Abstract
Doxorubicin-encapsulating liposomal formulations, known as Doxil, have been used for the treatment of Kaposi’s sarcoma and ovarian cancer. However, there is still a need for a drug delivery system for doxorubicin that limits the treatment’s side effects, namely, mucositis and hand-and-foot syndrome. The AG73 peptide derived from the laminin α1 chain is a ligand for syndecans, and syndecan-2 is highly expressed in some cancer cells. In this study, to develop a safer and more selective liposomal formulation, we prepared doxorubicin-encapsulating AG73 peptide-modified liposomes (AG73–Dox). First, we assessed the selectivity of AG73–Dox for cancer cells, including syndecan-2 over-expressing cells, using flow cytometry and confocal microscopy. AG73–Dox showed selective cellular uptake on cancer cells and enhancement of the intracellular uptake. Next, we examined the cytotoxicity of AG73–Dox using a WST assay. AG73–Dox exhibited a higher cytotoxicity against cancer cells than other control liposomes. In addition, we showed that the antitumor efficacy of AG73–Dox in vivo was better than that of free Dox. When we examined the biodistribution of liposomes, AG73 peptide-modified liposomes (AG73-L) tended to bind to intratumoral vessels and extravasated in the tumor tissue. Thus, further optimization of AG73-L toward tumor targeting may lead to a development of a useful tool for cancer therapy.
Keywords: Liposomes, Drug delivery, Antitumor, AG73 peptide
1. Introduction
The anthracycline anticancer drug doxorubicin (Dox) is generally used in chemotherapy for the treatment of various tumors, including breast cancer, leukemia, and soft-tissue sarcoma. Various mechanisms explain its therapeutic effect [22]. These mechanisms include DNA intercalation, lipid peroxidation, and inhibition of topoisomerase II. However, the use of free Dox is rather limited due to its severe side effects, including cardiotoxicity, hair loss, and nephrotoxicity [10,20,22,30]. Dox has been encapsulated in liposomes to reduce these problems associated with free Dox treatment [23]. Liposomes have been explored as a potential drug delivery carrier. Compared with the free drug, liposomal-encapsulated drugs typically exhibit a prolonged systemic circulation time and increased tumor localization by the enhanced permeability and retention (EPR) effect [15]. In addition, the toxicity of the liposomally encapsulated anticancer drugs could be diminished, as the drug cannot exert its activity when encapsulated in liposomes during bloodstream circulation [11,31]. In 1995, the liposomal Dox formulations Doxil and Caelyx were approved by the FDA for the treatment of AIDS-related Kaposi’s sarcoma and ovarian cancer. Although Doxil strongly reduced the cardiotoxicity of Dox in clinical trials, other side effects arose. Several patients suffered from mucositis and hand-and-foot syndrome due to the localization of the liposomes in skin capillaries [22]. Therefore, to develop safer and more selective liposomal formulations, many research groups have tried to develop tumor-targeting liposomes using transferrin, folic acid, RGD peptide, or antibodies as ligands [6,14,21,27–29].
The present study focused on AG73, which is a 12-amino-acid synthetic peptide derived from the globular domain of the laminin α1 chain. AG73 peptide is a ligand for syndecans, one of the major heparan sulfate-containing transmembrane proteoglycans [3,8,24]. Moreover, syndecan-2 is highly expressed in various cancer cell lines ([7,26,32]. Therefore, we tried to develop Dox-encapsulating AG73 peptide-modified liposomes (AG73–Dox) as novel tumor-targeting liposomes to enhance the intracellular uptake of anticancer drugs and treatment efficacy.
In this study, we prepared AG73–Dox and assessed the selectivity of AG73–Dox for cancer cells, including syndecan-2 over-expressing cells. Furthermore, we examined the antitumor effects of AG73–Dox in vitro and in vivo.
2. Materials and methods
2.1. Materials
1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), 1,2-distearoylphosphatidylethanolamine-methoxy-polyethyleneglycol (DSPE–PEG2000–OMe), and 1,2-distearoyl-sn-glycero-3-phosphatidylethanolamine–polyethyleneglycol–maleimide (DSPE–PEG2000–Mal) were purchased from NOF Corporation (Tokyo, Japan). Doxorubicin (Dox) was purchased from SIGMA-Aldrich Co. (St. Louis, MO, USA). For cell culture, Dulbecco's Modified Eagle's Medium (DMEM) was purchased from Kohjin Bio Co., Ltd. (Tokyo, Japan). Fetal bovine serum (FBS) was purchased from Equitech Bio Inc. (Kerrville, TX, USA). All other materials were used without further purification.
2.2. Preparation of liposomes
Liposomes composed of DSPC and DSPE–PEG2000–OMe at a molar ratio of 94:6 were prepared by a reverse-phase evaporation method. In brief, all reagents were dissolved in 1:1 (v/v) chloroform/diisopropylether. Three hundred millimolar citrate buffer (pH 4.0) was then added to the lipid solution, and the mixture was sonicated and evaporated at 65 °C. The organic solvent was completely removed, and the size of the liposomes was adjusted to approximately 150 nm using extruding equipment and sizing filters (pore sizes: 100 and 200 nm, Nuclepore Track-Etch Membrane, Whatman plc, UK). For the fluorescent labeling of the lipid membrane, 1,1-dioctadecyl-3,3,3,3-tetramethyl-indocarbocyanine perchlorate (DiI) was also added (1 mol% of total lipids). After the sizing, the liposomes were passed through a 0.45-μm pore-size filter (Syringe filter, ASAHI TECHNOGLASS Co., Chiba, Japan) for sterilization.
2.3. Preparation of Dox-encapsulating liposomes
The Dox-encapsulating liposomes were prepared by a remote loading method with a pH gradient [4,19]. In brief, liposomes were passed through a Sephadex G-50 (GE Healthcare UK Ltd., Buckinghamshire, England) spin column that was equilibrated with N-[2-hydroxyethyl]piperazine-NV-[2-ethanesulfonic acid] (HEPES)-buffered saline (HBS; 20 mM HEPES, 150 mM NaCl, pH 7.5) to exchange the external buffer. The eluted liposomes had a transmembrane pH gradient with pH 4.0 inside and pH 7.5 outside the liposomes. The eluted liposomes were incubated with Dox (at a Dox:lipid molar ratio of 1:5) at 65 °C for 30 min. To remove the unencapsulated Dox, the mixture was passed through a Sephadex G-50 spin column. The Dox-encapsulating liposomes were stored at 4 °C until use. The lipid concentration was measured using Phospholipid C test Wako (Wako Pure Chemical Industries, Ltd., Osaka, Japan), and the Dox concentration was determined by measuring the absorbance at 480 nm (Infinite M1000, TECAN, Männedorf, Switzerland) in a 1% Triton X-100 solution and comparing the absorbance value to the standard curve. The encapsulated efficiency was calculated according to the following equation:
where (D/L)30 min is the ratio of Dox to lipid concentration after the liposomes were passed through the Sephadex G-50 spin column and (D/L)initial is the ratio of Dox to lipid at the initial concentration.
2.4. Preparation of Dox-encapsulating AG73 peptide-modified liposomes
The Cys–AG73 peptide (CGG–RKRLQVQLSIRT) and a scrambled Cys–AG73T control peptide (CGG–LQQRRSVLRTKI) were synthesized manually using the 9-fluorenylmethoxycarbonyl (Fmoc)-based solid-phase strategy and were prepared in the COOH terminal amide form and purified by reverse-phase high-performance liquid chromatography. Dox-encapsulating liposomes composed of DSPC, DSPE–PEG2000–OMe, and DSPE–PEG2000–Mal at a molar ratio of 94:4:2 were prepared by a reverse-phase evaporation method and a remote loading method. For coupling, AG73 peptide at a molar ratio of 2-fold DSPE–PEG2000–Mal was added to the Dox-encapsulating liposomes, and the mixture was incubated for 24 h at 4 °C to conjugate the cysteine of the Cys–AG73 peptide with the maleimide of the Dox-encapsulating liposomes using a thioether bond. The resulting AG73 peptide-conjugated Dox-encapsulating liposomes (AG73–Dox) were passed through a Sephadex G-50 spin column to remove any excess peptide. AG73–Dox was modified with 6 mol% PEG and 2 mol% peptides. The particle size and zeta potential of the liposomes were measured using NICOMP 380 ZLS (Particle Sizing Systems, Santa Barbara, CA, USA).
2.5. Cell lines and animals
293T human embryonic kidney carcinoma cells that stably overexpressed syndecan-2 (293T-Syn2) and murine colorectal carcinoma cells (colon26) were cultured in DMEM that was supplemented with 10% FBS, penicillin (100 U/ml), streptomycin (100 μg/ml), and puromycin (0.4 or 100 μg/ml) at 37 °C in a humidified 5% CO2 atmosphere.
Male BALB/c mice (6 weeks old) were purchased from Tokyo Laboratory Animals Science Co., Ltd. (Tokyo, Japan). Animal use and relevant experimental procedures were approved by the Tokyo University of Pharmacy and Life Science Committee on the Care and Use of Laboratory Animals.
2.6. Flow cytometry analysis for the intracellular uptake of Dox-encapsulating AG73 peptide-modified liposomes
The intracellular uptake of liposomes was determined by flow cytometry analysis [9]. 293T-Syn2 cells (1×105 cells/well) were seeded in a 24-well plate and incubated for 48 h at 37 °C in 5% CO2. Colon26 cells (5×104 cells/well) were also seeded in a 24-well plate and incubated for 24 h at 37 °C in 5% CO2. The medium was then replaced with Dox-encapsulating liposomes (Dox–PEG), AG73–Dox, or Dox-encapsulating AG73T peptide-modified liposomes (AG73T–Dox) that were diluted with culture medium for a final Dox concentration of 20 μg/ml. The plates were incubated for 1 h at 37 °C. The medium was removed; subsequently, each cancer cell line was washed with phosphate-buffered saline (PBS), and the cell samples were examined by flow cytometry using an FACScan (Becton Dickinson, San Jose, CA, USA). Cell-associated Dox was excited with an argon laser (488 nm). Data were collected in 10,000 gated events and analyzed with the CELL Quest software program.
2.7. Confocal microscopy analysis
293T-Syn2 cells (2 or 5×104 cells/well) were seeded in a 24-well plate and incubated for 48 h at 37 °C in 5% CO2. Colon26 cells (5×104 cells/well) were also seeded in a 24-well plate and incubated for 24 h at 37 °C in 5% CO2. Then, the medium was replaced with Dox–PEG, AG73–Dox, AG73T–Dox, or free Dox diluted with culture medium for a final Dox concentration of 10 or 20 μg/ml. The plates were incubated for 4 h at 37 °C. The medium was removed, and subsequently, each cancer cell line was washed with PBS and then fixed with 4% paraformaldehyde for 1 h at 4 °C (293T-Syn2) or for 15 min at room temperature (colon26). For nuclear staining, the cells were treated with DAPI for 1 h. Fluorescence images of the cells were analyzed using an FV1000-D confocal microscope (OLYMPUS, Tokyo, Japan).
2.8. Cytotoxicity studies
The cytotoxicity of liposomes was determined using the WST assay. 293T-Syn2 and colon26 suspensions (1×104 cells/well for 293T-syn2 and 3×103 cells/well for colon26) were added to Dox–PEG, AG73–Dox, AG73T–Dox, or free Dox with a serial concentration of Dox ([Dox]=1–40 μg/ml). Then, the suspension was incubated for 4 h at 37 °C in 5% CO2. After 4 h of incubation at 37 °C, the cells were washed, and fresh DMEM was added. The cells were then seeded in a 96-well plate and incubated for 48 h at 37 °C in 5% CO2. After incubation, 10 μL of the cell-counting solution (WST-8, Dojindo Laboratories, Tokyo, Japan) was added to each well and was incubated for 2 h (293T-Syn2) or 1 h (colon26) at 37 °C in 5% CO2. Cell viability was assessed by measuring the absorbance at 450 nm with a reference absorbance at 650 nm (Infinite M1000, TECAN, Männedorf, Switzerland). Cell viability was calculated according to the following formula:
2.9. In vivo antitumor efficacy
Colon26 cells (1×106 cells/mouse) were inoculated subcutaneously in the right flank of mice. Five days after tumor inoculation (when the tumor volume reached approximately 50 mm3), HBS buffer used as a vehicle (control), Dox, Dox–PEG, or AG73–Dox was administered via a tail vein of the mice (n=4). The injected dose of Dox in each administration was 2 mg/kg (approximately 10 μmol/kg dose of lipid). Various formulations were given every other day for a total of 5 doses. The size of tumors and the body weight of each mouse were monitored, and the tumor volume was calculated using the following equation:
2.10. Intratumoral localization and biodistribution of liposomes
Colon26 cells (1×106 cells/mouse) were inoculated subcutaneously in the right flank of mice. Seven days after tumor inoculation (when the tumor volume reached approximately 100–200 mm3), DiI-labeled liposomes (lipid concentration: 10 μmol/kg) were administered via a tail vein of the mice (n=6). At 6 h after injection of the liposomes, the mice were sacrificed, and the tumors and organ tissues (heart, spleen, liver, and kidney) were dissected. These tissues were fixed in 10% paraformaldehyde substituted with 20% sucrose and then embedded in optimal cutting temperature compound (Sakura Finetech, Co., Ltd., Tokyo, Japan) and frozen at −80 °C. Each tumor and organ tissue section was prepared with a width of 10 μm and mounted on a poly-l-lysine coated slide. For immunohistochemistry, the tumor section was blocked with 10% lapine serum in TBS for 30 min at 4 °C and incubated with anti-mouse CD31 antibody (BD Pharmingen) overnight at 4 °C. Subsequently, the section was incubated with Alexa Fluor 488 rabbit anti-rat IgG (Molecular Probes) for 1 h at room temperature. Each tumor and organ section was then mounted with VECTASHIELD Hard Set Medium with DAPI (VECTOR LABORATORIES, INC, USA) and fluorescently observed with BZ-8100 (KEYENCE, Japan).
3. Results and discussion
3.1. Characteristics of Dox-encapsulating AG73 peptide-modified liposomes
First, we sought to prepare Dox-encapsulating AG73 peptide-modified liposomes (AG73–Dox). As shown in Table 1, the mean particle diameter of Dox–PEG, AG73–Dox, or AG73T–Dox ranged from 130 to 170 nm with a relatively narrow distribution (Fig. 1). The zeta potential of the Dox-encapsulating liposomes was slightly negative in value. The efficiency for the remote loading of Dox into the liposomes was 90–95% with a drug/lipid ratio of 1:5 (molar ratio). The encapsulated efficiency also remained unchanged even in the case of peptide modification. After one month of storage at 4 °C, the encapsulated efficiency was less than 5%.
Table 1.
Size and ζ potential of Dox-encapsulating peptide-modified liposomes.
| Liposomes | Mean diameter (nm)±S.D. | ζ potential (mV)±S.D. |
|---|---|---|
| Dox–PEG | 131.7±6.4 | −0.52±0.16 |
| AG73–Dox | 161.5±14.8 | −0.32±0.20 |
| AG73T–Dox | 134.6±6.3 | −0.37±0.36 |
Fig. 1.
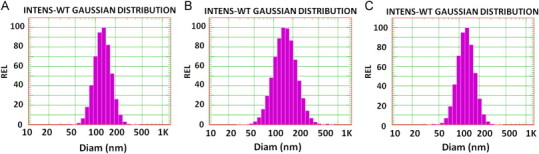
Size distribution of Dox-encapsulating peptide-modified liposomes obtained using DLS: Size distribution of Dox–PEG (A), AG73–Dox (B), and AG73T–Dox (C).
3.2. Cellular uptake of Dox-encapsulating AG73 peptide-modified liposomes
To examine the selective cellular uptake of Dox transfected by liposomes via the syndecan-2 receptor, the amount of Dox uptake into cancer cells including syndecan-2 overexpressing cells was evaluated by flow cytometry analysis. As shown in Fig. 2, the cellular uptake of AG73–Dox on both 293T-Syn2 and colon26 was higher than that of Dox–PEG or AG73T–Dox. The laminin-derived AG73 peptide is known as a ligand for syndecans, and it has been reported that syndecan-2 is highly expressed in various cancer cells [3,5,8,24,26]. In addition, the AG73 peptide has been shown to bind to the heparin sulfate side chains of syndecan [8]. Therefore, to verify that AG73–Dox can bind to syndecan-2 on the surface of cancer cells, the cancer cells were treated with AG73–Dox and heparin (Fig. 3). Our data showed that the cellular uptake of AG73–Dox was down-regulated by the treatment with heparin. However, the cellular uptake of Dox–PEG or AG73T–Dox was not down-regulated by the treatment (data not shown). Therefore, these results suggested that AG73–Dox could effectively target cancer cells via the syndecan-2 receptor.
Fig. 2.
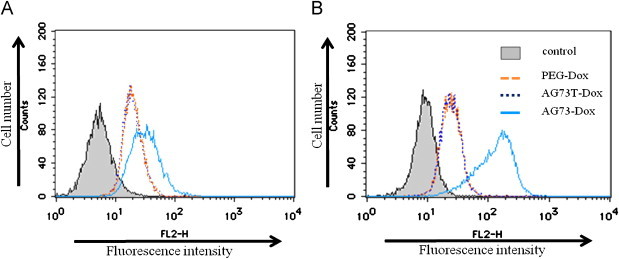
Cellular uptake of Dox-encapsulating peptide-modified liposomes on the cancer cells 293T-Syn2 (A) and colon26 (B).
The cells were treated with Dox-encapsulating liposomes (PEG–Dox), Dox-encapsulating AG73 peptides (AG73–Dox), or AG73T peptide-modified liposomes (AG73T–Dox) ([Dox]=20 μg/ml) for 1 h at 37 °C. After incubation, the cells were washed, and their fluorescence intensities were measured by flow cytometry.
Fig. 3.
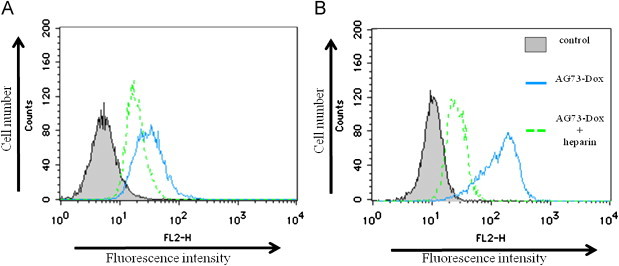
Effect of heparin on cellular uptake activity of AG73–PEG on the cancer cells 293T-Syn2 (A) and colon26 (B).
The cells were treated with AG73–PEG ([Dox]=20 μg/ml) and heparin (10 μg/ml) or only AG73–Dox for 1 h at 37 °C. After incubation, the cells were washed, and their fluorescence intensities were measured by flow cytometry.
3.3. Intracellular localization of Dox-encapsulating AG73 peptide-modified liposomes using confocal laser scanning microscopy
To further elucidate the intracellular uptake of Dox, the intracellular localization of Dox was evaluated after the treatment of cells with Dox–PEG, AG73–Dox, AG73T–Dox, or free Dox using confocal microscopy. As shown in Fig. 4, the cells treated with AG73–Dox showed a strong red fluorescence, whereas cells treated with Dox–PEG or AG73T–Dox showed a faint red fluorescence. The intracellular localization of Dox in the cells treated with AG73–Dox could be observed on the surface of the cell membrane and in the cytoplasm with a slight colocalization in the nuclei. However, these localizations after the treatment with AG73–Dox were blocked by heparin. A punctuated pattern could also be detected in the cytoplasm of both cancer cells (indicated by white arrows in Fig. 4), which suggested that the Dox localized in the endosomes [12]. Thus, these results suggested that AG73–Dox could enhance the intracellular uptake of Dox compared with Dox–PEG or AG73T–Dox.
Fig. 4.
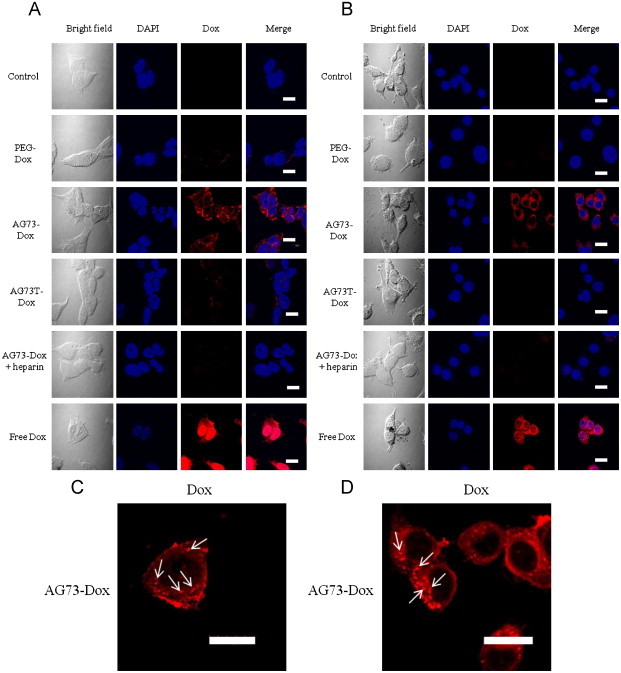
Confocal microscopy images of 293T-Syn2 (A and C) and colon26 (B and D) cells incubated with PEG–Dox, AG73–Dox, AG73T–Dox, or free Dox ([Dox]=10 μg/ml: 293T-Syn2, 20 μg/ml: colon26) for 4 h at 37 °C.
After incubation, the cells were washed and fixed. The cells were then treated with DAPI (blue) for nuclear staining. Blue: fluorescence of DAPI. Red: fluorescence of Dox. Pink: the merged blue and red that indicates the colocalization of DAPI and Dox. The arrows indicate that Dox localizes in the endosomes (C and D). Scale bars represent 20 μm.
3.4. Cytotoxicity of Dox-encapsulating AG73 peptide-modified liposomes
To examine the therapeutic efficiency of AG73–Dox, the cytotoxicity of Dox-encapsulating liposomes against cancer cells was evaluated using a WST assay. The cytotoxicity studies were initially performed with empty liposomes on the cancer cells to determine whether the liposomes themselves contributed to the cytotoxicity. The concentration of lipids tested was matched to the amount of lipids used in the drug-encapsulating formulations. At the concentration of lipids used, the cell viability was more than 80% that of the control (data not shown). These data indicated that the empty liposomes alone did not have a significant cytotoxicity. As shown in Fig. 5, the cell viability was dependent on the concentration of Dox. Furthermore, the cytotoxicity of AG73–Dox was more sensitive than that of Dox–PEG or AG73T–Dox against both types of cancer cells. In particular, the cytotoxicity of AG73–Dox against 293T-Syn2 was notably higher in comparison with that against colon26. This result may be due to the fact that the ability and sensitivity of the intracellular uptake of free Dox into the cancer cells were distinct from those of 293T-Syn2 and colon26, as shown by confocal microscopy (Fig. 4), as it has been reported that colon26 exhibits a constitutive expression of mdr1a and mdr1b that encodes the drug efflux transporter P-glycoprotein [16].
Fig. 5.
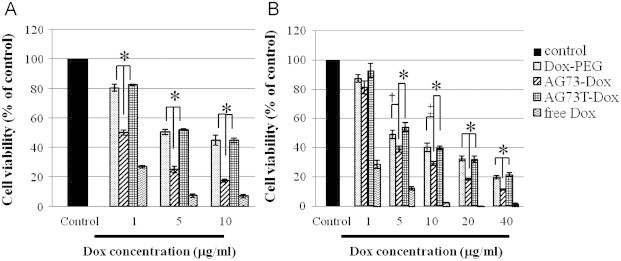
Cytotoxicity of Dox-encapsulating liposomes for 293T-Syn2 (A) or colon26 (B).
The cells were incubated with serial concentrations of Dox–PEG, AG73–Dox, AG73T–Dox, or free Dox for 4 h at 37 °C. After incubation, the cancer cells were washed, and fresh medium was added. The cells were seeded and further incubated for 48 h at 37 °C before the WST assay. †p<0.05; ‡p<0.01; *p<0.005. Data are represented as mean±SD.
3.5. Intracellular drug delivery of Dox-encapsulating AG73 peptide-modified liposomes
To understand the drug delivery and release behaviors of AG73–Dox, the cells were washed after the treatment of AG73–Dox and placed into fresh medium for further incubation at 37 °C. They were imaged using confocal microscopy at various time points after washing. As shown in Fig. 6, Dox was diffused through the 293T-Syn2 in a time-dependent manner and then was colocalized with the nuclei. This result could be due to the release of Dox from AG73–Dox inside endosomes with a lower pH. In addition, at 24 h post-incubation, we observed fragmentation of the nuclei, which is characteristic of apoptosis (indicated by white arrows in Fig. 6). Moreover, at 48 h post-incubation, we observed increased fragmentation of the nuclei. Therefore, these results suggested that after the cellular uptake of AG73–Dox, Dox was slowly released from AG73–Dox and was subsequently transferred to nuclei, which led to cytotoxicity (Fig. 5).
Fig. 6.
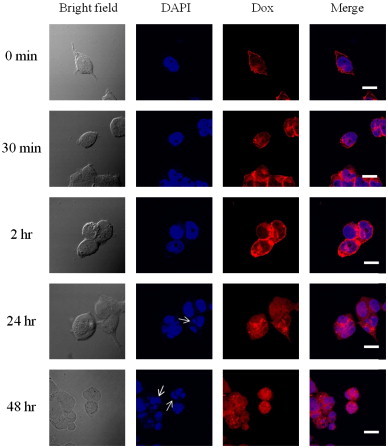
Confocal microscopy images of 293T-Syn2 after incubation with AG73–Dox. 293T-Syn2 (2×105/well) were seeded in a 24-well plate.
293T-Syn2 cells after 4 h of incubation ([Dox]=10 μg/ml) at 37 °C were washed and transferred into fresh medium for further incubation. The confocal microscopy images were taken after washing. The post-incubation times (0, 30 min, 2, 24, or 48 h) are also indicated above the figure. The cells were fixed and treated with DAPI (blue) for nuclear staining. Blue: fluorescence of DAPI. Red: fluorescence of Dox. Pink: the merged blue and red that indicates the colocalization of DAPI and Dox. The arrows indicate fragmentation of the nuclei. Scale bars represent 20 μm.
3.6. In vivo antitumor efficacy
Next, as AG73–Dox showed higher cellular uptake and cytotoxicity against cancer cells compared to Dox–PEG, the antitumor efficacy of AG73–Dox in vivo was evaluated in colon26 tumor-bearing mice at a dose of 2 mg Dox/kg body weight. As shown in Fig. 7, the Dox–PEG group had suppressed tumor growth compared with the free Dox group. These results showed that liposomal doxorubicin may be diffused passively into the tumor tissue by the EPR effect [15]. However, there was no significant difference between the Dox–PEG group and the free Dox group, whereas the tumor growth inhibition efficacy of the AG73–Dox group was better than that of the free Dox group (P<0.05). When the antitumor effect of Dox–PEG was compared with that of AG73–Dox, there was no significant difference in antitumor effect. We also monitored the body weights of the tumor-bearing mice to assess any side effects of AG73–Dox. The body weight change during the tumor treatment was not observed in the AG73–Dox group.
Fig. 7.
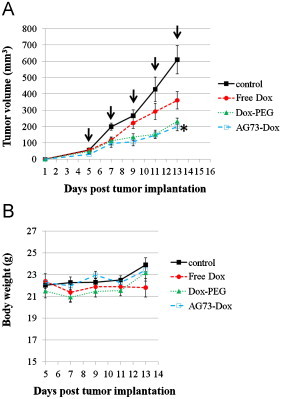
In vivo antitumor effects of various treatments against tumors in tumor-bearing mice (n=4). (A) Tumor growth curves for tumor-bearing mice. (B) Changes in body weight during the treatments.
The tumor-bearing mice were intravenously injected with HBS used as a vehicle (control), free Dox, Dox–PEG, or AG73–Dox ([Dox]=2 mg/kg) when the tumor volume reached about 50 mm3 (5 days after tumor inoculation). The tumor volumes and the body weights of the tumor-bearing mice were monitored. Each value is presented as the average with SE. The arrows show the days of injection. Significant differences were found between the AG73–Dox and free Dox groups and are marked as ⁎p<0.05.
3.7. Biodistribution of liposomes
To evaluate the targeting effect of AG73 peptide-modified liposomes in vivo, we examined the intratumoral localization of AG73 peptide-modified liposomes. As shown in Fig. 8, DiI-labeled PEG liposomes (PEG-L), which did not contain Dox, were leaked from intratumoral vessels and diffused in the tumor tissue, whereas DiI-labeled AG73 peptide-modified liposomes (AG73-L) were mainly bound to intratumoral vessels and were partially extravasated in the tumor. The AG73 peptide is a ligand for syndecan-2. Syndecan-2 is also highly expressed in human vascular endothelial cells [17]. Moreover, the cellular uptake of AG73–Dox in human umbilical vein endothelial cells was higher than that of Dox–PEG or AG73T–Dox, which was observed using flow cytometry (data not shown). In this study, there is little difference between antitumor effect of Dox–PEG and that of AG73–Dox. However, it seems that AG73–Dox tend to target not only tumor tissue but also intratumoral vessels (Figs. 7 and 8). To assess whether AG73-L is tumor selectivity, we injected DiI-labeled PEG-L or AG73-L and observed various other organs (heart, liver, spleen, and kidney) using fluorescence microscopy. As shown in Fig. 9, although the fluorescence intensity of AG73-L was slightly high in the heart compared to that of PEG-L, there was little difference between PEG-L and AG73-L in the other organs.
Fig. 8.
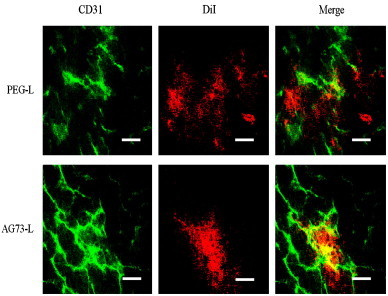
Localization of AG73 peptide-modified liposomes within the tumor.
The tumor-bearing mice (n=6) were intravenously injected with DiI-labeled PEG-liposomes (PEG-L) or DiI-labeled AG73 peptide-modified liposomes (AG73-L) when the tumor volume reached 100–200 mm3 (7 days after tumor inoculation). Liposomes were injected as lipids at a dose of 10 μmol/kg. At 6 h after injection, each tumor was dissected, and the tumor sections were then prepared in slices with widths of 10 μm each. Each section was stained with anti-CD31 antibody for the labeling of the endothelial cells. Green: CD31. Red: fluorescence of DiI. Yellow: the merged green and red indicates the colocalization of liposomes at the site of the vascular endothelial cells. Scale bars represent 50 μm.
Fig. 9.
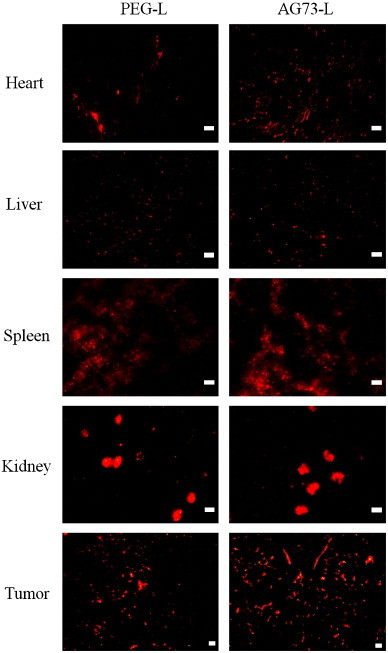
Biodistribution analysis by fluorescence imaging.
The tumor-bearing mice (n=6) were intravenously injected with PEG-L or AG73-L (lipid concentration: 10 μmol/kg). At 6 h after injection, each tissue was dissected, and sections were then prepared in slices with widths of 10 μm each. These sections were observed using a fluorescence microscope. Scale bars represent 50 μm.
Recently, to enhance the therapeutic effect of Dox, the drug delivery field has focused its attention on designing nanoparticles that are capable of releasing a drug efficiently when exposed to a specific triggering mechanism [1,2]. Such triggers include pH, light, ultrasound, enzymatic action, and heat [13]. Among the trigger-sensitive nanoparticle formulations that have been developed, ultrasound-sensitive liposomes (bubble liposomes) could function as a novel gene delivery tool by exposure to ultrasound [25,18]. Therefore, it is necessary to optimize the AG73–Dox for in vivo application by changing the peptide modification ratio or PEG ratio. Furthermore, the combination of AG73–Dox with bubble liposomes and ultrasound may enable enhancement of the therapeutic effect.
4. Conclusion
In this study, doxorubicin-encapsulating AG73 peptide-modified liposomes (AG73–Dox) were developed to increase the intracellular uptake of anticancer drugs and to achieve an improved therapeutic effect specifically against tumors. The AG73 peptide is a very suitable tumor-targeting molecule because it is known to be a ligand for syndecans, which are expressed in various cancer cells. The intracellular uptake of AG73–Dox was higher than that of control liposomes (PEG–Dox and AG73T–Dox). Moreover, AG73–Dox exhibited cytotoxicity and antitumor effects in vitro and in vivo. In addition, AG73 peptide-modified liposomes intended to bind intratumoral vessels within the tumor. Thus, further optimization of AG73-L toward tumor targeting may lead to a development of a useful tool for cancer therapy.
Acknowledgments
This work was supported in part by the Promotion and Mutual Aid Corporation for Private Schools of Japan.
References
- 1.Andresen T.L., Jensen S.S., Jørgensen K. Advanced strategies in liposomal cancer therapy: problems and prospects of active and tumor specific drug release. Prog Lipid Res. 2005;44:68–97. doi: 10.1016/j.plipres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Andresen T.L., Jensen S.S., Kaasgaard T., Jørgensen K. Triggered activation and release of liposomal prodrugs and drugs in cancer tissue by secretory phospholipase A2. Curr Drug Deliv. 2005;2:353–362. doi: 10.2174/156720105774370203. [DOI] [PubMed] [Google Scholar]
- 3.Carey D.J. Syndecans: multifunctional cell-surface co-receptors. Biochem J. 1997;327:1–16. doi: 10.1042/bj3270001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dos Santos N., Cox K.A., McKenzie C.A., van Baarda F., Gallagher R.C., Karlsson G. pH gradient loading of anthracyclines into cholesterol-free liposomes: enhancing drug loading rates through use of ethanol. Biochim Biophys Acta. 2004;1661:47–61. doi: 10.1016/j.bbamem.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Fears C.Y., Woods A. The role of syndecans in disease and wound healing. Matrix Biol. 2006;25:443–456. doi: 10.1016/j.matbio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Gabizon A., Shmeeda H., Horowitz A.T., Zalipsky S. Tumor cell targeting of liposome-entrapped drugs with phospholipid-anchored folic acid-PEG conjugates. Adv Drug Delivery Rev. 2004;56:1177–1192. doi: 10.1016/j.addr.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Han I., Park H., Oh E.S. New insights into syndecan-2 expression and tumourigenic activity in colon carcinoma cells. J Mol Histol. 2004;35:319–326. doi: 10.1023/b:hijo.0000032363.78829.4e. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman M.P., Nomizu M., Roque E., Lee S., Jung D.W., Yamada Y. Laminin-1 and laminin-2 G-domain synthetic peptides bind syndecan-1 and are involved in acinar formation of a human submandibular gland cell line. J Biol Chem. 1998;273:28633–28641. doi: 10.1074/jbc.273.44.28633. [DOI] [PubMed] [Google Scholar]
- 9.Hwang T., Han H.D., Song C.K., Seong H., Kim J.H., Chen X. Anticancer drug-phospholipid conjugate for enhancement of intracellular drug delivery. Macromol Symp. 2007;249–250:109–115. doi: 10.1002/masy.200750318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeon T.J., Lee J.D., Ha J.W., Yang W.I., Cho S.H. Evaluation of cardiac adrenergic neuronal damage in rats with doxorubicin-induced cardiomyopathy using iodine-131 MIBG autoradiography and PGP 9.5 immunohistochemistry. Eur J Nucl Med. 2000;27:686–693. doi: 10.1007/s002590050563. [DOI] [PubMed] [Google Scholar]
- 11.Lee R.J. Liposomal delivery as a mechanism to enhance synergism between anticancer drugs. Mol Cancer Ther. 2006;5:1639–1640. doi: 10.1158/1535-7163.MCT-06-C02. [DOI] [PubMed] [Google Scholar]
- 12.Lentacker I., Geers B., Demeester J., De Smedt S.C., Sanders N.N. Design and evaluation of doxorubicin-containing microbubbles for ultrasound-triggered doxorubicin delivery: cytotoxicity and mechanisms involved. Mol Ther. 2010;18:101–108. doi: 10.1038/mt.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S.D., Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm. 2008;5:496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- 14.Lukyanov A.N., Elbayoumi T.A., Chakilam A.R., Torchilin V.P. Tumor-targeted liposomes: doxorubicin-loaded long-circulating liposomes modified with anti-cancer antibody. J Control Release. 2004;100:135–144. doi: 10.1016/j.jconrel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Maeda H., Wu J., Sawa T., Matsumura Y., Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 16.Matsui Y., Kobayashi N., Nishikawa M., Takakura Y. Sequence-specific suppression of mda1a/mda1b expression in mice via RNA interference. Pharm Res. 2005;22:2091–2098. doi: 10.1007/s11095-005-8178-8. [DOI] [PubMed] [Google Scholar]
- 17.Mertens G., Cassiman J.J., Van den Berghe H., Vermylen J., David G. Cell surface heparan sulfate proteoglycans from human vascular endothelial cells. Core protein charcterization and antithrombin III binding properties. J Biol Chem. 1992;267:20435–20443. [PubMed] [Google Scholar]
- 18.Negishi Y., Endo Y., Fukuyama T., Suzuki R., Takizawa T., Omata D. Delivery of siRNA into the cytoplasm by liposomal bubbles and ultrasound. J Control Release. 2008;132:124–130. doi: 10.1016/j.jconrel.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Negussie A.H., Miller J.L., Reddy G., Drake S.K., Wood B.J., Dreher M.R. Synthesis and in vitro evaluation of cyclic NGR peptide targeted thermally sensitive liposome. J Control Release. 2010;143:265–273. doi: 10.1016/j.jconrel.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson L.E., Bedja D., Alvey S.J., Cardounel A.J., Gabrielson K.L., Reeves R.H. Protection from doxorubicin-induced cardiac toxicity in mice with a null allele of carbonyl reductase 1. Cancer Res. 2003;63:6602–6606. [PubMed] [Google Scholar]
- 21.Pan X.G., Wu G., Yang W.L., Barth R.F., Tjarks W., Lee R.J. Synthesis of cetuximab-immunoliposomes via a cholesterol-based membrane anchor for targeting of EGFR. Bioconjugate Chem. 2007;18:101–108. doi: 10.1021/bc060174r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patil R.R., Guhagarkar S.A., Devarajan P.V. Engineered nanocarriers ofdoxorubicin: a current update. Crit Rev Ther Drug Carrier Syst. 2008;25:1–61. doi: 10.1615/critrevtherdrugcarriersyst.v25.i1.10. [DOI] [PubMed] [Google Scholar]
- 23.Perez A.T., Domenech G.H., Frankel C., Vogel C.L. Pegylated liposomal doxorubicin (Doxil) for metastatic breast cancer: the Cancer Research Network, Inc., experience. Cancer Invest. 2002;20:22–29. doi: 10.1081/cnv-120014883. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki N., Ichikawa N., Kasai S., Yamada M., Nishi N., Morioka H. Syndecan binding sites in the laminin alpha1chain G domain. Biochemistry. 2003;42:12625–12633. doi: 10.1021/bi030014s. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki R., Takizawa T., Negishi Y., Hagisawa K., Tanaka K., Sawamura K. Gene delivery by combination of novel liposomal bubbles with perfluoropropane and ultrasound. J Control Release. 2007;117:130–136. doi: 10.1016/j.jconrel.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Tkachenko E., Rhodes J.M., Simons M. Syndecans: new kids on the signaling block. Circ Res. 2005;96:488–500. doi: 10.1161/01.RES.0000159708.71142.c8. [DOI] [PubMed] [Google Scholar]
- 27.Wu J., Liu Q., Lee R.J. A folate receptor-targeted liposomal formulation for paclitaxel. Int J Pharm. 2006;316:148–153. doi: 10.1016/j.ijpharm.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 28.Xiang G., Wu J., Lu Y., Liu Z., Lee R.J. Synthesis and evaluation of a novel ligand for folate-mediated targeting liposomes. Int J Pharm. 2008;356:29–36. doi: 10.1016/j.ijpharm.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiong X.B., Huang Y., Lu W.L., Zhang X., Zhang H., Nagai T. Enhanced intracellular delivery and improved antitumor efficacy of doxorubicin by sterically stabilized liposomes modified with a synthetic RGD mimetic. J Control Release. 2005;107:262–275. doi: 10.1016/j.jconrel.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 30.Yoo H.S., Park T.G. Folate receptor targeted biodegradable polymeric doxorubicin micelles. J Control Release. 2004;96:273–283. doi: 10.1016/j.jconrel.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Zamboni W.C. Liposomal, nanoparticle, and conjugated formulations of anticancer agents. Clin Cancer Res. 2005;11:8230–8234. doi: 10.1158/1078-0432.CCR-05-1895. [DOI] [PubMed] [Google Scholar]
- 32.Essner J.J., Chen E., Ekker S.C. Syndecan-2. Int J Biochem Cell Biol. 2006;38:152–156. doi: 10.1016/j.biocel.2005.08.012. [DOI] [PubMed] [Google Scholar]


