Significance
ErbB2 (v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2) is overexpressed in around 25% of breast cancers. The present study reveals that Erbin, an ErbB2-interacting protein that was thought to act as an antitumor factor, facilitates ErbB2-dependent proliferation of breast cancer cells and tumorigenesis in MMTV-neu transgenic mice. Disruption of the interaction decreases ErbB2-dependent proliferation, and deletion of the PDZ domain in Erbin hinders ErbB2-dependent tumor development in MMTV-neu mice. Erbin forms a complex with ErbB2, promotes its interaction with the chaperon protein HSP90, and thus prevents its degradation. ErbB2 and Erbin expression correlates in human breast tumor tissues. Thus, this study identifies the interaction of Erbin and ErbB2 as a novel drug target linking another clinical molecular target, HSP90, in ErbB2-positive breast cancer.
Keywords: ErbB2, Erbin, HSP90, breast cancer, stability
Abstract
ErbB2 (v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2), a receptor tyrosine kinase of the ErbB family, is overexpressed in around 25% of breast cancers. In addition to forming a heterodimer with other ErbB receptors in response to ligand stimulation, ErbB2 can be activated in a ligand-independent manner. We report here that Erbin, an ErbB2-interacting protein that was thought to act as an antitumor factor, is specifically expressed in mammary luminal epithelial cells and facilitates ErbB2-dependent proliferation of breast cancer cells and tumorigenesis in MMTV-neu transgenic mice. Disruption of their interaction decreases ErbB2-dependent proliferation, and deletion of the PDZ domain in Erbin hinders ErbB2-dependent tumor development in MMTV-neu mice. Mechanistically, Erbin forms a complex with ErbB2, promotes its interaction with the chaperon protein HSP90, and thus prevents its degradation. Finally, ErbB2 and Erbin expression correlates in human breast tumor tissues. Together, these observations establish Erbin as an ErbB2 regulator for breast tumor formation and progression.
The human epidermal growth factor receptor (HER) family of receptor tyrosine kinases, including epidermal growth factor receptor (EGFR, HER1, ErbB1), ErbB2 (v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2; HER2, neu), ErbB3 (HER3), and ErbB4 (HER4), has been implicated in tumor growth and progression. ErbB2, in particular, is overexpressed in around 25% of breast cancers, conferring high recurrence, malignant metastasis, and poor prognosis, (1, 2) and is also overexpressed in ovarian, stomach, and uterine tumors (3, 4). Upon ligand stimulation, ErbB receptors dimerize to activate downstream signaling (4). Unique in the ErbB family, ErbB2 does not have a ligand but is a preferred heterodimerization partner of other ErbB receptors in response to ligand stimulation. In mice, overexpression of ErbB2 or its activated form in mammary epithelium induces diffuse epithelial hyperplasia, mammary tumors, and lung metastases (5, 6). Unlike other ErbB members such as EGFR, which, upon activation, becomes internalized and sorted to lysosomes for degradation, ErbB2 is refractory to endocytosis and degradation (7, 8). The underlying mechanisms of ErbB2 stability are not well characterized.
Erbin is a cytoplasmic protein that contains leucine-rich repeats (LRR) and PSD95/Dlg1/zo-1 (PDZ) domain (thus named a LAP protein) (9, 10). Erbin interacts, via its single PDZ domain, specifically with ErbB2, but not with ErbB3, ErbB4, or EGFR (11, 12). It is colocalized with ErbB2 at the basolateral membranes of epithelial cells (11). In vitro studies are inconsistent regarding the role of Erbin in cell proliferation and tumorigenesis. It was thought to act as a tumor suppressor by inhibiting TGFβ or Erk signaling (13–15). On the other hand, knockdown of Erbin in HT-29 colon cancer cells appears to inhibit the formation of multicellular tumor spheroids (16).
Here we report that Erbin is expressed in mouse mammary epithelial cells and is implicated in ErbB2-dependent proliferation of breast cancer cells. Absence of Erbin delays breast tumor formation in transgenic MMTV-neu mice, an established model of breast tumorigenesis, indicating a role of Erbin in ErbB2-dependent tumorigenesis. This effect is specific for ErbB2 because loss of Erbin has no effect on breast tumor development induced by overexpression of polyomavirus middle T antigen (PyVT). We have investigated underlying mechanisms by in vitro and in vivo experiments. Our results indicate that Erbin increases ErbB2-dependent proliferation and tumorigenesis by promoting ErbB2 stability. Finally, a significant correlation between Erbin and ErbB2 expression was observed in human breast cancer tissues. Together, these observations identify Erbin as a positive regulator of ErbB2-dependent breast tumor formation and progression.
Results
Erbin Expression in Luminal Epithelial Cells of Mammary Glands.
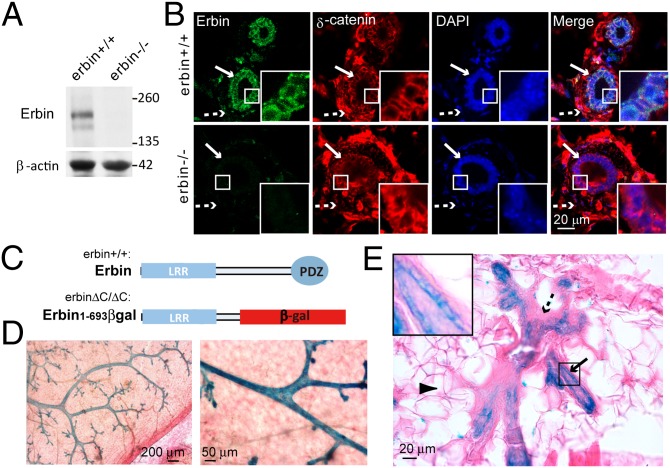
To assess the role of Erbin in ErbB2 tumorigenicity in vivo, we first determined Erbin expression in murine mammary glands. Mammary fat pads were isolated from adult wild-type mice (erbin+/+) and Erbin null (erbin−/−) mice and homogenized for protein extraction. Homogenates were subjected to Western blot analysis using affinity-purified anti-Erbin antibody. A dominant band that migrated at a predicted 180 kDa as well as a weak band with smaller molecular weight was identified (Fig. 1A). Both bands disappeared in samples isolated from erbin−/− mice, suggesting that they represent full-length Erbin and a likely proteolytic product. These results suggest that Erbin is expressed in mammary glands and is specifically recognized by the anti-Erbin antibody. To determine which cells express Erbin, sections of mammary glands were immunostained with anti-Erbin antibody. Myoepithelial and luminal epithelial cells expressed δ-catenin, a p120-catenin family protein known to be enriched at adherence junctions (17). Notably, Erbin immunoreactivity was restricted to δ-catenin–positive luminal epithelial cells (Fig. 1B). Specificity of the staining was demonstrated by absence of signal in sections isolated from erbin−/− mice (Fig. 1B). Furthermore, Erbin was colocalized with Keratin 8 (K8), a marker of luminal epithelial cells, but not with K14 or smooth muscle actin, two markers of myoepithelial cells (Fig. S1). These results indicate that Erbin expression is restricted in luminal epithelial cells of mouse mammary glands.
Fig. 1.
Erbin is expressed in luminal epithelial cells of mammary glands. (A) Expression of Erbin in mammary tissues. Mammary tissues of 3-mo-old mice were homogenized and blotted with affinity-purified anti-Erbin antibody. β-Actin served as loading control. (B) Localization of Erbin in luminal epithelial cells. Sections of mammary ducts were immunostained using anti-Erbin antibody. Arrows, luminal epithelial cells; dashed arrows, myoepithelial cells. (C) Schematic diagram of domain structures of wild-type Erbin and Erbin1–693βgal. Note that β-gal is expressed as a protein fused to truncated Erbin. (D) β-Gal activity was detected in mammary epithelial ducts in erbin+/ΔC mice. Mammary fat pads of erbin+/ΔC mice were stained whole mount for β-gal in situ activity. Shown are representative images at two magnifications. (E) β-Gal activity was confined to epithelial cells of the mammary gland from erbin+/ΔC mice. Arrowhead, adipocyte; Arrow, luminal epithelial cells; dashed arrow, myoepithelial cells. Areas in the small squares are enlarged in the Insets.
We next took advantage of our gene-trap erbin mouse model (erbin+/ΔC mice) to assess Erbin expression in the murine mammary gland. In erbin+/ΔC mice, one allele of the erbin gene is disrupted by a lacZ gene inserted in intron 20 leading to expression of a fusion protein, Erbin1–693βgal, which comprises the N-terminal region of Erbin (amino acids 1–693) fused to β-galactosidase (Fig. 1C). As expression of Erbin1–693βgal remains controlled by the promoter of the erbin gene, X-gal activity in Erbin1–693βgal faithfully tracks expression of Erbin in tissues. Erbin mutation did not cause apparent alteration in mammary gland structures in adult mice (Fig. S2). We therefore determined Erbin expression in breast fat pads of erbin+/ΔC mice subjected to X-gal staining (18). As shown in Fig. 1D, X-gal in situ assay revealed that the β-gal activity was detectable exclusively in mammary gland ducts but not in stromal cells. Staining of cross-sections indicated that β-gal activity was confined to luminal epithelial cells that formed a single layer lining mammary ducts, but not to myoepithelial cells or adipocytes (Fig. 1E). These results corroborate that Erbin is specifically expressed in luminal epithelial cells of mammary glands.
In Vivo Inhibition of ErbB2 Tumorigenicity by Loss of Erbin.
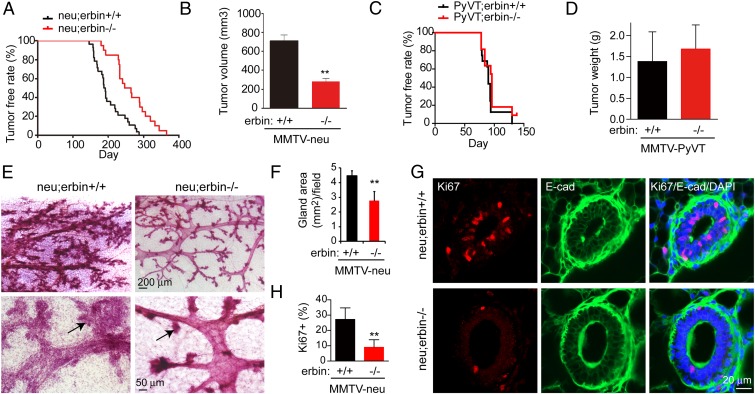
Considering the interaction between Erbin and ErbB2, we next investigated whether Erbin is necessary for breast cancer development that is promoted by ErbB2 in vivo. MMTV-neu mice express the rat homolog of ErbB2, neu, under the transcriptional control of the mouse mammary tumor virus (MMTV) promoter (5) and have served as a model for ErbB2-driven breast cancer (5, 6). MMTV-neu overexpression led to formation of adenocarcinoma in the mammary glands, revealed by hematoxylin and eosin (H&E) staining (Fig. S3). In a full congenic context with pure FVB mouse strain background, MMTV-neu;erbin−/− (neu;erbin−/−) mice developed tumors at a significantly slower rate than MMTV-neu;erbin+/+ (neu;erbin+/+) mice, indicating that absence of Erbin significantly delayed mammary tumor development (Fig. 2A, P < 0.0001). To assess the tumor growth rate, tumor volume was measured for each mouse 5 wk after tumor onset. Notably, tumor volume that developed after the same time span was significantly smaller in neu;erbin−/− mice compared with neu;erbin+/+ (Fig. 2B), demonstrating the decrease of tumor growth by ablation of Erbin. Lung metastases were found in neu;erbin+/+ and neu;erbin−/− mice 2 mo after breast tumor detection (Fig. S3).
Fig. 2.
Erbin mutation delays ErbB2-dependent tumorigenesis in vivo. (A) Kaplan–Meier survival plot. Log-rank (Mantel–Cox) test, P < 0.0001. Tumors were examined weekly in neu;erbin+/+ and neu;erbin−/− virgin littermates and sisters (n = 28 and 22, respectively). (B) Reduced tumor volumes in neu;erbin−/−, compared with neu;erbin+/+ littermates. Volumes were measured 5 wk after first detection of tumors for each mouse (n = 10 both groups; **P < 0.01). (C) Erbin mutation had no effect on tumorigenesis in MMTV-PyVT mice. Kaplan–Meier survival plot. Log-rank (Mantel–Cox) test, P > 0.05. Tumors were examined weekly in PyVT;erbin+/+ (n = 22) and PyVT;erbin−/− (n = 16) virgin littermates and sisters. (D) Erbin depletion had no effect on breast tumor growth in MMTV-PyVT mice. Tumors were weighed 3 wk after detection (n = 8; P > 0.05). (E) Hyperplasia in nontumor mammary glands of neu;erbin+/+ but not neu;erbin−/− littermates during diestrus phase at the same age. Tumor-free mammary glands were stained whole mount by hematoxylin. Arrows indicate branch buds of mammary ducts. (F) Quantitative analysis of data in E (n = 3; **P < 0.01). (G) Fewer Ki67-positive cells in nontransformed mammary glands of neu;erbin−/− mice. Tumor-free mammary gland sections were stained for E-cadherin (E-cad) and Ki67. (H) Quantitative analysis of results in G (n = 3; **P < 0.01).
To determine whether Erbin regulation of mammary gland tumorigenesis is specific for ErbB2-dependent tumorigenicity, we crossed erbin−/− mice with MMTV-PyVT mice that express the polyomavirus middle T antigen in mammary epithelial cells and induce similar luminal breast cancers (19, 20). MMTV-PyVT mice develop multifocal tumors at an early age with high incidence (21). Interestingly, loss of Erbin had no effect on tumor incidence (Fig. 2C), weight (Fig. 2D), fibrotic texture, or metastasis (Fig. S4A). These results indicate that Erbin ablation has no effect on mammary gland tumorigenesis due to overexpression of the polyomavirus middle T antigen. ErbB2 was not overexpressed in breast tumors in MMTV-PyVT mice, in contrast to its expression in MMTV-neu tumors (Fig. S4B). Together, these observations suggest that ablation of Erbin specifically delays ErbB2-dependent tumorigenesis.
Examination of tumors in mammary glands from neu;erbin+/+ and neu;erbin−/− mice did not show apparent difference at histopathological level (Fig. S3). However, nontumor mammary glands of neu;erbin+/+ mice showed epithelial hyperplasia and alveolar formation at pretumor stages, which were less present in neu;erbin−/− littermates when mammary glands were examined at same age (Fig. 2 E and F). This suggested that absence of Erbin delays or suppresses the tumor initiation. To test the hypothesis that Erbin accelerated tumorigenesis by promoting epithelial proliferation, we examined expression of Ki67, a nuclear proliferation marker, in nontransformed mammary glands from littermate mice at same age. As shown in Fig. 2 G and H, ∼27% of luminal epithelial cells in nontransformed mammary ducts from neu;erbin+/+ mice were positive in Ki67 staining, indicating an active proliferating status. In contrast, fewer cells in neu;erbin−/− mammary glands were stained by anti-Ki67 antibody (Fig. 2 G and H), suggesting that Erbin promotes the proliferation of luminal epithelial cells to initiate tumorigenesis.
Erbin Knockdown Suppresses ErbB2-Dependent Proliferation of Breast Cancer Cells.
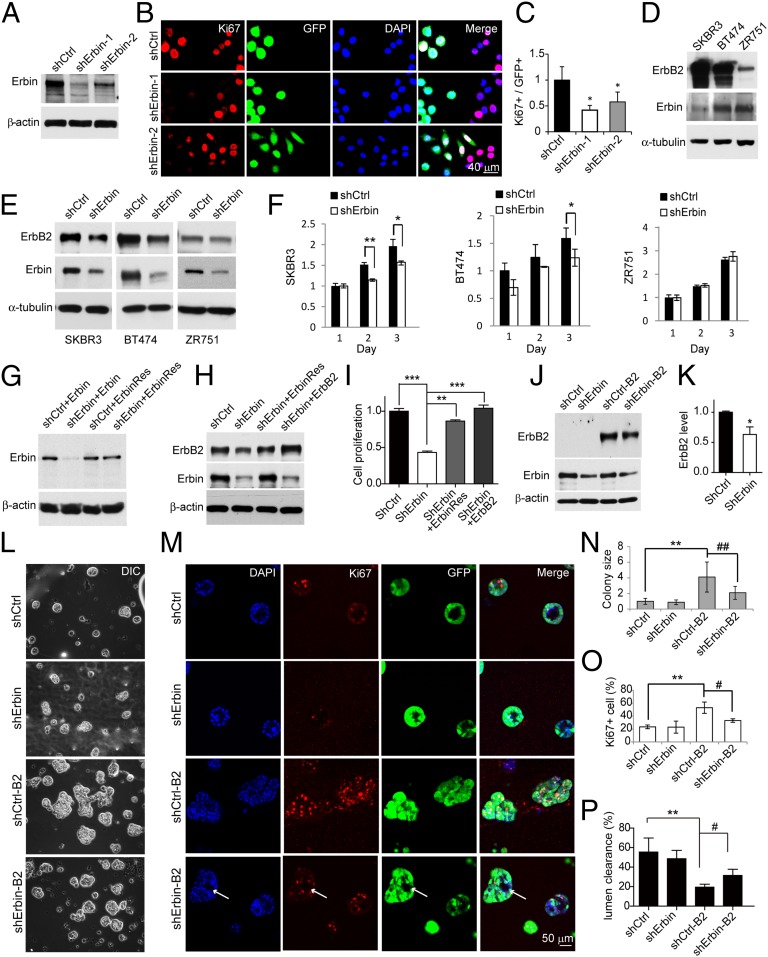
Given that ablation of Erbin delayed ErbB2-dependent tumor generation and growth in mouse models, we next asked if Erbin regulates ErbB2-dependent proliferation of breast cancer cells. SKBR3 cells, whose proliferation is dependent on high levels of ErbB2 (22, 23), were infected with lentiviruses encoding two different Erbin shRNAs (shErbin-1 and shErbin-2) or scrambled sequence (shCtrl) and stained for Ki67. Efficient repression of Erbin expression by shRNAs was tested by Western blotting (Fig. 3A). Ki67-positive cells were significantly reduced in shErbin virus-infected cells (identified by viral GFP) (Fig. 3 B and C), supporting the notion that Erbin depletion suppresses the proliferation of ErbB2-overexpressing cancer cells. To test this further, SKBR3 and BT474 cells (Fig. 3D)—whose proliferation is ErbB2-dependent (22, 23) as shown by cell growth inhibition with trastuzumab and PD158780 (Fig. S5A)—were infected by lentiviral shErbin-1 (18, 24) and sorted by FACS using GFP to establish stable cell populations. As shown in Fig. 3E, levels of Erbin in breast cancer cells were reduced by 70–90% in shErbin-infected cells, compared with those infected with shCtrl, which correlated with ErbB2 reduction. Growth of shErbin-BT474 and shErbin-SKBR3 cells was significantly reduced by day 2 or 3 of plating, compared with shCtrl-infected cells (Fig. 3F). As control, Erbin knockdown had little effect on cell proliferation of ZR751 (Fig. 3F and Fig. S5B), a cell line whose proliferation is independent of ErbB2 (23) (Fig. S5A). The effect of shErbin could be rescued by coexpressing ErbinRes, a shErbin-resistant construct (Fig. 3 G–I), excluding the possible off-target effect of shErbin. Moreover, ErbB2 expression was able to rescue shErbin-induced growth suppression in SKBR3 cells (Fig. 3 H and I), but not in ZR751 cells (Fig. S5 B and C).
Fig. 3.
Erbin promotes ErbB2-dependent breast cancer proliferation. (A) Erbin knockdown in SKBR3 cells by two Erbin shRNA constructs (shErbin). shCtrl, control shRNA. (B) shErbin reduced the number of Ki67-positive breast cancer cells. SKBR3 cells were infected with lentiviral shCtrl or shErbin and stained for Ki67. Infected cells were identified by expression of GFP. (C) Quantitative analysis of data in B (n = 3; *P < 0.05), compared with shCtrl. (D) ErbB2 expression in breast cancer cell lines. (E) Erbin knockdown in breast cancer cells. Breast cancer cells were infected with shCtrl or shErbin lentivirus. Lysates of GFP-positive cells were blotted with indicated antibodies. (F) shErbin suppression of ErbB2-dependent cell growth. BT474, SKBR3, and ZR751 cells were infected with shCtrl or shErbin lentivirus and sorted by FACS. Cell growth was monitored by MTS assay (n = 3; *P < 0.05; **P < 0.01). (G) Resistance of ErbinRes to Erbin knockdown. Myc-tagged Erbin or ErbinRes was cotransfected with shCtrl or shErbin in HEK293 cells. Lysates were blotted with anti-Myc or β-actin antibodies. (H and I) Rescue of shErbin-mediated ErbB2 reduction (H) and cell proliferation (I) by ErbinRes or exogenous ErbB2. SKBR3 cells were transfected with ShCtrl or shErbin without or with ErbinRes or ErbB2. Western blot and cell proliferation by MTS assay were examined 3 d after transfection (n = 3; **P < 0.01; ***P < 0.001). (J) Erbin knockdown reduced expression of Erbin and ErbB2 in MCF10A cells. MCF10A cells were infected with shCtrl or shErbin lentivirus and purified by FACS to yield shCtrl- and shErbin-MCF10A cells. They were transfected with Flag-ErbB2 and pcDNA3.1 that encodes the neomycin resistance gene and treated with G418 to yield shCtrl-B2 and shErbin-B2 cells. Lysates were blotted for Flag, Erbin, or β-actin. (K) Quantitative analysis of ErbB2 levels in J (n = 3; *P < 0.05). (L–P) Suppressed formation of ErbB2-dependent multiacini in Matrigel by Erbin knockdown. Cells described in J were cultured. Shown were phase (L) and confocal (M) images of acini 14 d in culture. Arrows, empty lumen in acini. (N) Reduced size in acinar structure by shErbin (normalized by shCtrl). n > 3; **P < 0.01, compared with shCtrl; ##P < 0.01, compared with shCtrl-B2. (O) Reduction in Ki67-positive cells by shErbin. n > 3; **P < 0.01, compared with shCtrl; #P < 0.05, compared with shCtrl-B2. (P) Increased lumen clearance by shErbin. n > 3; **P < 0.01, compared with shCtrl; #P < 0.05, compared with shCtrl-B2.
Human mammary epithelial MCF10A cells, when cultured on Matrigel, form acini-like structures with a single layer of polarized, growth-arrested cells and lumen in the center (25). Infection with viral shCtrl or shErbin had no effect on forming acini-like structures in MCF10A cells (Fig. 3 J–N). ErbB2 overexpression caused the formation of multiacini-like structures in MCF10A cells (Fig. 3 L and M) and increased the number of Ki67-positive cells (shCtrl-B2 versus shCtrl) (Fig. 3 M and O). Erbin reduction in naive MCF10A cells had little effect on the size of acini-like structures or the number of Ki67-positive cells (Fig. 3 L–O). In contrast, its reduction prevented ErbB2 from increasing the size of acini-like structures and the number of Ki67-positive cells (Fig. 3 L–O). Lumen clearance and hollow lumen phenotype were observed in Erbin knockdown acini-like structures (Fig. 3 M–P and Fig. S6). These results indicate that Erbin promotes ErbB2-dependent epithelial cell proliferation, consistent with the in vivo observations.
Ablation of Erbin Decreases ErbB2 Levels in Vivo.
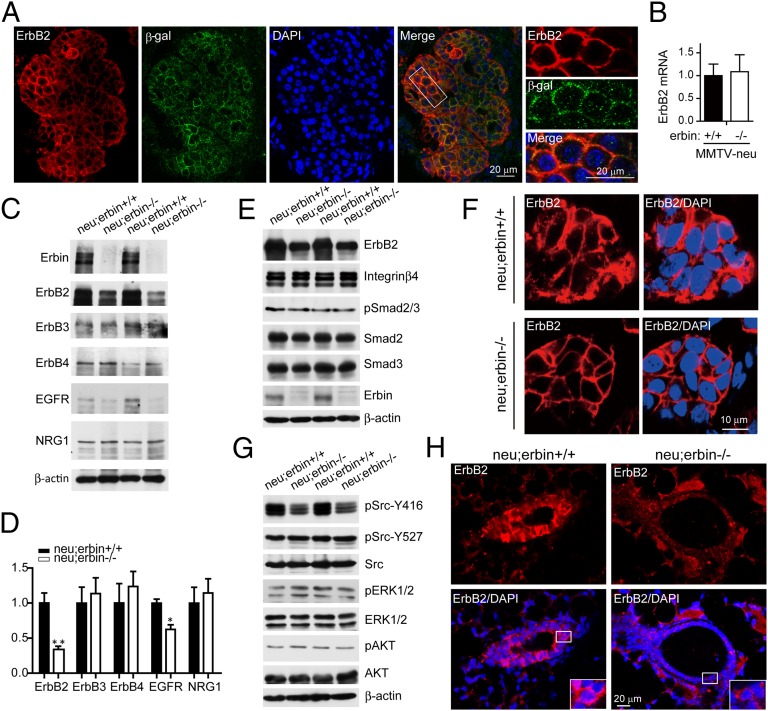
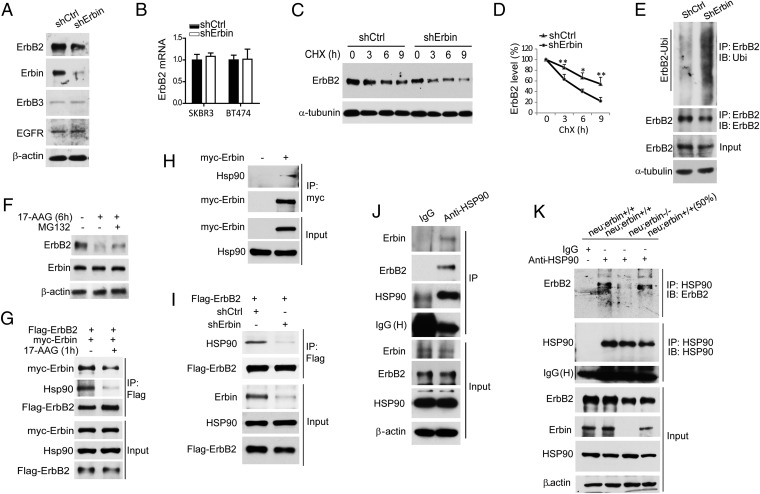
Next, we investigated possible mechanisms by which Erbin ablation suppresses ErbB2-dependent tumor development. First, we examined whether Erbin and ErbB2 colocalized in breast tumor cells. Because currently available anti-Erbin and ErbB2 antibodies are generated in rabbits, we took advantage of Erbin1–693β-gal expression in neu;erbin ΔC/ΔC mice and stained breast tumors of these mice with rabbit anti-ErbB2 antibody and chicken anti–β-gal antibody. Erbin1–693 has similar membrane location as full-length Erbin (Fig. 1 B and E and Fig. S7), consistent with the reports that truncation of the C terminus does not change the cellular distribution of Erbin (26, 27). Thus, the location of Erbin1–693βgal would represent that of Erbin in tumor cells. As shown in Fig. 4A, both ErbB2 and Erbin1–693βgal concentrated at membranes of tumor cells and were localized mostly into the same registries. Colocalization of Erbin and ErbB2 suggests that Erbin may regulate the function of ErbB2 at the plasma membrane.
Fig. 4.
Depletion of Erbin reduced ErbB2 protein levels in breast tumor. (A) Colocalization of Erbin and ErbB2 in breast tumor cells. Tumor sections from neu;erbinΔC/ΔC mice were costained by rabbit anti-ErbB2 and chicken anti–β-gal antibodies. (B) Erbin depletion did not alter ErbB2 mRNA levels in breast tumors. Total RNA was subjected to quantitative RT-PCR. Results were normalized to internal control α-tubulin (n = 3; P > 0.05). (C) Decrease of ErbB2 proteins in tumor tissues of neu;erbin−/− mice. Breast tumors were isolated from indicated genotypes, homogenized, and blotted for indicated proteins. (D) Quantitative analysis of protein levels in C (n = 3; *P < 0.05; **P < 0.01). (E) Characterization of Erbin-interacting proteins in tumor tissues. Homogenates were blotted for indicated proteins. (F) Reduced ErbB2 immunoreactivity in individual breast tumor cells in neu;erbin−/− mice. Tumor sections were stained by rabbit anti-ErbB2 antibody. (G) Reduced Src activity in tumor tissues of neu;erbin−/− mice. Homogenates were blotted for indicated pan- or phosphorylated proteins. (H) Reduced ErbB2 immunoreactivity in epithelial cells of nontransformed mammary glands in neu;erbin−/− mice. Mammary sections were stained by rabbit anti-ErbB2 antibody.
Next, we examined the expression of Erbin and ErbB2 levels in tumors in MMTV-neu or control mice. ErbB2 levels in breast tumors of MMTV-neu mice were excessive due to ErbB2 overexpression (5). However, Erbin loss caused a decrease in ErbB2 protein, but not mRNA, levels in MMTV-neu breast tumors (Fig. 4 B–D; n = 3; P < 0.05). Notably, ErbB2 basolateral localization identified in alveolar structures appeared to be similar in neu;erbin+/+ and neu;erbin−/− mice (Fig. S8). These observations demonstrate that Erbin contributes to the excessive protein amounts of ErbB2. Notably, no (in ErbB3, ErbB4, or NRG1) or mild (in EGFR) changes of control protein levels were observed for MMTV-neu tumors by Erbin depletion (Fig. 4 C and D). In addition to ErbB2, Erbin interacts with several proteins, some of which, including integrin β4, Smad2, and Smad3, have been implicated in tumorigenesis (28, 29). However, their protein level and/or phosphorylation in MMTV-neu–induced breast tumors was not changed by Erbin mutation (Fig. 4E), suggesting that they might not be involved.
To examine whether the reduction of ErbB2 in neu;erbin−/− tumors was a primary target or secondary to slowed tumor progression, we stained ErbB2 in breast tumors. As shown in Fig. 4F, ErbB2 levels were reduced in tumor cells. Together with Erbin and ErbB2 colocalization at membranes of tumor cells (Fig. 4A), these results suggest a possible cell-autonomous regulatory mechanism of Erbin. Next, we determined whether Erbin changes the level and activity of known ErbB2 downstream proteins in breast tumors. As shown in Fig. 4G, pErk and pAkt was similar in MMTV-neu–induced tumors of erbin wild-type and mutant mice, suggesting that the PI3K or Erk pathways are not altered by Erbin mutation. We examined phosphorylation of Src Y416 and Y527, the phosphorylation of which indicates activation and inactivation of the kinase, respectively (30). Intriguingly, phosphorylation of Y416, but not of Y527, was reduced in tumor lysates of Erbin mutant mice, compared with control mice (Fig. 4G). These results suggest that Erbin may be necessary for Src activation via ErbB2 during tumorigenesis. In addition, ErbB2 levels in mammary glands before epithelial hyperplasia were higher in neu;erbin+/+ mice than in neu;erbin−/− mice (Fig. 4H), suggesting a role of Erbin in tumor initiation.
Promotion of ErbB2 Stability by Erbin.
To investigate how Erbin regulates ErbB2 levels, we suppressed Erbin expression in BT474 breast cancer cells. As shown in Fig. 5A, levels of ErbB2, but not of EGFR or ErbB3, were reduced in BT474 cells by Erbin knockdown, identifying ErbB2 as a primary target. In addition, EGFR overexpression was unable to rescue the decrease of cell proliferation due to Erbin reduction (Fig. S9). Because EGFR does not bind to Erbin (11), this result suggests that EGFR reduction in neu;erbin−/− breast tumors may be secondary (Fig. 4 C and D). With no change in ErbB2 mRNA (Figs. 4B and 5B), Erbin may thus regulate ErbB2 protein by a posttranscriptional mechanism. To test this hypothesis, we determined if Erbin altered ErbB2 stability by treating BT474 cells with cycloheximide (CHX) to inhibit protein synthesis. Depletion of Erbin expedited ErbB2 degradation (Fig. 5 C and D), with half-life reduced from ∼9 h in shCtrl-expressing breast cancer cells to ∼4.5 h in shErbin-expressing breast cancer cells (n = 3, P < 0.05). This result suggests that Erbin stabilizes ErbB2 in breast cancer cells, which is consistent with an earlier report (18). In support of this notion, knockdown of Erbin promoted ErbB2 ubiquitination (Fig. 5E).
Fig. 5.
Erbin stabilizes ErbB2 by promoting ErbB2-HSP90 association. (A) Reduction in ErbB2, but not EGFR or ErbB3, in BT474 cells by shErbin. BT474 cells were infected with lentiviral shErbin, sorted by FACS, and analyzed for expression of indicated proteins by Western blot. (B) shErbin had no effect on ErbB2 mRNA levels in breast cancer cells. Total RNA was subjected to real time RT-PCR. Results were normalized to internal control α-tubulin. (C) Erbin is required for ErbB2 stabilization in breast cancer cells. Stable BT474 cells in A were treated by CHX (50 μM) for indicated times and analyzed for endogenous ErbB2 by Western blot. (D) Quantitative analysis of data in C (n = 3; *P < 0.05; **P < 0.01). (E) Erbin knockdown increased ErbB2 ubiquitination in breast cancer cells. HA-Ubiquitin was electroporated into shCtrl- or shErbin-BT474 stable cells. Cells were treated with MG132 (20 μM) for 2 h. ErbB2 complex in resulting lysates was examined using antibody against Ubiquitin (Ubi). (F) 17-AAG induced degradation of ErbB2 but not Erbin. HEK293 cells were transfected with Flag-ErbB2 and treated with 17-AAG with or without MG132 for 6 h. Lysates were blotted by indicated antibodies. (G) HSP90 is required for Erbin–ErbB2 interaction. HEK293 cells were transfected with Flag-ErbB2 and Myc-Erbin and treated with 17-AAG for 1 h. Lysates were subjected to immunoprecipitation with anti-Flag antibody, and the complex was blotted for Myc, Flag, and HSP90. (H) Association of Erbin with HSP90. HEK293 cells were transfected with Myc-Erbin. Lysates were subjected to immunoprecipitation using anti-Myc antibody, and precipitates were blotted with Myc or HSP90 antibodies. (I) Erbin is required for ErbB2-HSP90 association. HEK293 cells were transfected first with shCtrl or shErbin and 48 h later with Flag-ErbB2. The complex of Flag-ErbB2 was blotted for HSP90. (J) Ternary complex of ErbB2–Erbin–HSP90 in breast cancer cells. SKBR3 cells lysates were subjected to HSP90 precipitation, and precipitates were examined for the presence of Erbin and ErbB2. (K) Erbin is required for ErbB2–HSP90 interaction in vivo. Breast tumor lysates were subjected to immunoprecipitation with anti-HSP90 antibody, and precipitates were blotted with indicated antibodies.
ErbB2 is a client protein of HSP90, and disruption of the HSP90 complex by 17-allylamino-demethoxygeldanamycin (17-AAG) resulted in ErbB2 degradation (Fig. 5F), which was partially blocked by MG132, an inhibitor of the ubiquitination-proteasome pathway (31–33). Notably, 17-AAG had no effect on Erbin levels (Fig. 5F), suggesting a dissociation of Erbin from ErbB2 when the HSP90 complex is disrupted. Indeed, Erbin in the ErbB2 complex was reduced in cells within 1 h of 17-AAG treatment (Fig. 5G), a condition that does not alter ErbB2 levels but inhibits ErbB2–HSP90 interaction (34). This result suggests that HSP90 facilitates the Erbin-ErbB2 association. Intriguingly, Erbin also interacted with HSP90 in the absence of exogenous ErbB2 (Fig. 5H). The results suggest that ErbB2, HSP90, and Erbin may form a ternary complex. In this model, Erbin serves as a regulator for HSP90 to stabilize ErbB2 rather than a client of HSP90 because it was not degraded by 17-AAG treatment (Fig. 5G). When Erbin was reduced, little HSP90 was present in the ErbB2 complex (Fig. 5I), suggesting impaired interaction between HSP90 and ErbB2. Note that the ErbB2–Erbin–HSP90 ternary complex could be recovered in breast cancer cells (Fig. 5J). In tumors of neu;erbin−/− mice, the ErbB2-HSP90 association was reduced, in comparison with that in neu;erbin+/+ tumors (Fig. 5K). The reduction was not due to decreased ErbB2 levels in tumors because robust coprecipitation of ErbB2 and HSP90 was observed when a half amount of neu;erbin+/+ tumor lysates was used as input. The in vivo evidence supports the model that interaction of HSP90 with its client ErbB2 is impaired in tumors where Erbin expression was lost.
Role for Erbin–ErbB2 Interaction in Breast Cancer.
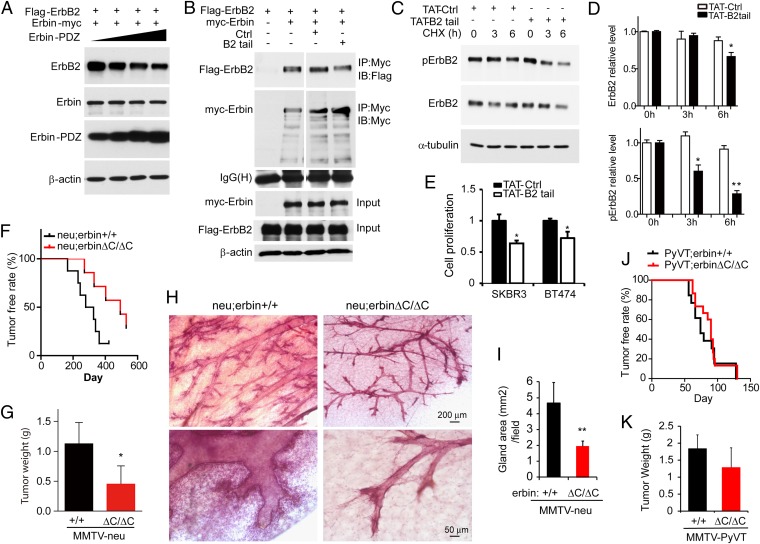
Erbin is known to form a complex with ErbB2 by interacting with its C-terminal region (via Erbin’s PDZ domain) (11, 12). To determine if the interaction is required for Erbin regulation of ErbB2 stability and activity, we explored the consequence of overexpressing Erbin and ErbB2 domains that are sufficient for interaction and may serve as dominant negative inhibitors. Intriguingly, coexpression of the PDZ domain of Erbin reduced ErbB2 levels (Fig. 6A), suggesting that the Erbin–ErbB2 interaction is required for Erbin activity. To test this hypothesis in breast cancer cells, we generated B2tail, a peptide that contains the C-terminal 15-aa residues of ErbB2 that is sufficient to interact with Erbin (12). B2tail was able to partially disrupt the Erbin–ErbB2 interaction (Fig. 6B). To facilitate cell membrane penetrance, we generated TAT-B2tail, a peptide that contains B2tail and additional 11-aa residues of the transactivator of transcription (TAT) of HIV (35). SKBR3 cells were treated with 20 μM TAT-B2tail for 1 h before the addition of CHX to inhibit protein synthesis. As shown in Fig. 6C, ErbB2 reduction was faster in TAT-B2tail–treated SKBR3 cells, compared with cells treated with TAT-Ctrl peptide (scrambled). Accordingly, phosphorylated ErbB2 (pErbB2), which represents activated ErbB2, was reduced in TAT-B2tail–treated cells, compared with cells treated with TAT-Ctrl (Fig. 6C). Note that TAT-B2tail–induced pErbB2 reduction was more dramatic than that of ErbB2 protein (Fig. 6 C and D), indicating additional impairment of activity. Importantly, TAT-B2tail but not control peptides significantly decreased proliferation of SKBR3 and BT474 cells about 40 and 35%, respectively (Fig. 6E). This result suggests that disruption of the Erbin–ErbB2 interaction increases ErbB2 degradation, reduces ErbB2 signaling, and inhibits ErbB2-dependent cell proliferation.
Fig. 6.
Requirement of the Erbin–ErbB2 interaction for ErbB2 stabilization and ErbB2-dependent tumorigenesis. (A) Decreased ErbB2 expression by the Erbin-PDZ domain. HEK293 cells were transfected with Flag-ErbB2 together with increasing doses of Myc-Erbin-PDZ. Cell lysates were blotted with antibodies against ErbB2 and Myc. (B) Disruption of the Erbin–ErbB2 interaction by B2tail. Lysates of transfected cells expressing Flag-ErbB2 or Myc-Erbin were incubated with control peptide (Ctrl) or B2tail. The Erbin complex was precipitated using anti-Myc antibody and probed for ErbB2. (C) Treatment with TAT-B2tail decreases ErbB2 stability and pErbB2 in human breast cancer cells. SKBR3 cells were incubated with 20 μM TAT-Ctrl or TAT-B2tail for 1 h before analysis of ErbB2 stability as in Fig. 5C. (D) Quantitative analysis of data in C (n = 3; *P < 0.05; **P < 0.01). (E) TAT-B2tail treatment inhibits proliferation of SKBR3 and BT474 cells. Cells were treated with 20 μM TAT-Ctrl or TAT-B2tail peptide for 24 h and analyzed by MTS assay (n = 3; *P < 0.05). (F) PDZ deletion inhibited tumorigenesis in MMTV-neu mice. neu;erbinΔC/ΔC and neu;erbin+/+ virgin littermates or sisters (n = 7 and 8, respectively) were examined weekly for tumors. Kaplan–Meier survival plot. Log-rank (Mantel–Cox) test, P < 0.0001. (G) Reduced tumor weight in neu;erbin ΔC/ΔC, compared with neu;erbin+/+ littermates. Tumors were isolated 5 wk after first detection of tumors (n = 7 and 8, respectively; *P < 0.05). (H) Reduced hyperplasia in nontumor mammary glands of neu;erbinΔC/ΔC mice. Shown is whole-mount hematoxylin staining of tumor-free mammary glands in neu;erbin+/+ and neu;erbinΔC/ΔC littermates during diestrus phase at the same age. (I) Quantitative analysis of data in H (n = 3; **P < 0.01). (J) PDZ deletion had no effect on tumorigenesis in MMTV-PyVT mice. PyVT;erbin+/+ (n = 23) and PyVT;erbinΔC/ΔC (n = 15) virgin littermates and sisters were examined for tumors. Kaplan–Meier survival plot. Log-rank (Mantel–Cox) test, P > 0.05. (K) PDZ deletion had no effect on breast tumor growth in MMTV-PyVT mice. Tumors were isolated 3 wk after detection (n = 8; P > 0.05).
Erbin-PDZ Domain-Mediated Interaction Is Important for Tumorigenesis in MMTV-neu Mice.
To investigate whether the interaction between Erbin and ErbB2 is crucial for mammary gland tumorigenesis and progression in vivo, MMTV-neu mice were crossed with erbinΔC/ΔC mice. As described above, erbinΔC/ΔC mice express Erbin1–693βgal that lacks 694–1,450 amino acids of Erbin, a sequence that comprises the PDZ domain. Thus, Erbin1–693βgal is unable to interact with ErbB2 (12). Notably, neu;erbinΔC/ΔC mice resembled almost all phenotypes observed in neu;erbin−/− mice, delayed tumor onset time (Fig. 6F), and compromised tumor growth rate (Fig. 6G), in comparison with congenic neu;erbin+/+ mice. These results indicate that the C-terminal region of Erbin including the PDZ domain is involved in mammary tumor development in MMTV-neu mice, in agreement with results of in vitro studies. Also as observed in neu;erbin−/− mice, neu;erbinΔC/ΔC mice showed no obvious epithelial hyperplasia of mammary glands at the same age as littermate controls before tumor onset (Fig. 6 H and I), suggesting a role of the Erbin C-terminal region in ErbB2-driven tumorigenesis. Finally, multifocal, fibrotic tumors were observed in PyVT;erbinΔC/ΔC at a similar rate and size as those in PyVT;erbin+/+ (Fig. 6 J and K), indicating that deletion of the C-terminal region in Erbin had no effect on mammary gland tumorigenesis due to overexpression of the polyomavirus middle T antigen. Together, these results support a working model where Erbin regulates stability and activity of ErbB2 and functions through a direct interaction.
Erbin and ErbB2 Protein Levels Are Correlated in Human Breast Tumors.
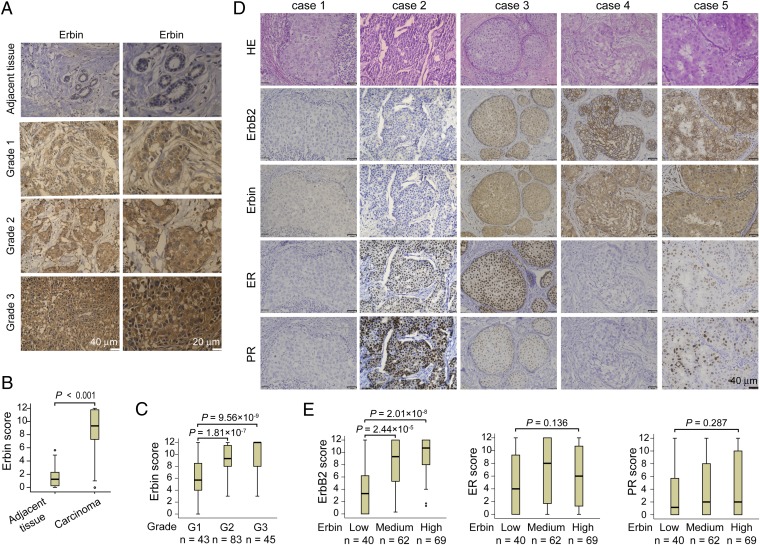
To determine if Erbin and ErbB2 levels correlate in primary breast tumor tissues, we examined Erbin expression in human breast samples and its relationship with ErbB2-overexpressing tumors. A total of 171 breast cancer specimens, mostly from invasive breast carcinomas, were analyzed by immunohistochemistry for ErbB2 and Erbin expression as well as estrogen receptor (ER) and progesterone receptor (PR) levels by using specific anti-Erbin antibodies (Fig. 1) (24). Preabsorption of the anti-Erbin antibody with the antigen reduced staining intensity to background levels in human cancer specimens (Fig. S10). Compared with adjacent normal tissues, Erbin staining was significantly higher in tumor tissues (Fig. 7 A and B). Erbin staining was categorized to three levels (low, scores 0–4; medium, scores 5–8; and high, scores 9–12) by the German semiquantitative scoring system that takes into consideration both staining intensity and area. Of 171 samples, 36% showed medium levels and 40% expressed high levels of Erbin (Table 1). Notably, there was a positive correlation between pathological grades and Erbin staining (Fig. 7C). Moreover, Erbin levels correlated with those of ErbB2, but not with those of ER or PR (Fig. 7 D and E and Table 1). These observations demonstrate an association between Erbin and human breast cancer and ErbB2 expression in tumor samples.
Fig. 7.
Correlation between Erbin and ErbB2 expression in human breast tumor tissues. (A) Representative human primary breast tumor tissues stained for Erbin. (B) Increased Erbin levels in breast tumors. Erbin expression scores are shown as box plots, with horizontal lines indicating the median; the bottom and top of boxes indicating the 25th and 75th percentiles, respectively; and the vertical bars indicating the range of data. Outliers are marked with a circle (n = 171; P < 0.001, Mann–Whitney U test). (C) Correlation of Erbin expression with pathological grades of tumors. Erbin expression scores are shown as box plots, as described in B. Sample numbers of different grades are described below each respective group. Data were analyzed by the Mann–Whitney U test. (D) Immunohistochemcial staining of human primary breast tumor tissues. Consecutive sections of tissues were subjected to H&E staining and immunostaining with antibodies against ErbB2, Erbin, ER, and PR. Case 1 showed weak staining for ErbB2, Erbin, ER, or PR. Case 2 was positive for ER and PR. Cases 3 and 5 were positive for ErbB2, Erbin, ER, and PR. Case 4 was positive for ErbB2 and Erbin. (E) Increased ErbB2, but not ER or PR, in tumor tissues with high levels of Erbin. Erbin expression levels were grouped into low, medium, or high levels as described in Results. ErbB2, ER, and PR expression scores are shown as box plots. Sample numbers of Erbin groups are described below respective group. Data were analyzed by the Mann–Whitney U test and the Kruskal–Wallis test.
Table 1.
Association of Erbin levels with different clinicopathologic characteristics
| Erbin expression | |||||
| Characteristics | n | Low (40): n (%) | Medium (62): n (%) | High (69): n (%) | P value |
| Age, y | |||||
| Mean | 171 | 49.5 | 50.2 | 47.1 | 0.154 |
| 95% CI | 46.1–52.8 | 48.0–52.5 | 44.9–49.4 | ||
| Primary tumor size | |||||
| ≤2 cm | 7 (17.9) | 16 (25.8) | 19 (28.4) | ||
| 2–5 cm | 168 | 25 (64.1) | 40 (64.5) | 41 (61.2) | 0.620 |
| ≥5 cm | 7 (17.9) | 6 (9.7) | 7 (10.4) | ||
| No. of positive nodes | |||||
| 0 | 13 (43.3) | 19 (37.3) | 17 (37.8) | ||
| 1–3 | 126 | 9 (30.0) | 15 (29.4) | 16 (35.6) | 0.867 |
| >3 | 8 (26.7) | 17 (33.3) | 12 (26.7) | ||
| Histologic grade | |||||
| G1 | 22 (55.0) | 17 (27.4) | 4 (5.8) | ||
| G2 | 171 | 11 (27.5) | 34 (54.8) | 38 (55.1) | 1.69 × 10−7*** |
| G3 | 7 (17.5) | 11 (17.7) | 27 (39.1) | ||
| Estrogen receptor | |||||
| Low | 20 (50.0) | 24 (38.7) | 29 (42.0) | ||
| Medium | 171 | 7 (17.5) | 9 (14.5) | 14 (20.3) | 0.604 |
| High | 13 (32.5) | 29 (46.8) | 26 (37.7) | ||
| Progesterone receptor | |||||
| Low | 29 (72.5) | 37 (59.7) | 42 (60.9) | ||
| Medium | 171 | 6 (15.0) | 11 (17.7) | 9 (13.0) | 0.493 |
| High | 5 (12.5) | 14 (22.6) | 18 (26.1) | ||
| ErbB2/Neu status | |||||
| Low | 26 (65.0) | 15 (24.2) | 5 (7.2) | ||
| Medium | 171 | 9 (22.5) | 14 (22.6) | 19 (27.5) | 7.08 × 10−7*** |
| High | 5 (12.5) | 33 (53.2) | 45 (65.2) | ||
This cohort of 171 patients includes 158 cases of invasive ductal carcinoma, 6 cases of ductal carcinoma in situ, 4 cases of invasive lobular carcinoma, and 3 cases of mixed-type carcinoma. The cases were classified into grade I (G1, 43 cases), grade II (G2, 83 cases), and grade III (G3, 45 cases) based on an assessment of tubule/gland formation, nuclear pleomorphism, and mitotic counts according to the WHO classification criteria (51–53). ***P < 0.001.
Discussion
Here we show that Erbin controls ErbB2 stability and activity and acts as a positive regulator in tumor generation and progression. Erbin is expressed in epithelial cells in mammary glands (Fig. 1). It promotes ErbB2-dependent growth of breast cancer cells (Fig. 3) and expedites tumorigenesis in MMTV-neu mice (Fig. 2). These effects are ErbB2-specific because Erbin is not required for tumor onset and progression in MMTV-PyVT mice. Suppression of Erbin expression or disruption of its interaction with ErbB2 inhibits ErbB2-dependent tumor development or proliferation of breast cancer cells (Fig. 6). These effects are probably driven by the ability of Erbin to promote ErbB2 stability (Fig. 4). Finally, there is a significant correlation between Erbin and ErbB2 expression in human breast tumor tissues and tumor grades (Fig. 7 and Table 1). Together, these observations indicate that Erbin facilitates breast tumor development.
ErbB2 levels correlate with recurrence, malignant metastasis, and poor prognosis (1–3). Effective drugs directly targeting ErbB2 include trastuzumab and pertuzumab, two humanized antibodies directed against the extracellular domain of ErbB2 (36–38), and lapatinib, an inhibitor of EGFR and ErbB2 kinase (39). However, cancer cells often escape from trastuzumab or lapatinib treatment via intrinsic or de novo pathways (1, 40). For example, ErbB2-positive breast tumors may become resistant to trastuzumab by alternative splicing or by proteolytic cleavage that generates p95HER2, an intracellular truncated form of ErbB2 (41). Thus, the interaction of Erbin and ErbB2 might serve as a previously unidentified drug target for ErbB2-positive breast cancer.
Mechanisms that lead to high ErbB2 levels in breast cancer have begun to be unraveled. In addition to gene amplification, recent studies suggest a role of posttranscriptional regulation (42–44). ErbB2 is thought to be resistant to endocytosis and degradation (7, 8). It interacts with chaperon proteins HSP90 and HSP70 and two different E3 ligases, CHIP and c-Cbl. Inhibition of HSP90 leads to its dissociation from ErbB2 and activates ErbB2 internalization and interaction with HSP70 and the E3 ligase CHIP, leading to ubiquitination and degradation (32, 33, 45, 46). It was unclear how the degradation pathway of ErbB2 was regulated. Earlier reports showed that C-terminal truncation or loss of the PDZ-binding motif of ErbB2 increased its endocytosis and degradation (42, 47). Here we provide evidence that the PDZ domain containing protein Erbin is required for ErbB2 stability. Erbin overexpression increases the stability of ErbB2 (12, 18) whereas knockdown of Erbin reduces its half-life and consequently decreases the proliferation of ErbB2-expressing breast cancer cells (Figs. 3 and 4). These observations indicate that Erbin maintains ErbB2 levels in breast cancer cells for tumor development. In a working model, Erbin interacts with ErbB2 to promote its association with HSP90, a protein necessary for ErbB2 stability.
HSP90 has been targeted for breast cancer therapy, but its plethora clients may cause side effects of HSP90 inhibitors (48). Recently, lipid raft-associated flotillins were found to be complexed with ErbB2 and HSP90 (49). Depletion of flotillin-1 or -2 disrupted the complex and destabilized ErbB2, suggesting that the regulation of HSP90 chaperon activity on ErbB2 is tightly controlled (49). Flotillin-2 expression positively correlated with ErbB2 and potentially predicts prognosis of breast cancer (49). Together with our findings, regulation of the HSP90 chaperon activity on ErbB2 appears to be important in breast cancer development. Notably, Erbin is present in lipid rafts (12, 24, 27). It remains to be determined whether Erbin and flotillin-1 or -2 are coordinated to regulate HSP90–ErbB2 interaction or other HSP90 client proteins such as macrophage migration inhibitory factor (50) in breast tumor development.
Our observation that ablation or mutation of Erbin had no effect on tumor onset and progression in MMTV-PyVT mice indicates that Erbin specifically regulates ErbB2-dependent tumor growth. Erbin levels were conspicuously higher in breast tumors of both MMTV-neu mice (Fig. S11) and patients (Fig. 7 and Table 1) and correlated with ErbB2 levels and tumor grades, suggesting a previously unidentified diagnostic and therapeutic target for breast cancers. How Erbin is increased in ErbB2-dependent carcinomas warrants further investigation. These observations, however, suggest a positive feedback loop between Erbin and ErbB2, which eventually participates in tumorigenesis.
Materials and Methods
Animals, constructs, antibodies and reagents, human breast tissue specimens, cell culture and transfection, lentivirus packaging and infection, Matrigel 3D culture, MTS [3-(4,5-dimethyl-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] assay, immunofluorescence and immunohistochemistry, whole-mount hematoxylin staining of mammary glands, X-gal in situ assay, protein interaction and Western blotting, quantitative RT-PCR, and statistical analysis are described in SI Materials and Methods.
Animal experiments were approved by the Institutional Animal Care and Use Committee of the Georgia Regents University and the French Guidelines for Animal Handling (Committee for Animal Experimentation of Provence no. 14 of Marseille, 2-091009). Human primary breast specimens of paraffin-embedded tissue blocks were obtained from the Department of Pathology and a tissue bank at the Center for Experimental Medicine, the First Affiliated Hospital of Nanchang University, China. Specimens were collected and processed in compliance with protocols approved by the Institutional Review Board of Nanchang University. Informed consent was provided by human subjects in the course of this research.
Supplementary Material
Acknowledgments
We thank Dr. Quansheng Du (Georgia Regents University) for lentivirus constructs and suggestions; Dr. Dihua Yu (MD Anderson Cancer Center) for breast cancer cell lines; William King (Georgia Regents University) for assistance on flow cytometry; and Dr. Shuang Huang (Georgia Regents University), Dr. Christophe Ginestier (INSERM), Dr. Bruno Chetaille (INSERM), Cécile Meunier (INSERM), members of the Animal and Cell Imaging Facilities of Cancer Research Center of Marseille, especially Patrick Gibier, and members of the L.M. and W.-C.X. laboratories for discussion. This work was supported in part by grants from the NIH (to L.M. and W.-C.X.) and by the La Ligue Contre le Cancer (J.-P.B.) and by SIRIC [INCa-DGOS-Inserm 6038 (to J.-P.B.)], Ministry of Research (fellowship to W.T.), and National Natural Science Foundation of China [NSFC 31371075 (to Y.T.)]. J.-P.B. is a scholar of Institut Universitaire de France.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.K.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1407139111/-/DCSupplemental.
References
- 1.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: Understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3(5):269–280. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 2.Yu D, Hung MC. Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene. 2000;19(53):6115–6121. doi: 10.1038/sj.onc.1203972. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 4.Yarden Y, Pines G. The ERBB network: At last, cancer therapy meets systems biology. Nat Rev Cancer. 2012;12(8):553–563. doi: 10.1038/nrc3309. [DOI] [PubMed] [Google Scholar]
- 5.Guy CT, et al. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89(22):10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988;54(1):105–115. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- 7.Baulida J, Kraus MH, Alimandi M, Di Fiore PP, Carpenter G. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem. 1996;271(9):5251–5257. doi: 10.1074/jbc.271.9.5251. [DOI] [PubMed] [Google Scholar]
- 8.Lenferink AE, et al. Differential endocytic routing of homo- and hetero-dimeric ErbB tyrosine kinases confers signaling superiority to receptor heterodimers. EMBO J. 1998;17(12):3385–3397. doi: 10.1093/emboj/17.12.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403(6770):676–680. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- 10.Santoni MJ, Pontarotti P, Birnbaum D, Borg JP. The LAP family: A phylogenetic point of view. Trends Genet. 2002;18(10):494–497. doi: 10.1016/s0168-9525(02)02738-5. [DOI] [PubMed] [Google Scholar]
- 11.Borg JP, et al. ERBIN: A basolateral PDZ protein that interacts with the mammalian ERBB2/HER2 receptor. Nat Cell Biol. 2000;2(7):407–414. doi: 10.1038/35017038. [DOI] [PubMed] [Google Scholar]
- 12.Huang YZ, Wang Q, Xiong WC, Mei L. Erbin is a protein concentrated at postsynaptic membranes that interacts with PSD-95. J Biol Chem. 2001;276(22):19318–19326. doi: 10.1074/jbc.M100494200. [DOI] [PubMed] [Google Scholar]
- 13.Dai F, et al. Erbin inhibits transforming growth factor beta signaling through a novel Smad-interacting domain. Mol Cell Biol. 2007;27(17):6183–6194. doi: 10.1128/MCB.00132-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai P, Xiong WC, Mei L. Erbin inhibits RAF activation by disrupting the sur-8-Ras-Raf complex. J Biol Chem. 2006;281(2):927–933. doi: 10.1074/jbc.M507360200. [DOI] [PubMed] [Google Scholar]
- 15.Huang YZ, Zang M, Xiong WC, Luo Z, Mei L. Erbin suppresses the MAP kinase pathway. J Biol Chem. 2003;278(2):1108–1114. doi: 10.1074/jbc.M205413200. [DOI] [PubMed] [Google Scholar]
- 16.Dardousis K, et al. Identification of differentially expressed genes involved in the formation of multicellular tumor spheroids by HT-29 colon carcinoma cells. Mol Ther. 2007;15(1):94–102. doi: 10.1038/sj.mt.6300003. [DOI] [PubMed] [Google Scholar]
- 17.Anastasiadis PZ, Reynolds AB. The p120 catenin family: Complex roles in adhesion, signaling and cancer. J Cell Sci. 2000;113(Pt 8):1319–1334. doi: 10.1242/jcs.113.8.1319. [DOI] [PubMed] [Google Scholar]
- 18.Tao Y, et al. Erbin regulates NRG1 signaling and myelination. Proc Natl Acad Sci USA. 2009;106(23):9477–9482. doi: 10.1073/pnas.0901844106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herschkowitz JI, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8(5):R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai KV, et al. Initiating oncogenic event determines gene-expression patterns of human breast cancer models. Proc Natl Acad Sci USA. 2002;99(10):6967–6972. doi: 10.1073/pnas.102172399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: A transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12(3):954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kao J, Pollack JR. RNA interference-based functional dissection of the 17q12 amplicon in breast cancer reveals contribution of coamplified genes. Genes Chromosomes Cancer. 2006;45(8):761–769. doi: 10.1002/gcc.20339. [DOI] [PubMed] [Google Scholar]
- 23.Neve RM, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10(6):515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tao Y, et al. Erbin interacts with TARP γ-2 for surface expression of AMPA receptors in cortical interneurons. Nat Neurosci. 2013;16(3):290–299. doi: 10.1038/nn.3320. [DOI] [PubMed] [Google Scholar]
- 25.Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3(9):785–792. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izawa I, Nishizawa M, Hayashi Y, Inagaki M. Palmitoylation of ERBIN is required for its plasma membrane localization. Genes Cells. 2008;13(7):691–701. doi: 10.1111/j.1365-2443.2008.01198.x. [DOI] [PubMed] [Google Scholar]
- 27.Legouis R, et al. Basolateral targeting by leucine-rich repeat domains in epithelial cells. EMBO Rep. 2003;4(11):1096–1102. doi: 10.1038/sj.embor.7400006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu S, Simin K, Khan A, Mercurio AM. Analysis of integrin beta4 expression in human breast cancer: Association with basal-like tumors and prognostic significance. Clin Cancer Res. 2008;14(4):1050–1058. doi: 10.1158/1078-0432.CCR-07-4116. [DOI] [PubMed] [Google Scholar]
- 29.Xie W, et al. Alterations of Smad signaling in human breast carcinoma are associated with poor outcome: A tissue microarray study. Cancer Res. 2002;62(2):497–505. [PubMed] [Google Scholar]
- 30.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4(6):470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 31.Xu W, et al. Sensitivity of mature Erbb2 to geldanamycin is conferred by its kinase domain and is mediated by the chaperone protein Hsp90. J Biol Chem. 2001;276(5):3702–3708. doi: 10.1074/jbc.M006864200. [DOI] [PubMed] [Google Scholar]
- 32.Xu W, Mimnaugh EG, Kim JS, Trepel JB, Neckers LM. Hsp90, not Grp94, regulates the intracellular trafficking and stability of nascent ErbB2. Cell Stress Chaperones. 2002;7(1):91–96. doi: 10.1379/1466-1268(2002)007<0091:hngrti>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou P, et al. ErbB2 degradation mediated by the co-chaperone protein CHIP. J Biol Chem. 2003;278(16):13829–13837. doi: 10.1074/jbc.M209640200. [DOI] [PubMed] [Google Scholar]
- 34.Xu W, Yuan X, Beebe K, Xiang Z, Neckers L. Loss of Hsp90 association up-regulates Src-dependent ErbB2 activity. Mol Cell Biol. 2007;27(1):220–228. doi: 10.1128/MCB.00899-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: Delivery of a biologically active protein into the mouse. Science. 1999;285(5433):1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 36.Baselga J, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol. 1996;14(3):737–744. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 37.Cobleigh MA, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 38.Agus DB, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2(2):127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 39.Geyer CE, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 40.Abramson V, Arteaga CL. New strategies in HER2-overexpressing breast cancer: Many combinations of targeted drugs available. Clin Cancer Res. 2011;17(5):952–958. doi: 10.1158/1078-0432.CCR-09-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scaltriti M, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99(8):628–638. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 42.Lerdrup M, et al. Endocytic down-regulation of ErbB2 is stimulated by cleavage of its C-terminus. Mol Biol Cell. 2007;18(9):3656–3666. doi: 10.1091/mbc.E07-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magnifico A, et al. Protein kinase Calpha determines HER2 fate in breast carcinoma cells with HER2 protein overexpression without gene amplification. Cancer Res. 2007;67(11):5308–5317. doi: 10.1158/0008-5472.CAN-06-3936. [DOI] [PubMed] [Google Scholar]
- 44.Vernimmen D, Gueders M, Pisvin S, Delvenne P, Winkler R. Different mechanisms are implicated in ERBB2 gene overexpression in breast and in other cancers. Br J Cancer. 2003;89(5):899–906. doi: 10.1038/sj.bjc.6601200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Austin CD, et al. Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol Biol Cell. 2004;15(12):5268–5282. doi: 10.1091/mbc.E04-07-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu W, et al. Chaperone-dependent E3 ubiquitin ligase CHIP mediates a degradative pathway for c-ErbB2/Neu. Proc Natl Acad Sci USA. 2002;99(20):12847–12852. doi: 10.1073/pnas.202365899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shelly M, et al. Polar expression of ErbB-2/HER2 in epithelia. Bimodal regulation by Lin-7. Dev Cell. 2003;5(3):475–486. doi: 10.1016/j.devcel.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10(8):537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pust S, et al. Flotillins as regulators of ErbB2 levels in breast cancer. Oncogene. 2013;32(29):3443–3451. doi: 10.1038/onc.2012.357. [DOI] [PubMed] [Google Scholar]
- 50.Schulz R, et al. Inhibiting the HSP90 chaperone destabilizes macrophage migration inhibitory factor and thereby inhibits breast tumor progression. J Exp Med. 2012;209(2):275–289. doi: 10.1084/jem.20111117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ellis IO, et al. 2003. Pathology and genetics of tumours of the breast and female genital organs. World Health Organization Classification of Tumours, eds Tavassoli F, Devilee P, (WHO, Geneva), pp 14–41.
- 52.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 53.Robbins P, et al. Histological grading of breast carcinomas: A study of interobserver agreement. Hum Pathol. 1995;26(8):873–879. doi: 10.1016/0046-8177(95)90010-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.