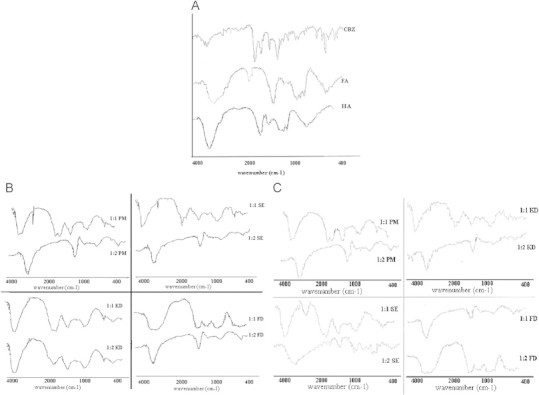
(A) FT-IR spectra of CBZ, HA and FA. (B) FT-IR spectra of different HA–CBZ complexes. (C) FT-IR spectra of different FA–CBZ complexes.
In FT-IR spectra, the interactions were less prominent in complexes prepared by physical mixture and solvent evaporated, indicating lesser degree of complexometric interaction. On the other hand freeze dried and kneading complexes of both the types were exhibiting better interaction. Comparing the different ratios, 1:2 ratio complexes were exhibiting more dispersed spectra and greater degree of interaction; likewise HA complexes were appearing more promising than FA complexes.
3.1.3. Powder X-ray diffraction
XRD of carbamazepine shows various peaks at different angles with most intense peaks at 15.41° (100%) followed by 13.18° (83%), 27.84° (66%), 27.32° (60%) and 27.66° (56%) (Fig. 5), revealing the crystalline nature of carbamazepine. The X-ray diffraction patterns of fulvic acid show peaks at angles 39° (90%) and 47° (100%) but it was more amorphous in nature. Similarly HA also showed amorphous nature in XRD diffractograms.
Fig. 5.
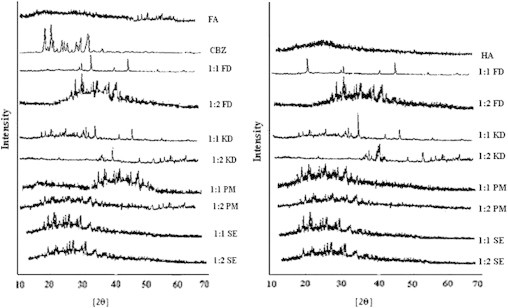
X-ray powder diffraction patterns of drug, HA, FA and different complexes.
In XRD studies, little crystallinity was observed in all the complexes but it was very less in freeze dried and kneading complexes for both the complexing agents. Similarity between different process was also observed when compared different HSs (HA and FA). Comparing the different ratios, 1:2 ratio turns out to be a better option since in the better performing processes (kneading and freeze drying) it was showing lesser crystallinity and good inclusion of drug molecule.
3.1.4. Mass spectroscopy
Results of mass spectrometric analysis also show a satisfactory result (Fig. 6A and B). Significant amount of noises was present in the spectra that were expected considering the macromolecular and polyionic nature of the molecules. But spectra from both types of complexes were funneling some common conclusions. Spectra were showing peaks of un-complexed drug (m/z∼ 237) at around 40% of relative intensity. Similarly, unused complexing agents were also observed (m/z∼ 2406.13 for humic acid and 1220.52 for fulvic acid). Most intense peaks in both the spectra were around 1:2 complexing ratios. 1:1 ratio was also observed in both the spectra with appreciable relative intensity. Thus it seems from the study that all the complexing agents were not consumed in the complexation and both 1:1 and 1:2 ratios were generated from the study. Positive results from other studies indicate that unconsumed macromolecules were imparting their positive effects in some other way.
Fig. 6.
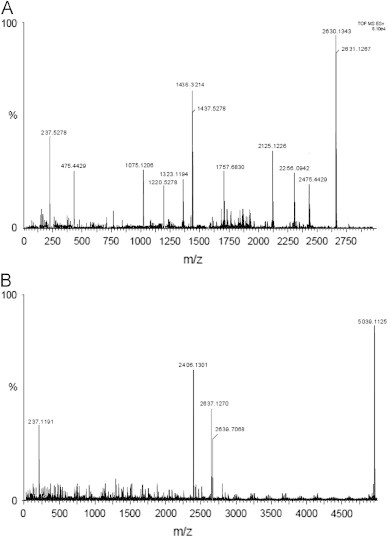
(A) Mass spectra of fulvic acid–CBZ complex. (B) Representative mass spectra of humic acid–CBZ complex.
3.2. Conformational analysis by computational method
Molecular modeling has shown that complexes of CBZ–HA and CBZ–FA are stable. The differences in energy of stabilization between the two complexes are marginal. It revealed that humic/fulvic acids have the ability for complexation with carbamazepine. Intermolecular hydrogen bonds observed contribute to the stability of the molecule. In the case of carbamazepine as shown in Fig. 7, amide hydrogen is oriented away from the carbonyl group but is approaching towards one of the aromatic moieties. Fig. 8 shows the energy minimized structure of fulvic acid. This structure shows at least five intramolecular H-bonds. Three out of five intramolecular H-bonds are OH…O type, which means that these are strong H-bonds. These hydrogen bonds are supposed to increase the stability of the molecule, while a drug complex optimization with fulvic acid shows that the carbamazepine is stabilized by a strong NH…N interaction with fulvic acid (Fig. 9).
Fig. 7.
Energy minimized structure of carbamazepine.
Fig. 8.
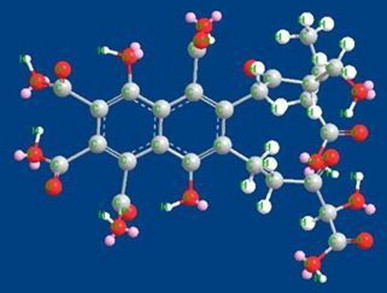
Energy optimized structure of fulvic acid.
Fig. 9.
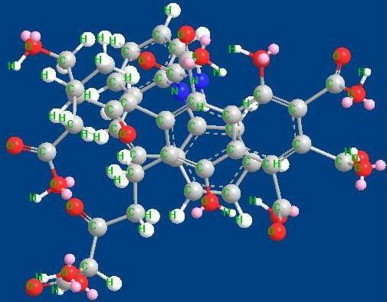
Carbamazepine complexed with fulvic acid.
Although the exact structure of humic acid is not yet characterized, a probable structure is modeled in this study. Total potential energies of the humic acid and the drug molecule are compared. Total potential energy of the humic acid using Chem 3D-Ultra 8.0 software comes to around −45.896 kcal/mol (Fig. 10) while a complex of carbamazepine and humic acid is stabilized at −22.584 (Fig. 11), which is more stable as CBZ alone. Energy optimization of carbamazepine resulted in −6.84 kcal/mol. In this complex H of amide of CBZ has an H-bond with OH of HA.
Fig. 10.
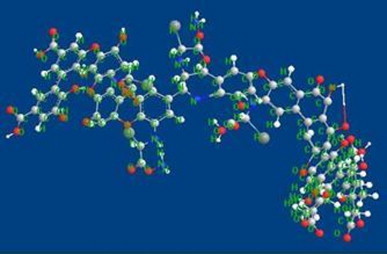
Energy minimized model of humic acid model.
Fig. 11.
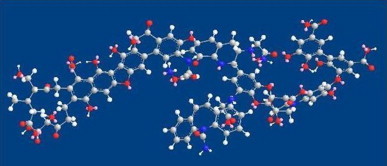
Carbamazepine complexed with humic acid.
3.3. Determination of carbamazepine in complexes (at equilibrium in water)
Carbamazepine is practically insoluble in water, aqueous saturation solubility of carbamazepine was found to be 12.65 μg/ml. Complexation of carbamazepine greatly increased the solubility (Table 1). Again by all the methods, freeze drying turns out to be a better technique and 1:2 ratio a better option. It was assumed that size of the complexes was less than 0.22 μm as it was the size of “Millipore” filter.
Better performance of 1:2 ratios may be credited to the development of CMC in the aqueous media. Because in a similar study [33], CMC of humic acid like substances was found to form micelles at a concentration of 2 g/L. This work also reports the amount of drug solubilized by per gram of HA like substances, which is in accordance with our findings. Here fulvic acid appears to leave a more pronounced effect on the solubility of the drug. Humic substances offer both types of interaction like metal ion interaction due to the presence of various functional groups and inclusion of hydrophobic moieties [34], [35]. Regarding the different binding capacities of HA in comparison with FA, it is evident from literature that HA binds the model chemicals more than FA. There is roughly a tenfold decrease moving from humic acid samples to fulvic acid samples [25]. But our finding regarding the increased solubility of a hydrophobic substance shows a different result. The reason may be the fact that the sorption/complexation of humic substances is not its intrinsic property; it generally depends on pH values [36], [37] and on the presence of other ions [38]. As on different pH ranges these behave differently.
3.4. Release of carbamazepine from complex
The release profiles of pure carbamazepine and complexes, prepared by different methods studied for 60 min, are shown in Fig. 12A and B. Active pharmaceutical ingredients have an intrinsic dissolution rate that is dependent on its solubility and particle size [39], which was showing 34% in 60 min and attaining plateau then after. Among all the methods freeze drying and kneading were performing the best release (∼80%). Maintaining the results of previous findings 1:2 ratio bestowed better. Release profile of humic acid complex was little better than that of fulvic acid. Among the different techniques employed the physical mixture method appears to be complexing the least. We could conclude that better complexing interaction resulted in higher aqueous concentration in a given time period. The result corroborates the data obtained from solubility analysis and different instrumental analyses (Figs. 3–6).
Fig. 12.
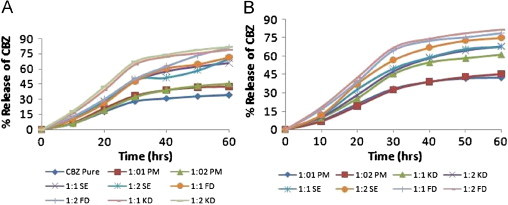
(A) Release profile of carbamazepine and FA complexes. (B) Release profile of HA complexes.
3.5. Comparison of anticonvulsant activity
From all the previous mentioned studies it was very much obvious that the complexes developed by kneading and freeze-drying methods showed promising results. So, these were chosen for further pharmacodynamic study (Table 2). Study of MES activity showed that due to complexation there was about three-fold increase in potency of carbamazepine when compared to pure drug. All the complexes have shown 75% inhibition to electroshock.
Table 2.
Comparative MES activity of optimized complexes in swiss albino mice.
| Substance | Dose | % Inhibition |
|---|---|---|
| Control | – | 0 |
| CBZ | 30 mg/kg body wt. | 100 |
| FA | 102 mg/kg body wt. | 0 |
| HA | 550 mg/kg body wt. | 0 |
| 1:2 KD (HA–CBZ) | Eq. to 10 mg/kg body wt. of CBZ | 75 |
| 1:2 KD (FA–CBZ) | Eq. to 10 mg/kg body wt. of CBZ | 75 |
| 1:2 FD (HA–CBZ) | Eq. to 10 mg/kg body wt. of CBZ | 75 |
| 1:2 FD (FA–CBZ) | Eq. to 10 mg/kg body wt. of CBZ | 75 |
Control was given only 0.2 ml of glycerin. HA and FA were dispersed in the same volume of glycerin.
The data obtained in this study are in accordance with previous findings and permeation study. FA and HA were also given to mice to check the antiepileptic potential and found zero inhibition. Thus, our optimized complexes were exhibiting better performance in crossing blood brain barrier (BBB), which may be attributed to increased solubility, passive diffusion gradient and lesser ionic character. Formation of aggregates in humic material is also well known [40], which may lead to increased local concentration of drug.
3.6. Permeation study across rat gut sac
The permeability of optimized complexes across gut sac was significantly increased (∼2.9–3.8 times) as compared to carbamazepine suspension in water in 24 h (Fig. 13). The permeation profile of complex shows two patterns, i.e. in initial 10 h there was a sharp increase in permeation but after that a plateau was observed.
Fig. 13.
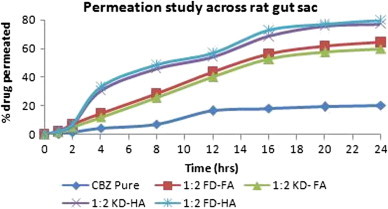
Permeation study of carbamazepine and optimized complexes.
Considering the permeation of complexes across the intestinal membrane, two opposing forces (concentration gradient and aggregation of humic substances) act against each other. The one that predominates influences the result. Initially, permeation increased steeply because there was an increasing concentration gradient across the sac but after sometime (10 h) it attains plateau, as the gradient falls. In spite of having larger size, HA was showing a better permeability in both the methods of complexation because of its structure. In aqueous media HA is less charged [41] and more hydrophobic [31], which aids in its permeation across intestinal mucosa.
3.7. Ageing study
After ageing freeze dried complexes of HA–CBZ complex (1:1 and 1:2) and FA–CBZ complex (1:1 and 1:2) showed only single spots. But the position of spots was variable, which may be due to different polarities [25] of complexing agents (HA and FA). Pure CBZ showed the Rf value of 0.5 while the average Rf values for fulvic acid complexes (1:1 and 1:2) were around 0.6. Average Rf values for humic acid complexes (1:1 and 1:2) were around 0.45. This study indicates the stability of developed complexes during the study.
3.8. Biochemical estimations of brain tissues
3.8.1. Thiobarbituric acid reactive substances estimation
TBARS levels were significantly elevated in PTX-treated group (1.13±0.064 vs. 4.49±0.14) (p<0.01). TBARS levels were also significantly elevated in the CBZ treated group. TBARS levels were significantly decreased to the normal in all the groups treated with the carbamazepine complexes (groups 4–7). {F (8, 45)=245.21}. Among the entire complexed groups the TBARS levels were effectively normalized with the CBZ–HA (1:2 KD) treated group (Table 3).
Table 3.
Picrotoxin induced oxidative stress studies of the developed complexes and CBZ.
| Group treatment | TBARS (μmol MDA/mg protein) | GSH (μmol/mg protein) |
|---|---|---|
| 1. Normal saline (NS, 10 ml/kg, i.p.) | 1.13±0.064** | 97.29±1.99** |
| 2. PTX (3.5 mg/kg, s.c) | 4.49±0.14†† | 29.24±1.12†† |
| 3. CBZ (30 mg/kg, p.o) | 2.1±0.06** | 90.96±1.59 |
| 4. CBZ–HA (1:2)–KD (30 mg/kg, p.o.) | 1.17±0.05** | 108.96±1.86**†† |
| 5. CBZ–FA (1:2)–KD (30 mg/kg, p.o.) | 1.36±0.03** | 99.79±1.74** |
| 6. CBZ–HA (1:2)–FD (30 mg/kg, p.o.) | 1.52±0.05†† | 97.80±2.26** |
| 7. CBZ–FA (1:2)–FD (30 mg/kg, p.o.) | 1.62±0.04†† | 94.96±1.34** |
| 8. HA per se (640 mg/kg body wt.) | 1.12±0.05 | 101.63±1.40 |
| 9. FA per se (335 mg/kg body wt.) | 1.15±0.07 | 99.79±1.30 |
HA and FA were also given as control.
Data expressed as Mean±SEM, ANOVA followed by Dunnett's t-test for multiple comparison, ††p<0.01 vs Normal Saline (NS), **p<0.01 vs CBZ.
Picrotoxin (PTX) treatment significantly enhances the TBARS level compared with the saline control group. This finding is in agreement with earlier findings, which point to the development of oxidative stress in epilepsy. Enhanced lipid peroxidation as a result of oxidative stress produces convulsion, which leads to the inactivation of glutathione synthase, thus permitting abnormal build up of excitatory neurotransmitter glutamic acid. CBZ treated group significantly decreased the TBARS level compared to the PTX group. This decrease in the TBARS levels was further augmented upon complexation of CBZ with the complexing agents (HA and FA). Augmented reduction in TBARS level could be attributed to the enhanced bioavailability of CBZ. This is consistent with the previous findings, which reported enhanced bioavailability upon complexation with humic substances [12].
3.8.2. Tissue glutathione
GSH PTX treatment resulted in significant decrease in glutathione level when compared with the saline control group and CBZ treated group (97.29±1.99 vs. 29.24±1.12) (p<0.01). GSH levels were significantly normalized with the treatment with CBZ complexes (groups 4–7) (Table 3). Among the entire CBZ complexed group, CBZ–HA (1:2 KD) was found to have a better effect on the normalization of GSH.
PTX treatment leads to significant lipid peroxidation, which is evident from the significant increase in the TBAR levels of PTX-treated group and in turn generation of free radicals, which are quenched by the glutathione. Thus, GSH levels were significantly lowered in PTX-treated group compared to the normal control group. CBZ treatment significantly restored glutathione level to near normal. Complexation of CBZ with HA and FA further enhanced the glutathione level. Among all the groups treated with CBZ complex, the CBZ–HA groups (kneading or freeze dried) were found to be much effective in reversing the glutathione level.
This could be as a result of enhanced bioavailability of CBZ due to complexation. Further there are reports [16] of free radicals scavenging activity of HA and FA and thus the enhanced antioxidant activity of CBZ complex can also be explained by synergistic activity of HA and FA.
3.9. Pharmacokinetic study
The pharmacokinetic profiles of API and the optimized complexes (FA (1:2) and HA (1:2) freeze dried complexes) are shown in Fig. 14. Both the complexes were showing better absorption of carbamazepine than API and are comparable to each other. The complexes took 30 more minutes to attain Cmax and also the slope of absorption phase of API was sharper than the complexes. It may be explained by cogitating over the absorption phase. Till the attainment of plasma concentration around 570 ng/ml, the slope was almost the same or complexes were performing somewhat better. It could be attributed to the passive distribution or inherent permeability of molecule as was also evident from the mass spectrometer that some drug molecules were un-entrapped. After this point the absorption phase arose, but in a staggering fashion. Here, solubilization effect of humic substance dominates and also the release of entrapped carbamazepine from the complex shows its effect to uplift the Cmax. FA complex got some higher jump in Cmax because of the pH independent solubility. In highly acidic (stomach) pH humic acid shows lesser solubility than fulvic acid while at alkaline (intestine) both show nearly the same solubility. The elimination pattern of pure carbamazepine was obvious and in accordance with the literature. The elimination of complexes was showing some sustain release characteristics. Not any major difference in elimination pattern was observed due to nearly the same solubility. But the smaller molecular size would have played a role in the distribution and faster elimination. The standard deviation bars are omitted from the graph to have a clear understanding of the pharmacokinetic profile. Other parameters of pharmacokinetics are given in Table 4.
Fig. 14.
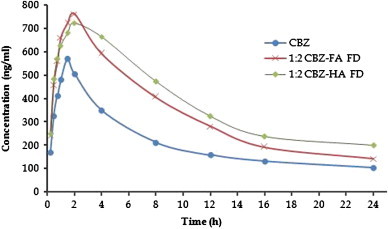
Graphical representation of mean plasma concentration of CBZ and complexes (n=6) after oral administration.
Table 4.
Pharmacokinetic profiles of pure and complexed carbamazepine.
| CBZ | 1:2 CBZ–FA FD | 1:2 CBZ–HA FD | |
|---|---|---|---|
| Cmax (ng/ml) | 572.58 | 762.73 | 724.51 |
| Tmax (h) | 1.5 | 2.0 | 2.0 |
| Half life (h) | 11.24 | 11.85 | 15.75 |
| AUC0−t (ng h/ml) | 5047.9 | 8107.2 | 9183.1 |
| AUC0−i (ng h/ml) | 8032.4 | 10,756.46 | 14,444.40 |
| Elimination rate constant (h−1) | 0.034 | 0.053 | 0.037 |
4. Conclusion
The present study demonstrates that both HA and FA have sufficient potentials to be explored as pharmaceutical excipients for bioavailability enhancement. Complexation of CBZ with FA and HA indicates its beneficial effect on enhancing brain permeability, which is presumed to decrease the amount of CBZ taken per se, and hence reduction of side effects encountered. CBZ–HA (1:2) complex appeared as the best performing complex in different in vitro and in vivo studies. Further, since FA and HA have demonstrated good antioxidant activity, they will take care of oxidative stress produced in seizures. However, further studies involving different experimental animals are also needed to make more conclusive statement on these complexing agents.
Acknowledgment
The authors are grateful to Prof. A. Wahab, Department of Physics (Jamia MiIlia Islamia), New Delhi, for providing access to X-ray diffraction facility. They are also grateful to Novartis Pharmaceuticals Ltd., India, and Dabur Research Foundation, Ghaziabad, India, for providing the gift sample of carbamazepine and rock shilajit, respectively.
References
- 1.Kong Y.C., Butt P.P.H., Ng K.H., Cheng K.F., Camble R.C., Malla S.B. Chemical studies on a Naplese pancea: Shilajit. Int J Crude Drug Res. 1987;25:179–187. [Google Scholar]
- 2.Ghosal S. In: Research and development of indigeneous drugs. Vohara S.B., Dandiya P.C., editors. Institute of History of Medicine and Medical Research; New Delhi: 1989. The facets and facts of shilajit. [Google Scholar]
- 3.Ghosal S. Chemistry of Shilajit, an immunomodulatory Ayurvedic rasayan. Pure Appl Chem. 1990;62:1285–1288. [Google Scholar]
- 4.Anderson H.A., Bick W., Hepburn A., Stewart M. In: Hayes M.H.B., MacCarthy P., Malcolm R.L., Swift R.S., editors. Vol. 1989. Wiley-Interscience; Chichester, UK: 1989. Humic substances II; pp. 223–253. (Search of structure). [Google Scholar]
- 5.Wershaw R.L., Thorn K.A., Pmckney D.J., Rice J.A., Hemond H.F. Elsevier Applied Science; 1986. Application of a membrane model to the secondary structure of humic materials in peat. Peat and water: aspects of water retention and dehydrating in peat. [Google Scholar]
- 6.Kobayashi Y., Ito S., Itai S., Yamamoto K. Physicochemical properties and bioavailability of carbamazepine polymorphs and dehydrate. Int J Pharm. 2000;193(2):137–146. doi: 10.1016/s0378-5173(99)00315-4. [DOI] [PubMed] [Google Scholar]
- 7.Wu C.Y., Benet L.Z. Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22(1):11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]
- 8.Koester L.S., Xavier C.R., Mayorga P., Valquiria L. Influence of β-cyclodextrin complexation on carbamazepine release from hydroxypropyl methylcellulose matrix tablets. Eur J Pharm Biopharm. 2003;55(1):85–91. doi: 10.1016/s0939-6411(02)00127-3. [DOI] [PubMed] [Google Scholar]
- 9.El-Zein H., Riad L., El-Bary A. Enhancement of carbamazepine dissolution: in vitro and in vivo evaluation. Int J Pharm. 1998;168(2):209–220. [Google Scholar]
- 10.Li H., Jin Y., Nie Y. Application of alkaline treatment for sludge decrement and humic acid recovery. Biores Tech. 2009;100(24):6278–6283. doi: 10.1016/j.biortech.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Vlachou M., Papaïoannou G. Preparation and characterization of the inclusion complex of furosemide with hydroxypropyl-beta-cyclodextrin. J Biomater Appl. 2003;17(3):197–206. doi: 10.1177/0885328203017003557. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal S.P., Anwer M.K., Aqil M. Complexation of furosemide with fulvic acid extracted from shilajit: a novel approach. Drug Dev Ind Pharm. 2008;34(5):506–511. doi: 10.1080/03639040701744053. [DOI] [PubMed] [Google Scholar]
- 13.Anwer M.K., Agarwal S.P., Ali A, Sultana Y. Molecular complexes of aspirin with humic acid extracted from shilajit and their characterization. J Incl Phenom Macrocycl Chem. 2009;67(1–2):209–215. [Google Scholar]
- 14.Cazali N., Tran A., Treluyer J.M., Rey E., d'Athis P., Vincent J., et al. Inhibitory effect of stiripentol on carbamazepine and saquinavir metabolism in human. Br J Clin Pharmacol. 2003;56(5):526–536. doi: 10.1046/j.1365-2125.2003.01919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boobis A.R., Burley D., Davies D.M., Davies D.S., Harrison P.I., Orme M.L.E., et al. Therapeutic drugs. 2nd ed. Churchill Livingstone; New York: 1991. [Google Scholar]
- 16.Santos A., Botero W.G., Bellin L.C., de Oliveira L.C., Rocha J.C., Mendonça A.G.R., et al. Interaction between humic substances and metallic ions: a selectivity study of humic substances and their possible therapeutic application. J Braz Chem Soc. 2007;18(4):824–830. [Google Scholar]
- 17.Loftsson T., Brewster M.E. Drug solubilization and stabilization. J Pharm Sci. 1996;85:1017–1025. doi: 10.1021/js950534b. [DOI] [PubMed] [Google Scholar]
- 18.Yogeeswari P., Sriram D., Saraswat V., Ragavendran J., Kumar M., Murugesan M., et al. Synthesis and anticonvulsant and neurotoxicity evaluation of N4-phthalimido phenyl (thio) semicarbazides. Eur J Pharm Sci. 2003;20:341–346. doi: 10.1016/j.ejps.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Achliya G.S., Wadodkar S.G., Darle A.K. Evaluation of CNS activity of Bramhi Ghrita. Ind J Pharm. 2005;37:33–36. [Google Scholar]
- 20.Ahmed M.O., El-Gibaly I., Ahmed S.M. Effect of cyclodextrins on the physicochemical properties and antimycotic activity of clotrimazole. Int J Pharm. 1998;171:111–121. [Google Scholar]
- 21.Gandhimathi M., Ravi T.K. Rapid HPTLC analysis of oxcarbazepine in human plasma. J Plan Chrom Mod TLC. 2008;21(6):437–439. [Google Scholar]
- 22.Ohkawa H., Ohishi Ν., Yagi Κ. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 23.Ellman G. Tissue sulphydryl groups. Arch Biochem Biophys. 1959;32:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Y., Chiang H., Wulster-Radcliffe, Hilt M.R., Wong P., Kissinger C.B., et al. Liquid chromatography/tandem mass spectrometry for the determination of carbamazepine and its main metabolite in rat plasma utilizing an automated blood sampling system. J Pharm Biomed Anal. 2005;38:119–125. doi: 10.1016/j.jpba.2004.11.058. [DOI] [PubMed] [Google Scholar]
- 25.Paolid F.D., Kukkonen J. Binding of organic pollutants to humic and fulvic acids: influence of pH and the structure of humic material. Chemosphere. 1997;34(8):1693–1704. [Google Scholar]
- 26.Steinberg C.E.W., Xu Y., Lee S.K., Freitag D., Kettmp A. Effect of dissolved humic material (DHM) on bioavailability of some organic xenobiotics to Daphnia magna. Chem Spec Bioav. 1993;5:l–9. [Google Scholar]
- 27.Fnmd R., Ludemann H.D. The quantitative analysis of solution- and CPMAS-C-13 NMR spectra of humic material. Sci Total Environ. 1989;81/82:157–168. [Google Scholar]
- 28.Yadav V.R., Suresh S., Devi K., Yadav S. Effect of cyclodextrin complexation of curcumin on its solubility and antiangiogenic and anti-inflammatory activity in rat colitis model. AAPS Pharm Sci Tech. 2009;10:752–762. doi: 10.1208/s12249-009-9264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarasija S., Shivakumar H.N., Kumar K. Effect of β cyclodextrin complexation on the solubility and dissolution rate of carbamazepine from tablets. Ind J Pharm Sci. 2006;68:301–307. [Google Scholar]
- 30.Pietro M., Paola C. Thermal analysis for the evaluation of the organic matter evolution during municipal solid waste aerobic composting process. Thermo Acta. 2004;413:209–214. [Google Scholar]
- 31.Moura M.N., Martín M.J., Burguillo F.J. A comparative study of the adsorption of humic acid, fulvic acid and phenol onto Bacillus subtilis and activated sludge. J Haz Mater. 2007;149(1):42–48. doi: 10.1016/j.jhazmat.2007.02.074. [DOI] [PubMed] [Google Scholar]
- 32.Schnitzer M. Chemical, spectroscopic and thermal methods for the classification and characterization of fulvic substances. In: Proceedings of the international meeting on humic substances. Nieuwersluis; 1972.
- 33.Adani F., Tambone F., Davoli E., Scaglia B. Surfactant properties and tetrachloroethene (PCE) solubilisation ability of humic acid-like substances extracted from maize plant and from organic wastes: a comparative study. Chemosphere. 2010;78(8):1017–1022. doi: 10.1016/j.chemosphere.2009.11.039. [DOI] [PubMed] [Google Scholar]
- 34.Hasset J.P., Milicic E. Determination of equilibrhun and rate constants for binding of polychlorinated biphenyl congener by dissolved humic substances. Environ Sci Technol. 1985;19:638–643. doi: 10.1021/es00137a010. [DOI] [PubMed] [Google Scholar]
- 35.McCarthy J.F., Jiienez B.D. Interactions between polycyclic aromatic hydrocarbons and dissolved humic material: binding and dissociation. Environ Sci Tech. 1985;19:1072–1076. doi: 10.1021/es00141a008. [DOI] [PubMed] [Google Scholar]
- 36.Wang X.K., Chen C.L., Du J.Z., Tan X.L., Xu D., Yu S.M. Effect of pH and aging time on the kinetic dissociation of 234Am (III) from humic acid-coated γ-Al2O3: a chelating resin exchange study. Environ Sci Tech. 2005;39:7084–7088. doi: 10.1021/es0506307. [DOI] [PubMed] [Google Scholar]
- 37.Wang X.K., Zhou X., Du J.Z., Hu W.P., Chen C.L., Chen Y.X. Using of chelating resin to study the kinetic desorption of Eu(III) from humic acid–Al2O3 colloid surfaces. Surf Sci. 2006;600:478–483. [Google Scholar]
- 38.Mandal R., Salam M.S.A., Murimboh J., Hassan. N.M., Chakrabarti C.L., Back M.H. Competition of Ca (II) and Mg (II) with Ni (II) for binding by a well-characterized fulvic acid in model solutions. Environ Sci Tech. 2000;34:2201–2208. [Google Scholar]
- 39.Skoug J.W., Halstead G.W., Theis D.L., Freeman J.E., Fagan D.T. Strategy for the development and validation of dissolution tests for solid oral dosage forms. Pharm Tech. 1996;20:58–68. [Google Scholar]
- 40.Sanchez-Marín P., Lorenzo J.I., Blust R., Beiras R. Fulvic acids increase dissolved lead bioavailability for marine invertebrates. Environ Sci Techn. 2007;41(16):5679–5684. doi: 10.1021/es070088h. [DOI] [PubMed] [Google Scholar]
- 41.Milne C.J., Kinniburgh D.G., Tipping E. Generic NICA– Donnan model parameters for proton binding by humic substances. Environ Sci Tech. 2001;35:2049–2059. doi: 10.1021/es000123j. [DOI] [PubMed] [Google Scholar]


