Significance
Several developmental and physiological processes require that cells display a controlled ability to migrate in an orientated manner. This capacity is also reacquired by certain cancer cells during their progression toward aggressiveness that allows them to establish distant metastases. The Rho GTPases are instrumental in the control of orientated migration. Here, we demonstrate that the estrogen-related receptor α (ERRα), whose high expression correlates with tumor aggressiveness and poor prognosis, decreases the stability and activity of the RHOA protein and induces orientated cell migration. Together with other published data, our results show that inactivation of ERRα may reduce tumor aggressiveness.
Keywords: cell migration, nuclear receptor, RhoA, protein stability, ERR
Abstract
Several physiopathological processes require orientated cellular migration. This phenomenon highly depends on members of the RHO family of GTPases. Both excessive and deficient RHO activity impair directional migration. A tight control is thus exerted on these proteins through the regulation of their activation and of their stability. Here we show that the estrogen-related receptor α (ERRα) directly activates the expression of TNFAIP1, the product of which [BTB/POZ domain-containing adapter for Cullin3-mediated RhoA degradation 2 (BACURD2)] regulates RHOA protein turnover. Inactivation of the receptor leads to enhanced RHOA stability and activation. This results in cell disorientation, increased actin network, and inability to form a lamellipodium at the migration edge. As a consequence, directional migration, but not cell motility per se, is impaired in the absence of the receptor, under pathological as well as physiological conditions. Altogether, our results show that the control exerted by ERRα on RHOA stability is required for directional migration.
Deciphering the multiple phenomena and pathways leading to the establishment of metastasis is a key challenge for the understanding of cancer. The process of metastasis involves several components in the primary tumor such as the capacity for cells to dissociate from their original tissue [which includes the loss of E-cadherin–mediated intercellular junctions during the epithelial–mesenchymal transition (EMT)], invade their immediate environment, intravasate into (and extravasate out of) vessels, survive into circulation, and nest in target tissues. The acquisition of cell migration is required to achieve several of these processes.
The Rho family of GTPases is instrumental in regulating cell motility and invasive phenotype in vivo and in vitro (1–3). RAC1 regulates the formation of the membrane protrusions (lamellipodia) at the leading edge of the migrating cells, whereas RHOA regulates the assembly of actin stress fibers through its downstream effectors mDIA and ROCKs and limits the extent of the lamellipodium (4–7). Rho GTPases oscillate between an inactive (GDP bound) and an active (GTP bound) form. Guanine nucleotide exchange factors (GEFs) catalyze the exchange of GDP for GTP, whereas GTPase activating proteins (GAPs) stimulate GTP hydrolysis, thus promoting Rho GTPases activation and deactivation, respectively (8). In addition, regulation of protein stability and degradation has emerged as a particularly important level of control of Rho GTPase activity (9). For instance the degradation of GTP-bound RHOA is specifically induced by SMURF1, the overexpression of which leads to loss of actin stress fibers and reduced cell migration (10). Another protein complex containing CULLIN3 and BTB/POZ domain-containing adapter for Cullin3-mediated RhoA degradation (BACURD protein 1 and 2) negatively impacts on the stability of GDP-bound RHOA (11). siRNA-mediated inactivation of CULLIN3 or BACURDs leads to RHOA overexpression, which strikingly also results in reduced cell migration. Altogether, this demonstrates that not only RHOA activation but also its expression must be tightly regulated for appropriate cellular migration. However, the upstream regulators of these processes have not been identified so far.
The estrogen-related receptor α (ERRα) is an orphan member of the nuclear receptor superfamily of transcription factors (12, 13), the high expression of which is correlated to a poor prognosis in breast cancer as well as in other types of tumors (14–17). ERRα controls the expression of genes involved in oxidative phosphorylation, lipid metabolism, and the tricarboxylic acid cycle (18, 19). These effects are exerted in tissues with important energy demands such as the liver, skeletal muscle, heart, and adipose tissue. Interestingly expression of a subset of ERRα-regulated genes (in particular “metabolic” ones) negatively correlates with disease-free survival in patients with breast cancer (20). Furthermore, ERRα has recently been suggested to mediate the switch toward aerobic glycolysis, referred to as the Warburg effect, which is particularly crucial for various cell types, including cancer cells (21–24). However, the activities of ERRα in cancer cells may not be limited to its direct impact on metabolism. Indeed deactivating ERRα using specific inverse agonists inhibits the proliferation of tumor cells in vitro or assayed as xenografts, possibly via an increased expression of the p21 cell cycle inhibitor (25, 26). Moreover ablation of ERRα in knock-out mice delays ERBB2-induced mammary gland tumorigenesis (27). Furthermore overexpression of ERRα in xenografted breast cancer cells increases their metastatic capacities (28), in correlation with a massive induction of tumoral angiogenesis, likely reflecting the regulation of VEGF by ERRα (29, 30). The angiogenic effect displayed in vivo by ERRα may however impair the detection of other regulatory functions exerted by the receptor on cell migration and invasion. Indeed inactivation of ERRα impairs in vitro migration of breast cancer cells (31, 32). In this respect, an autoregulatory loop involving ERRα and the Wnt11/β-cat pathway has been documented that leads to increased chemotactic migration. However, the effects of ERRα on cell motility per se (understood as the intrinsic capacity of a cell to move) have not been investigated.
Using inactivation approaches as well as phenocopy and complementation experiments, we show here that ERRα is required for cell orientation, lamellipodium formation, and directional migration. At the molecular level, we demonstrate that ERRα directly regulates the expression of BACURD2, leading to controlled RHOA stability, activation, and downstream activity. ERRα is thus an upstream regulator of RHOA stability, maintaining its expression appropriately low to allow orientated cell migration.
Results
Inhibiting ERRα Induces Cell Disorientation.
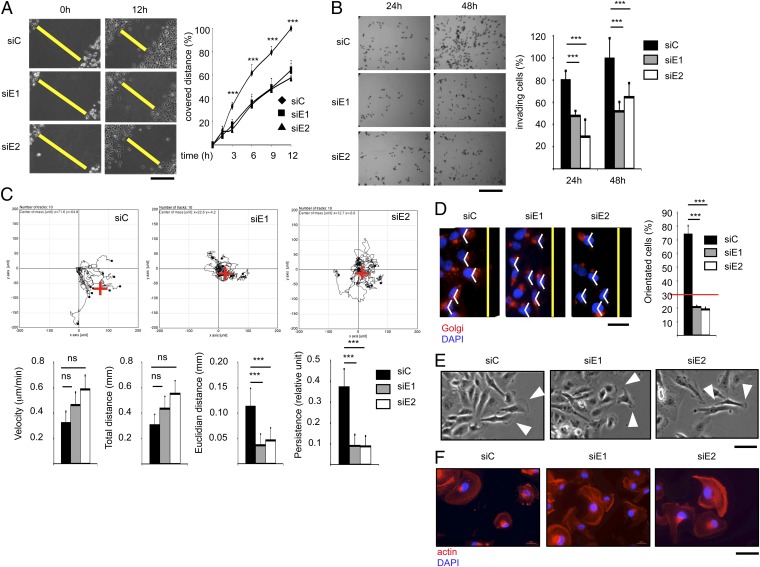
To analyze the effects of ERRα on cell migration, we used a transient knockdown approach in MDA-MB231 human mammary carcinoma cells using two different siRNA (Fig. S1A). Compared with controls, ERRα-deficient cells displayed a reduced capacity to close a wound inflicted to the cell monolayer (Fig. 1A), as well as to invade a 3D gel mimicking the extracellular matrix (Fig. 1B). To determine the origin of these perturbations, migrating cells were individually followed by time-lapse videomicroscopy in wound healing assays (Fig. 1C). ERRα inhibition did not impact on cellular velocity nor did it influence the total distance covered per time unit. In contrast the absence of the receptor resulted in reduced persistent, directional movement, suggesting cell disorientation. To evaluate this possibility, we first analyzed the localization of the Golgi apparatus (GA), which, upon initiation of directional migration, reorients between the nucleus and the migration front (33). We found that the GA was randomly localized in ERRα-deficient migrating cells in contrast to controls (Fig. 1D). Migrating cells progress by dynamically projecting a single lamellipodium, containing a dense actin meshwork toward the migration front (7). Upon ERRα knockdown, cells displayed an increased number of protrusions, which were narrower and randomly localized relative to the migration front (Fig. 1E and Fig. S1B). In addition, the actin network was not concentrated in limited membrane territories but rather spanned the whole cell surface (Fig. 1F). Similar cellular phenotypes were obtained when ERRα was stably knocked down in cell populations using two unrelated shRNA (Fig. S1 A–H).
Fig. 1.
Loss of ERRα induces cell disorientation. (A) Confluent layer of control- (siC) or siERRα (siE1, siE2)-transfected cells was scratch wounded; phase contrast microphotographs were taken. Wound extents are shown as yellow bars. Quantification is displayed as percentage of maximal migration (extent of wound closure in siC-treated cells after 12 h); data are means of three experiments (four fields per experiment) ±SD. ***P < 0.005. (Scale bar, 200 μm.) (B) siRNA-transfected cells were submitted to Transwell assays. Quantified data represent mean of three independent experiments (performed in triplicate) ±SD and are expressed relative to maximal invasion (siC at 48 h). ***P < 0.005. (Scale bar, 200 μm.) (C) siRNA-transfected cells were individually followed by time-lapse videomicroscopy in would healing assays over 12 h. (Upper) Graphic representations of cell migratory behavior. Red cross represents the calculated mean arrival point. (Lower) Quantification of the migratory parameters. Euclidean distance represents the linear distance from the start to the arrival point. Persistence is the ratio between Euclidean and total distance. Data are mean ± SD, n = 10 cells. ***P < 0.005. ns, nonsignificant. (D) Golgi apparatus (GA) orientation 4 h after monolayer wounding. The yellow lines indicate the wound orientation. A 120°-angle based on the nucleus (DAPI staining) is displayed in white. Quantification is displayed as percentage of orientated cells (i.e., GA within the 120° angle). Data are mean of three experiments ±SD. ***P < 0.005, n > 40 cells per condition. (Scale bar, 25 μm.) (E) Cell protrusions 2 h after monolayer wounding analyzed by phase contrast. White arrowheads show major cell protrusions facing the wound. (Scale bar, 25 μm.) (F) Actin network stained with phalloidin. Nuclei were DAPI counterstained. (Scale bar, 50 μm.)
Inhibiting ERRα Enhances RhoA Stability and Activation.
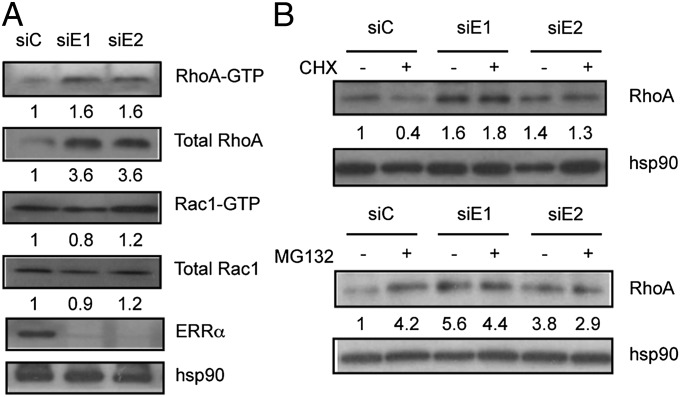
Formation of the lamellipodium is dynamically regulated by the activity of two RhoGTPases (1). RAC1 controls the formation of the lamellipodium at the leading edge, whereas RHOA acts to limit its width and expansion as well as to regulate the assembly of actin stress fibers (4, 6, 34–36). The phenotype of ERRα-deficient cells may thus result from excessive RHOA activity and/or reduced RAC1 activity. To investigate this hypothesis, we analyzed the expression and activation of these proteins (Fig. 2A). We found that ERRα knockdown did not affect RAC1 expression and activation. In contrast the levels of total RHOA, but not RHOB or RHOC, as well as of GTP-bound RHOA were strongly elevated in the absence of the receptor (Fig. 2A and Fig. S2A). Interestingly transient RHOA overexpression in ERRα wild-type cells resulted in impaired wound closure and reduced persistent migration with an increase of narrow protrusions per cell (Fig. S2 B–E). Thus, ERRα deficiency can be phenocopied by RHOA overexpression, suggesting that the latter event mediates the cellular defects observed in the absence of the receptor.
Fig. 2.
Loss of ERRα enhances RhoA protein stability. (A) Expression of activated (GTP bound) RhoA and Rac1 in siRNA-treated cells examined by immunoprecipitation. Total RhoA, Rac1, ERRα, and hsp90, were also analyzed in cell lysates before immunoprecipitation. (B) RhoA expression in siRNA-transfected cells treated with cycloheximide (CHX) or MG132. Quantifications (absolute for GTP-bound proteins, relative to hsp90 levels for total proteins) are relative to control conditions.
Modulation of RHOA degradation is instrumental in the regulation of cell polarity (9, 10). We thus tested the possibility that ERRα may impact on RHOA stability. Blocking protein synthesis using cycloheximide resulted in a decrease in RHOA protein steady state level reflecting its degradation (Fig. 2B). Interestingly, this decrease was not observed upon ERRα knockdown, indicating that RHOA is stabilized in the absence of the receptor. In addition, blocking the proteasome using MG132 increased RHOA steady-state levels under control conditions, as expected. In contrast this treatment failed to enhance RHOA expression in ERRα-deficient cells, indicating that proteasome-mediated degradation of RHOA was reduced in the absence of the receptor.
Together this shows that the absence of ERRα impairs proteasome-dependent RHOA degradation, resulting in excess activated RHOA that disrupts lamellipodium formation. ERRα is a transcription factor, acting in the nucleus, for which there is no evidence of direct modulation of protein degradation processes. We postulated that the receptor indirectly controls RHOA stability through the transcriptional regulation of a factor directly involved in RHOA degradation.
ERRα Directly Regulates TNFAIP1/BACURD2, a Regulator of RHOA Stability.
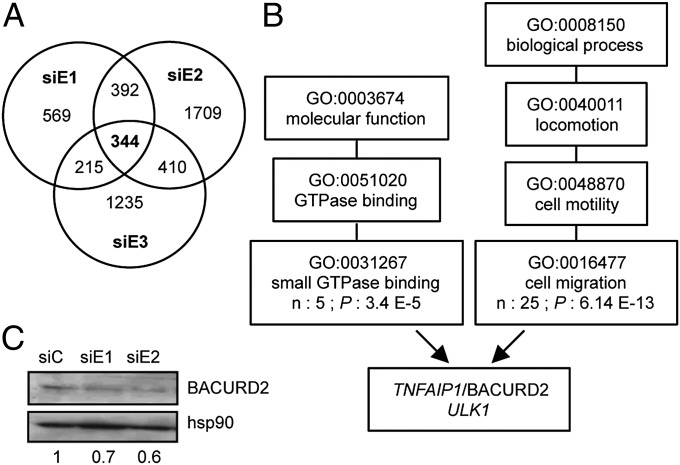
To identify this factor, we performed an unbiased transcriptomic analysis by RNA-sequencing (RNA-seq) comparing siERRα-treated cells to control ones. To increase stringency in this experiment, we included a third siRNA directed against ERRα (siE3), which was first validated in Western blot (Fig. S3A). Genes were considered down- or up-modulated upon individual siERRα when their residual expression was <0.75- or >1.5-fold over control, respectively. We obtained a list of 344 genes whose expression was independently modulated by all three siERRα (Fig. 3A and Table S1). The transcriptomic experiment was further validated by independent quantitative PCR (QPCR) experiments comparing siERRα to siC-treated cells (Table S1). Gene Ontology (GO) analysis was then performed on up- and down-modulated genes to identify enriched categories whose GO term could be relevant to the molecular and cellular phenotypes observed above. Analysis under “molecular function” and “biological process” produced highly significant enrichments, respectively, under the GO terms “small GTPase binding” (5 genes) and “cell migration” (25 genes), with two genes in common (Fig. 3B and Table S1).
Fig. 3.
TNFAIP1/BACURD2 is a direct target of ERRα. (A) Summary of the RNA-sequencing analysis. Number of genes down- and up-regulated by each siERRα is displayed as well as the intersected lists. Lists of genes up- or down-modulated by the three siRNAs are available in Table S1. (B) Summary of Gene Ontology analysis. Genes commonly modulated by all siERRα were analyzed for molecular function and involvement in biological processes using GOEAST. Number of genes (n) and P values are shown for the relevant GO terms. Genes in common under terms GO:0031267 and GO:0016477 are listed. (C) Expression of BACURD2 protein in siRNA-treated cells with quantification relative to hsp90 and to control conditions.
Among these, we focused on TNFAIP1 because it encodes a protein (hereafter referred to as BACURD2), which induces RHOA (but not RAC1, RHOB, or RHOC) proteasomal degradation (11). Down-regulation of TNFAIP1 expression in the absence of ERRα was confirmed at the mRNA level by RT-QPCR experiments as well as at the protein level by Western blotting (Fig. 3C and Fig. S3B). In agreement with our transcriptomic data, the mRNA expression of the closely related KCTD13/BACURD1 was not affected by ERRα deficiency nor was those of SMURF1 and -2, which encode other proteins involved in the control of RHOA degradation (Fig. S3B and Table S1), indicating a specific effect of the receptor on TNFAIP1. Chromatin immunoprecipitation (ChIP) experiments indicated that ERRα bound to the TNFAIP1 gene in close vicinity to the putative transcriptional start site (Fig. S3 C and D). This is in agreement with published ChIP-chip data showing a binding of ERRα in the corresponding TNFAIP1 genomic region in SKBr3 cells (27). We next determined the effects of TNFAIP1 inactivation in wild-type cells (Fig. S3 E–I). siRNA-mediated BACURD2 deficiency led to enhanced RHOA protein expression, cell disorientation, altered protrusions, and reduced persistent migration in wound healing assays. Thus, inactivating TNFAIP1 phenocopied ERRα deficiency. In addition, overexpression of BACURD2 protein in wild-type MDA-MB231 cells led to a decrease in RHOA expression and reduced migration abilities in wound closure assays (Fig. S3J). Altogether, this suggests that the BACURD2 protein is involved in the effects of the receptor on cell motility.
A Molecular Cascade Driven by ERRα Regulates Cell Orientation and Migration.
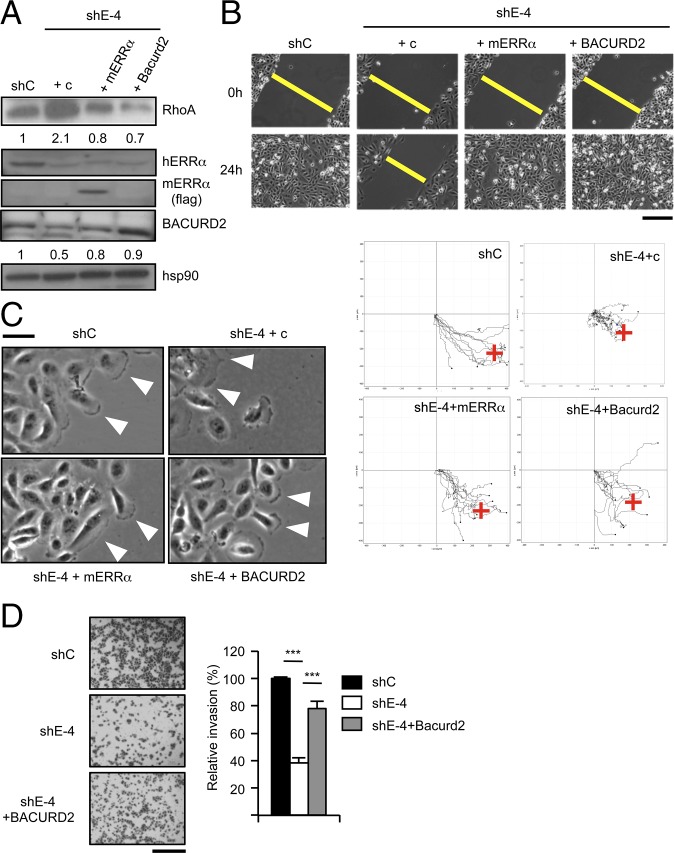
We have postulated a cascade in which ERRα activates the expression of TNFAIP1, which in turn regulates RHOA protein stability, leading to orientated, persistent cellular migration. To investigate the relevance of this cascade, we performed rescue experiments in cell populations in which ERRα has been stably knocked down (characterized in Fig. S1). Interestingly these cells displayed reduced BACURD2- and increased RHOA expression, which were normalized upon reintroduction of ERRα (in a version not targeted by the shRNA) or BACURD2 (Fig. 4A). The ability to close a wound and to sustain persistent migration was also restored in these cells (Fig. 4B and Fig. S4A). In addition, ERRα- or BACURD2-reexpressing cells mainly displayed a single broad protrusion orientated toward the migration front as well as normalized cortical actin network (Fig. 4C and Fig. S4 B and C). In contrast, a transcriptionally inactive ERRα mutant was unable to restore RHOA protein levels or persistent migration (Fig. S5 A and B). Cell capacity to invade a 3D matrix was also near completely (78%) restored by reintroduction of BACURD2 in ERRα-deficient cells as evidenced by Transwell assays (Fig. 4D). In conclusion, the original cell migration capacities were rescued upon ERRα- or BACURD2 reexpression, validating the functionality of the ERRα/BACURD2/RHOA cascade.
Fig. 4.
Complementations restore migration upon ERRα deficiency. Pooled cells stably transfected with ERRα-specific shRNA-encoding vector (shE-4; Fig. S1) and with flagged mouse ERRα or flagged human BACURD2 or empty vector (c) and analyzed in populations. Unrelated sh-transfected populations (shC) were used as controls. (A) Expression of RhoA, ERRα, and BACURD2 proteins, with hsp90 used as a control. Quantifications are relative to hsp90 and control conditions. (B, Upper) Wound healing assays. (Scale bar, 200 μm.) (Lower) Time-lapse microscopy analysis of cell migration. (C) Cell protrusion analysis. (D) Transwell assays. Quantification shows mean ± SD of three independent experiments performed in triplicate. ***P < 0.005. (Scale bar, 200 μm.)
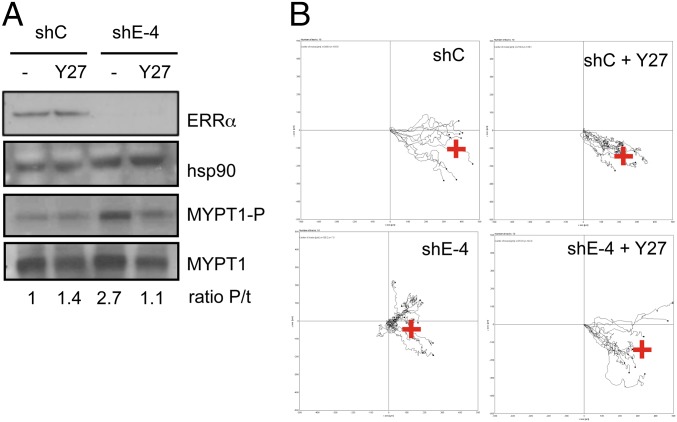
We next questioned whether the ERRα-deficiency phenotype could be rescued downstream of RHOA. Indeed we observed that the absence of ERRα resulted in enhanced activity of the RHOA effector ROCK, as evidenced by an increased phosphorylation level of its direct target MYPT1 (5) (Fig. 5A). This observation is consistent with ROCK being a major RHOA effector in limiting the extent of cell protrusions (4), a phenomenon exacerbated in the absence of ERRα as described above. Treating ERRα-deficient cells with the selective ROCK inhibitor Y27632 reduces MYPT1 phosphorylation level and restored persistent migration (Fig. 5 A and B and Fig. S4D). Taken together, these data strongly suggest that the BACURD2/RHOA/ROCK cascade constitutes a unique pathway, the deregulation of which is responsible for the impaired migration induced by ERRα deficiency.
Fig. 5.
Inhibition of ROCK activity restores migration upon ERRα deficiency. (A) Cells treated with Y27232 (Y27) or vehicle were analyzed for MYPT1 and phosphoMYPT1 expression. Quantification (ratio P/t) indicates the ratio phosphoMYPT1/total MYPT1 relative to control conditions. (B) Control (shC) or ERRα-deficient (shE-4) cells, treated or not with Y27232, were analyzed by videomicroscopy in wound healing assays. Red cross represents the calculated mean arrival point.
ERRα Controls Physiological Migration Through Conserved Mechanisms.
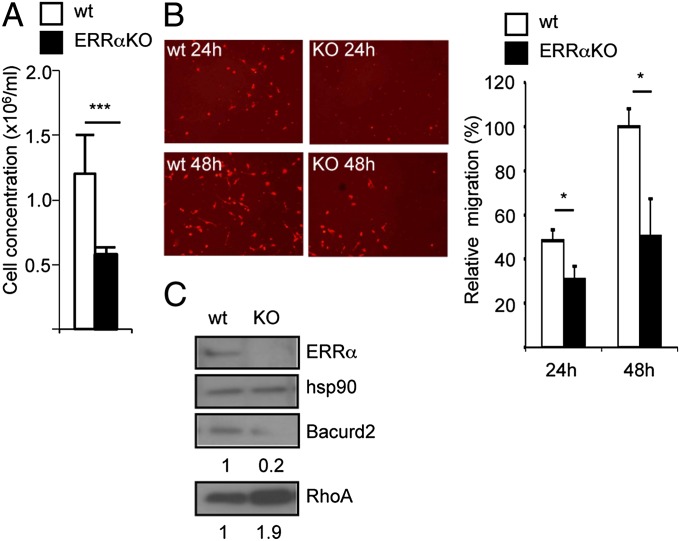
Finally we investigated the relevance of the cellular and molecular processes controlled by ERRα in other cell systems. siRNA-mediated ERRα inactivation in PC3 prostate cancer cells reduced their ability to close a wound, with a concomitant decrease in TNFAIP1 expression (at the mRNA and protein levels) and increased RHOA protein level (Fig. S6 A–C). We next turned to a nonpathological cell model. We first examined the in vivo migration of mouse macrophages into the peritoneum after thioglycolate injection. The number of cells recovered after such a treatment was considerably reduced in ERRαKO mice compared with wild type (Fig. 6A). However, this defect could be due, among others, to an altered inflammatory response in mutant animals. To circumvent this possibility, mouse bone marrow macrophages were analyzed ex vivo for chemotactic migration, a behavior that requires a fine regulation of RHOA expression (37–40). We found that cells displayed reduced migration abilities when originating from ERRαKO animals compared with wild-type ones (Fig. 6B). This is unlikely to result from impaired differentiation in the absence of the receptor as evidenced by comparable expression of differentiation markers (Table S2). In contrast we found that bacurd2 expression was greatly reduced in ERRαKO cells at the mRNA and protein level (Fig. 6C and Fig. S6D). Rhoa protein expression, but not that of its corresponding mRNA, was enhanced in mouse ERRαKO cells. Together, this suggests that ERRα regulates cell migration through a similar pathway whether under physiological or pathological conditions.
Fig. 6.
ERRα deficiency in mouse impairs macrophage migration. (A) Concentration of macrophages harvested in WT or ERRαKO mouse peritoneum 3 d after thioglycolate injection. Data are mean of two independent experiments ±SD. ***P < 0.005, n = 6 mice. (B) Ex vivo migration assay using bone marrow macrophages from WT or ERRαKO untreated animals. Quantifications are percentages of maximal migration (WT cells after 48 h); data are means of three experiments ±SD; *P < 0.05. (C) Expression of bacurd2 and RhoA proteins in wild-type vs. ERRαKO animals with ERRα and hsp90 used as controls. Quantifications are relative to hsp90 levels and wild-type conditions.
Discussion
In this report, we show that the orphan nuclear receptor ERRα is required for orientated cellular migration. This is consistent with a previous report showing that knockdown of ERRα during the early stages of zebrafish embryonic development results in inhibition of cell migration (41). Our data are also in agreement with results published by others, indicating that cell migration is considerably affected by ERRα deficiency (32). A molecular mechanism has been proposed linking ERRα to the activation of Wnt11-elicited pathway leading to increased Msx1 and N-cadherin expression. However, we were unable to detect Wnt11, Msx1, and N-cadherin in our cell system through RNA-sequencing as well as by QPCR experiments (Table S1), suggesting that Wnt11-independent pathways that are instrumental in cell migration are also controlled by ERRα.
ERRα directly regulates the expression of the TNFAIP1 gene, the protein product of which (BACURD2) controls RHOA turnover (11). BACURD2 primarily induces the degradation of GDP-bound RHOA. Reduced BACURD2 expression should thus lead to a relative accumulation of GDP-bound RHOA unless it is rapidly converted to GTP-bound isoform by a RHOGEF(s) present in nonlimiting amounts. Interestingly loss of CULLIN3 (which is part of the BACURD2-containing degradation complex) leads to enhanced total but also GTP-bound RHOA (11), indicating that RHOA activation is not a limiting step. This is consistent with a generally high expression of RHOGEFs in cancer cells (8), as well as with our results, which show that the absence of ERRα results in an increased amount of total and activated RHOA. Accordingly, this results in enhanced activity of the RHOA downstream effector ROCK1. Of note, our transcriptomic analysis did not reveal any regulation of the expression of RHOGEFs or RHOGAPs by ERRα, suggesting that activation of RHOA is not a limiting step in this cell system.
The increased activated RHOA resulting from ERRα deficiency leads to excessive actin network and inability to form a single large protrusion at the migration front (4, 34, 42–44). The expression of total RHOB, RHOC, and RAC1, as well as the level of activated RAC1, are not regulated by ERRα. This is in agreement with the demonstration that BACURD2 regulates RHOA stability, not that of RAC1, RHOB, and RHOC (11). The effects of ERRα deficiency can be phenocopied by independently inactivating BACURD2 or by overexpressing wild-type RHOA. Importantly the defects resulting from the absence of ERRα can be rescued at various levels of the molecular cascade downstream of the receptor, e.g., by reintroducing ERRα itself or BACURD2, or by down-modulating the activity of the downstream RHOA effector ROCK1. Altogether our results validate the cascade controlled by the receptor, as well as its unique and straightforward nature.
On the one hand, RHOA expression and activity is required for oriented cellular migration (1, 3). For instance the hypoxia-inducible factors have recently been shown to activate RHOA expression to induce cell migration (45). On the other hand, excess activity of this protein results in impaired cell polarity and motility (4, 10, 35, 42, this report). These results show that the amount and/or activity of RHOA must be prevented from overstepping a certain threshold. The FRA1 transcription factor exerts such a control by negatively regulating the activity of β1-integrin leading to low RHOA activity without affecting the expression of the protein (34). As discussed above, it seems unlikely that ERRα regulates RHOA activation (understood as the switch from GDP to GTP binding). Rather the receptor limits the amount of RHOA protein by inducing BACURD2 protein, which is itself directly involved in the modulation of RHOA stability. These activities of ERRα depend on its transcriptional capacities as evidenced by its binding to TNFAIP1 promoter and by the inability of a transactivation-deficient mutant of ERRα to rescue the absence of the receptor. This regulation is not limited to human cancer cells because macrophages originating from ERRαKO mice display reduced migration potential with associated decreased bacurd2 and enhanced rhoa expression.
Epidemiological studies have shown that high expression of ERRα is associated with a negative outcome in various tumor types (14–17). These include breast cancers, where a regulatory loop involving ERBB2 signaling has been documented (27, 46, 47). In addition ERRα promotes angiogenesis likely via the regulation of VEGF expression (28, 30). Several reports have shown that ERRα is instrumental in controlling energy metabolism in tissues such as the liver, heart, or skeletal muscle through regulation of mitochondrial biogenesis, oxidative phosphorylation, glycolysis, pyruvate metabolism, and the TCA cycle (18, 19, 48). In addition recent results have shown that ERRα mediates the switch undergone by many cancer cells from mitochondrial to glycolytic metabolism referred to as the Warburg effect (20, 21, 23). All these “energetic” activities apparently require an interaction with a member of the PGC-1 family of coactivators. Our transcriptomic and GO analyses of ERRα targets did not reveal a high enrichment in energetic categories. However, it must be stressed that, as evidenced by RNA-sequencing, the cell system used for these studies does not express PGC-1α, and only low levels of PGC-1β, as compared for instance with members of the NCOA coactivator family. This confirms the absolute dependence of ERRα on PGC-1 family members for energetic regulations. In addition this strongly suggests that, in the absence of these coactivators, ERRα displays nonmetabolic activities. For instance, ERRα-regulated genes were found enriched for several GO categories related to (cell–cell or cell–substrate) adhesion, migration, and motility, all processes that are highly relevant to cancer progression. The mechanisms through which these genes are regulated by ERRα and in particular, which coactivator is involved, remain to be determined.
In summary, our results, taken together with published literature, establish ERRα as an orchestrator of several independent physiopathological processes which all contribute to the acquisition of a highly aggressive phenotype by cancer cells. Given the diversity of processes it controls, ERRα represents a promising therapeutic target in aggressive tumors.
Materials and Methods
pIRES-mERRα and pIRES-hBACURD2 were constructed by inserting the corresponding cDNA fragments from pSGFlag-mERRα or pCS2-hBACURD2-Flag (provided by F. Shao, National Institute of Biological Sciences, Beijing) into the pIRES2-GFP vector (Clontech). Antibodies were from Gentex (hERRα, GTX108166; ROCK1, GTX113266), ProteinTech (BACURD2, 10096–1), Santa Cruz (RhoA, sc-418; normal rabbit IgG, sc-2027), BD Biosciences (Rac1, 610928), Enzo (hsp90, ADI-SPA-830), Sigma-Aldrich (Flag-M2, F33165), Promega (mHRP, 715–035-151; rabbit HRP, 711–035-152), Bethyl (MYPT, A300-88A), and Cell Biolabs (P-T696-MYPT, 241504). Custom anti-ERRα antiserum (see Fig. S3C for characterization) was produced (Eurogentec) in rabbits, using the human ERRα N-terminal domain fused to GST as an antigene.
Detailed materials and methods are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Frédéric Flamant and Ali Badache for their critical reading of the manuscript, Structure Fédérative de Recherche Biosciences Gerland-Lyon Sud (UMS3444/US8) platforms Plateau de Biologie Expérimentale de la Souris (in particular Nadine Aguilera) for helping in performing animal experimentations, Plateau Technique Imagerie Microscopie (in particular Christophe Chamot) for helping in microscopy, and the ProfilExpert platform for helping with RNA-Seq. This work was supported by grants from Ligue contre le Cancer (comités Rhône and Drôme), Association pour la Recherche sur le Cancer, Cancéropôle Lyon Auvergne Rhône-Alpes (to J.-M.V.), by a grant from the Canadian Institutes for Health Research (MOP-64275, to V.G.), and by General Research Fund from Hong Kong Research Grants Council (461009, to F.L.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.P.M. is a guest editor invited by the Editorial Board.
Data deposition: The sequencing data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE49110).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1402094111/-/DCSupplemental.
References
- 1.Jaffe AB, Hall A. Rho GTPases: Biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 2.Ellenbroek SIJ, Collard JG. Rho GTPases: Functions and association with cancer. Clin Exp Metastasis. 2007;24(8):657–672. doi: 10.1007/s10585-007-9119-1. [DOI] [PubMed] [Google Scholar]
- 3.Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582(14):2093–2101. doi: 10.1016/j.febslet.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 4.Worthylake RA, Burridge K. RhoA and ROCK promote migration by limiting membrane protrusions. J Biol Chem. 2003;278(15):13578–13584. doi: 10.1074/jbc.M211584200. [DOI] [PubMed] [Google Scholar]
- 5.Riento K, Ridley AJ. Rocks: Multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4(6):446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 6.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440(7087):1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 7.Insall RH, Machesky LM. Actin dynamics at the leading edge: From simple machinery to complex networks. Dev Cell. 2009;17(3):310–322. doi: 10.1016/j.devcel.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Vigil D, Cherfils J, Rossman KL, Der CJ. Ras superfamily GEFs and GAPs: Validated and tractable targets for cancer therapy? Nat Rev Cancer. 2010;10(12):842–857. doi: 10.1038/nrc2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nethe M, Hordijk PL. The role of ubiquitylation and degradation in RhoGTPase signalling. J Cell Sci. 2010;123(Pt 23):4011–4018. doi: 10.1242/jcs.078360. [DOI] [PubMed] [Google Scholar]
- 10.Wang HR, et al. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science. 2003;302(5651):1775–1779. doi: 10.1126/science.1090772. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, et al. Cullin mediates degradation of RhoA through evolutionarily conserved BTB adaptors to control actin cytoskeleton structure and cell movement. Mol Cell. 2009;35(6):841–855. doi: 10.1016/j.molcel.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Giguère V. Orphan nuclear receptors: From gene to function. Endocr Rev. 1999;20(5):689–725. doi: 10.1210/edrv.20.5.0378. [DOI] [PubMed] [Google Scholar]
- 13.Horard B, Vanacker JM. Estrogen receptor-related receptors: Orphan receptors desperately seeking a ligand. J Mol Endocrinol. 2003;31(3):349–357. doi: 10.1677/jme.0.0310349. [DOI] [PubMed] [Google Scholar]
- 14.Ariazi EA, Jordan VC. Estrogen-related receptors as emerging targets in cancer and metabolic disorders. Curr Top Med Chem. 2006;6(3):203–215. doi: 10.2174/1568026610606030203. [DOI] [PubMed] [Google Scholar]
- 15.Stein RA, McDonnell DP. Estrogen-related receptor alpha as a therapeutic target in cancer. Endocr Relat Cancer. 2006;13(Suppl 1):S25–S32. doi: 10.1677/erc.1.01292. [DOI] [PubMed] [Google Scholar]
- 16.Bianco S, Sailland J, Vanacker JM. ERRs and cancers: Effects on metabolism and on proliferation and migration capacities. J Steroid Biochem Mol Biol. 2012;130(3-5):180–185. doi: 10.1016/j.jsbmb.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Deblois G, Giguère V. Oestrogen-related receptors in breast cancer: Control of cellular metabolism and beyond. Nat Rev Cancer. 2013;13(1):27–36. doi: 10.1038/nrc3396. [DOI] [PubMed] [Google Scholar]
- 18.Giguère V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev. 2008;29(6):677–696. doi: 10.1210/er.2008-0017. [DOI] [PubMed] [Google Scholar]
- 19.Villena JA, Kralli A. ERRalpha: A metabolic function for the oldest orphan. Trends Endocrinol Metab. 2008;19(8):269–276. doi: 10.1016/j.tem.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang CY, et al. The metabolic regulator ERRα, a downstream target of HER2/IGF-1R, as a therapeutic target in breast cancer. Cancer Cell. 2011;20(4):500–510. doi: 10.1016/j.ccr.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eichner LJ, et al. miR-378(*) mediates metabolic shift in breast cancer cells via the PGC-1β/ERRγ transcriptional pathway. Cell Metab. 2010;12(4):352–361. doi: 10.1016/j.cmet.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Tennessen JM, Baker KD, Lam G, Evans J, Thummel CS. The Drosophila estrogen-related receptor directs a metabolic switch that supports developmental growth. Cell Metab. 2011;13(2):139–148. doi: 10.1016/j.cmet.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai Q, Lin T, Kamarajugadda S, Lu J. Regulation of glycolysis and the Warburg effect by estrogen-related receptors. Oncogene. 2013;32(16):2079–2086. doi: 10.1038/onc.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, et al. HIF- and non-HIF-regulated hypoxic responses require the estrogen-related receptor in Drosophila melanogaster. PLoS Genet. 2013;9(1):e1003230. doi: 10.1371/journal.pgen.1003230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bianco S, et al. Modulating estrogen receptor-related receptor-alpha activity inhibits cell proliferation. J Biol Chem. 2009;284(35):23286–23292. doi: 10.1074/jbc.M109.028191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chisamore MJ, Wilkinson HA, Flores O, Chen JD. Estrogen-related receptor-α antagonist inhibits both estrogen receptor-positive and estrogen receptor-negative breast tumor growth in mouse xenografts. Mol Cancer Ther. 2009;8(3):672–681. doi: 10.1158/1535-7163.MCT-08-1028. [DOI] [PubMed] [Google Scholar]
- 27.Deblois G, et al. Transcriptional control of the ERBB2 amplicon by ERRalpha and PGC-1β promotes mammary gland tumorigenesis. Cancer Res. 2010;70(24):10277–10287. doi: 10.1158/0008-5472.CAN-10-2840. [DOI] [PubMed] [Google Scholar]
- 28.Fradet A, et al. Dual function of ERRα in breast cancer and bone metastasis formation: implication of VEGF and osteoprotegerin. Cancer Res. 2011;71(17):5728–5738. doi: 10.1158/0008-5472.CAN-11-1431. [DOI] [PubMed] [Google Scholar]
- 29.Ao A, Wang H, Kamarajugadda S, Lu J. Involvement of estrogen-related receptors in transcriptional response to hypoxia and growth of solid tumors. Proc Natl Acad Sci USA. 2008;105(22):7821–7826. doi: 10.1073/pnas.0711677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stein RA, Gaillard S, McDonnell DP. Estrogen-related receptor alpha induces the expression of vascular endothelial growth factor in breast cancer cells. J Steroid Biochem Mol Biol. 2009;114(1–2):106–112. doi: 10.1016/j.jsbmb.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein RA, et al. Estrogen-related receptor α is critical for the growth of estrogen receptor-negative breast cancer. Cancer Res. 2008;68(21):8805–8812. doi: 10.1158/0008-5472.CAN-08-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dwyer MA, et al. WNT11 expression is induced by estrogen-related receptor α and β-catenin and acts in an autocrine manner to increase cancer cell migration. Cancer Res. 2010;70(22):9298–9308. doi: 10.1158/0008-5472.CAN-10-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridley AJ, et al. Cell migration: Integrating signals from front to back. Science. 2003;302(5651):1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 34.Vial E, Sahai E, Marshall CJ. ERK-MAPK signaling coordinately regulates activity of Rac1 and RhoA for tumor cell motility. Cancer Cell. 2003;4(1):67–79. doi: 10.1016/s1535-6108(03)00162-4. [DOI] [PubMed] [Google Scholar]
- 35.Wildenberg GA, et al. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127(5):1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 36.Machacek M, et al. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461(7260):99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones GE. Cellular signaling in macrophage migration and chemotaxis. J Leukoc Biol. 2000;68(5):593–602. [PubMed] [Google Scholar]
- 38.Worthylake RA, Burridge K. Leukocyte transendothelial migration: Orchestrating the underlying molecular machinery. Curr Opin Cell Biol. 2001;13(5):569–577. doi: 10.1016/s0955-0674(00)00253-2. [DOI] [PubMed] [Google Scholar]
- 39.Kim JS, et al. Transforming growth factor-beta1 regulates macrophage migration via RhoA. Blood. 2006;108(6):1821–1829. doi: 10.1182/blood-2005-10-009191. [DOI] [PubMed] [Google Scholar]
- 40.Aflaki E, et al. Impaired Rho GTPase activation abrogates cell polarization and migration in macrophages with defective lipolysis. Cell Mol Life Sci. 2011;68(23):3933–3947. doi: 10.1007/s00018-011-0688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bardet PL, Horard B, Laudet V, Vanacker JM. The ERRalpha orphan nuclear receptor controls morphogenetic movements during zebrafish gastrulation. Dev Biol. 2005;281(1):102–111. doi: 10.1016/j.ydbio.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 42.Simpson KJ, et al. Identification of genes that regulate epithelial cell migration using an siRNA screening approach. Nat Cell Biol. 2008;10(9):1027–1038. doi: 10.1038/ncb1762. [DOI] [PubMed] [Google Scholar]
- 43.Lim Y, et al. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J Cell Biol. 2008;180(1):187–203. doi: 10.1083/jcb.200708194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Z, et al. Migration of epithelial cells on laminins: RhoA antagonizes directionally persistent migration. Eur J Cell Biol. 2011;90(1):1–12. doi: 10.1016/j.ejcb.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Gilkes DM, et al. Hypoxia-inducible factors mediate coordinated RhoA-ROCK1 expression and signaling in breast cancer cells. Proc Natl Acad Sci USA. 2014;111(3):E384–E393. doi: 10.1073/pnas.1321510111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Barry JB, Giguère V. Epidermal growth factor-induced signaling in breast cancer cells results in selective target gene activation by orphan nuclear receptor estrogen-related receptor α. Cancer Res. 2005;65(14):6120–6129. doi: 10.1158/0008-5472.CAN-05-0922. [DOI] [PubMed] [Google Scholar]
- 47.Deblois G, et al. Genome-wide identification of direct target genes implicates estrogen-related receptor α as a determinant of breast cancer heterogeneity. Cancer Res. 2009;69(15):6149–6157. doi: 10.1158/0008-5472.CAN-09-1251. [DOI] [PubMed] [Google Scholar]
- 48.Deblois G, St-Pierre J, Giguère V. The PGC-1/ERR signaling axis in cancer. Oncogene. 2013;32(30):3483–3490. doi: 10.1038/onc.2012.529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.