Significance
Stress can increase susceptibility to developing psychiatric disorders, including depression. Understanding the neurobiological mechanisms underlying stress resilience and susceptibility is key to identifying novel targets for the development of more effective treatments for stress-related psychiatric disorders. Here we show that specific isoforms of GABAB receptor subunits differentially regulate stress resilience. Specifically, GABAB(1a)−/− mice are more susceptible whereas GABAB(1b)−/− mice are more resilient to stress-induced anhedonia and psychosocial stress-induced social withdrawal, two features of depression. Furthermore, GABAB(1b)−/− mice were resilient to stress-induced decreases in the survival of newly born cells in the adult hippocampus, and hippocampal GABAB(1b) expression was increased in a genetic mouse model of depression. Taken together, GABAB receptor subunit isoforms may represent novel therapeutic targets for stress-related disorders.
Keywords: neurogenesis, depression, anxiety, antidepressant
Abstract
Stressful life events increase the susceptibility to developing psychiatric disorders such as depression; however, many individuals are resilient to such negative effects of stress. Determining the neurobiology underlying this resilience is instrumental to the development of novel and more effective treatments for stress-related psychiatric disorders. GABAB receptors are emerging therapeutic targets for the treatment of stress-related disorders such as depression. These receptors are predominantly expressed as heterodimers of a GABAB(2) subunit with either a GABAB(1a) or a GABAB(1b) subunit. Here we show that mice lacking the GABAB(1b) receptor isoform are more resilient to both early-life stress and chronic psychosocial stress in adulthood, whereas mice lacking GABAB(1a) receptors are more susceptible to stress-induced anhedonia and social avoidance compared with wild-type mice. In addition, increased hippocampal expression of the GABAB(1b) receptor subunit is associated with a depression-like phenotype in the helpless H/Rouen genetic mouse model of depression. Stress resilience in GABAB(1b)−/− mice is coupled with increased proliferation and survival of newly born cells in the adult ventral hippocampus and increased stress-induced c-Fos activation in the hippocampus following early-life stress. Taken together, the data suggest that GABAB(1) receptor subunit isoforms differentially regulate the deleterious effects of stress and, thus, may be important therapeutic targets for the treatment of depression.
Although chronic and/or severe stress is a risk factor for the development of several different psychiatric disorders including depression and anxiety, many individuals remain resilient to such negative effects of stress. The mechanisms underlying this resilience are not yet fully understood, although it is thought to involve a complex interplay between several environmental factors such as social support and biological and genetic risk factors (1, 2). Currently, there is an impetus to determine the neural substrates underlying stress resilience and susceptibility, as these are poised to be key novel targets for the development of more effective treatments for depression and anxiety disorders.
Accumulating evidence suggests that GABAB receptors may be important therapeutic targets for the treatment of stress-related psychiatric disorders such as anxiety and depression (3–5). Functional GABAB receptors are composed of heterodimers of GABAB(1) and GABAB(2) subunits (6). Interestingly, the GABAB(1) subunit is expressed as different isoforms, and in the brain the predominant isoforms are GABAB(1a) and GABAB(1b) (6). Mice deficient in GABAB(1a) and GABAB(1b) exhibit differential cognitive and conditioned fear responses, indicating a potential role for these isoforms in psychiatric illness (7–10). Recent postmortem brain studies report alterations in the expression of GABAB receptor subunits in depression (4, 11), and clinical studies suggest that neurophysiology deficits in GABAB receptors may play a role in major depression (12) and the antidepressant response (13). In addition, mice lacking functional GABAB receptors exhibit an antidepressant-like phenotype and increased anxiety (14, 15), and pharmacological blockade of these receptors induces antidepressant-like behavior (16–18). GABAB receptor antagonists have also recently been shown to increase cell proliferation in the adult hippocampus (16), which is an important regulator of stress- and antidepressant-related neuroplasticity. However, the specific role of GABAB receptor isoforms in stress sensitivity is unclear. Therefore, we assessed the susceptibility and resilience to stress during either early life (maternal separation) or adulthood (psychosocial stress) in GABAB(1a)−/− and GABAB(1b)−/− mice.
Results
GABAB(1b)−/− Mice Are Resilient Whereas GABAB(1a)−/− Mice Are More Susceptible to Social Avoidance Behavior Following Social Defeat Stress.
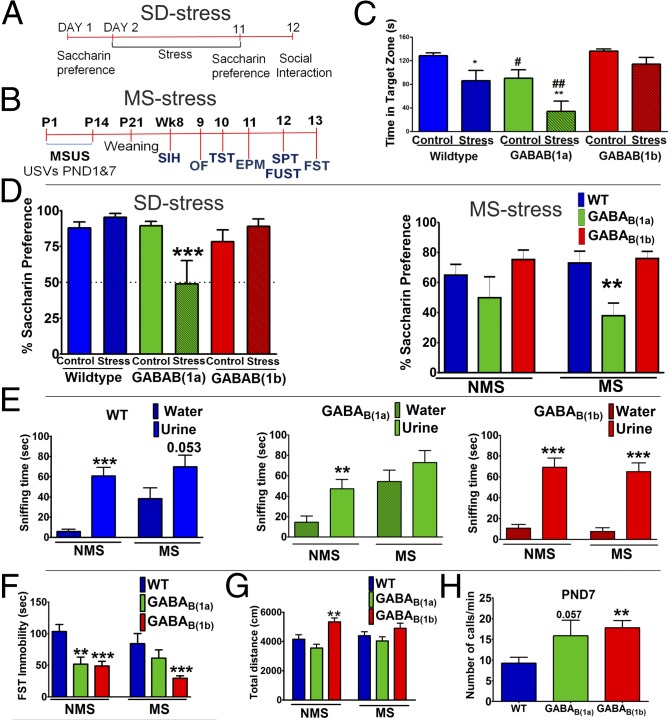
In the social interaction test (Fig. 1C), there was a significant effect of social defeat (SD) stress [F(1,56) = 15.357, P < 0.001] and genotype [F(2,56) = 12.843, P < 0.001] on social avoidance behavior. SD stress decreased time spent in the interaction zone in the presence of a social target in WT (P = 0.018) and GABAB(1a)−/− mice (P = 0.004), but this effect was greater in GABAB(1a)−/− mice [SD stress GABAB(1a)−/− vs. SD stress WT, P = 0.006]. In contrast, GABAB(1b)−/− mice were resilient to SD stress-induced social avoidance. Interestingly, nonstressed GABAB(1a)−/− mice spent less time in the interaction zone in the presence of a social target than nonstressed WT and GABAB(1b)−/− mice (P = 0.036). There were no group differences in time spent in the interaction zone in the absence of a social target (Fig. S1A). Social avoidance was not due to altered locomotor activity (Fig. S1B).
Fig. 1.
GABAB(1b)−/− mice are more resilient whereas GABAB(1a)−/− mice are more susceptible to stress-induced changes in behavior. (A) Social defeat stress experimental design. (B) Maternal separation experimental design. (C) GABAB(1b)−/− mice are resilient whereas GABAB(1a)−/− mice are more susceptible to SD stress-induced social avoidance (n = 8–10 males). (D) SD stress (n = 9–10 males) or MS stress (n = 6–14 females) reduced saccharin preference in GABAB(1a)−/− but not WT or GABAB(1b)−/− mice. (E) GABAB(1b)−/− mice are resilient to MS stress-induced anhedonia in the female urine sniffing test (n = 12–14 males). (F) Immobility in the forced swimming test (n = 6–14 females). (G) Distance traveled by female mice in open field (n = 4–14). (H) On PND7, GABAB(1b)−/− and GABAB(1a)−/− mice emit more USVs during MS than WT mice (n = 55–80 males and females). Data are presented as mean ± SEM. *SD stress experiment (C and D): compared with a nonstressed animal of the same genotype. #SD stress experiment (C): compared with WT of the same stress condition; #P < 0.05; ##P < 0.01. *FUST (E): compared with time spent sniffing water. *All other MS stress experiments (D and F–H): compared with WT of the same stress condition. *P < 0.05; **P < 0.01; ***P < 0.001.
GABAB(1b)−/− Mice Are More Resilient than GABAB(1a)−/− Mice to Stress-Induced Anhedonia.
The effects of SD stress or maternal separation (MS) on saccharin preference as a measure of anhedonia are illustrated in Fig. 1D. In the SD stress experiment, there was a significant genotype [F(2,56) = 4.589, P = 0.015] and genotype x stress interaction [F(2,56) = 7.182, P = 0.002]. Specifically, SD stress decreased saccharin preference in GABAB(1a)−/− (P = 0.001) but not WT (P = 0.469) or GABAB(1b)−/− mice (P = 0.294). Before SD stress, there were no differences in saccharin preference between groups (Fig. S1C).
GABAB(1a)−/− mice were also more susceptible to MS-induced anhedonia than GABAB(1b)−/− mice. The effects of MS on anhedonia were measured using the saccharin preference test and the female urine sniffing test. Analysis of saccharin preference in female mice over 24 h (Fig. 1D) revealed an effect of genotype [F(2,50) = 8.107, P < 0.001]. Specifically, MS reduced saccharin preference in GABAB(1a)−/− mice compared with MS WT (P < 0.01) and MS GABAB(1b)−/− (P < 0.01) mice. Similar results were obtained when preference was measured over 36- and 48-h periods (Fig. S2 A and B). The effect of MS in male mice on preference to sniff female urine is illustrated in Fig. 1E. Nonseparated (NMS) WT [t(25) = 6.048, P < 0.001], NMS GABAB(1a)−/− [t(21) = 2.966, P < 0.01], and NMS GABAB(1b)−/− [t(25) = 6.071, P < 0.001] mice spent more time sniffing urine than water. However, MS abolished urine preference in GABAB(1a)−/− mice [t(22) = 1.132, P = 0.2698] and attenuated preference in WT mice [t(26) = 2.029, P = 0.053]. GABAB(1b)−/− mice were resilient to this anhedonic effect of MS [t(25) = 6.708, P < 0.001].
Maternally Separated Female GABAB(1b)−/− Mice Retain an Antidepressant-Like Phenotype in the Forced Swimming Test.
The forced swimming test (FST) and tail suspension test (TST) were used to examine the effects of MS and GABAB(1) receptor subunit disruption on antidepressant-like behavior. In the FST in female mice (Fig. 1F), there was a significant effect of genotype [F(2,56) = 15.084, P < 0.001]. Both NMS GABAB(1a)−/− (P < 0.01) and NMS GABAB(1b)−/− mice (P < 0.001) displayed decreased immobility compared with NMS WT mice. However, following MS, only GABAB(1b)−/− mice maintained this reduced immobility (P < 0.001), thus suggesting that female GABAB(1b)−/− mice are more resilient to the effects of early-life stress. The reduced immobility in MS GABAB(1b)−/− female mice is unlikely due to increased locomotor activity, because activity in the open field did not differ across any of the female MS groups (Fig. 1G). In contrast, NMS female GABAB(1b)−/− mice were hyperactive in the open field (Fig. 1G; genotype effect [F(2,58) = 8.949, P < 0.0001]); thus we cannot exclude the possibility that this contributed to reduced immobility under nonseparated conditions. Male GABAB(1b)−/− mice exhibited reduced immobility in both the FST and TST irrespective of MS (Fig. S2 C and E), but this effect may have been confounded by increased locomotor activity (Fig. S2D). Interestingly, NMS GABAB(1a)−/− mice exhibited differential phenotypes in the FST and TST irrespective of sex whereby immobility was increased in the TST but decreased in the FST (Fig. 1F and Fig. S2 C, E, and F).
Stress Resilience in GABAB(1b)−/− Mice Is Not Associated with Higher Levels of Maternal Care or Significant Alterations in the Stress-Induced Corticosterone Response.
The level of maternal care that a pup receives can program stress sensitivity later in adulthood (19). To exclude the possibility that the resilient phenotype of GABAB(1b)−/− mice is due to increased maternal care, we measured high maternal care behaviors (Fig. S3A) and time spent off-nest (Fig. S3B) in WT, GABAB(1a)−/−, and GABAB(1b)−/− dams of both nonseparated and separated pups on postnatal day (PND) 7. GABAB(1b)−/− dams did not provide more maternal care. On the contrary, GABAB(1a)−/− dams provided higher maternal care than GABAB(1b)−/− and WT dams, irrespective of whether their pups had undergone MS (Fig. S3).
Stress-induced effects on plasma corticosterone diverged across genotypes and sexes. Interestingly, in males (Fig. S3C), stress-induced corticosterone levels were attenuated in GABAB(1a)−/− mice and enhanced in GABAB(1b)−/− mice. In females (Fig. S3D), there were no genotype differences. However, MS had no effect on this parameter in any genotype or sex.
Anxiety Levels in Adulthood Are Unaffected by Early-Life Stress and GABAB(1) Receptor Subunit Isoforms.
GABAB receptors have been reported to play a role in innate anxiety (14, 15). However, the contribution of specific GABAB(1) isoforms to this effect is less obvious (20). Here, neither MS nor GABAB(1) receptor subunit isoform disruption nor MS coupled with GABAB(1) receptor subunit isoform disruption affected anxiety levels in adult males or females, as measured by behavior in the elevated plus maze, defecation in the open field, as well as stress-induced hyperthermia (Figs. S4 and S5). However, a genotype effect on anxiety was observed when we measured the number of ultrasonic vocalizations (USVs) emitted during MS on PND7 [F(2,157) = 5.130, P < 0.01; Fig. 1H] but not on the first day of separation (PND1) [F(2,51) = 1.023, P = 0.362; Fig. S5E]. Specifically, on PND7, GABAB(1b)−/− mice emitted more USVs than WT (P < 0.05) mice. Similarly, GABAB(1a)−/− mice exhibited a trend toward emitting more USVs compared with WT mice (P = 0.057). Interestingly, mice deficient in either isoform failed to show the habituation to separation-induced anxiety between PND1 and PND7 observed in WT mice (Fig. 1H and Fig. S5E).
Stress-Induced Neural Activation Is Enhanced in GABAB(1b)−/− Mice.
Toward identifying the neural circuitry underlying the differential stress susceptibility between GABAB(1a)−/− and GABAB(1b)−/− mice, we measured the expression of c-Fos, an established marker of neural activation, in response to acute restraint stress in adult WT, GABAB(1a)−/−, and GABAB(1b)−/− mice, with and without prior MS. Restraint stress was induced by placing mice in ventilated tubes for a period of 2 h, and animals were killed 2 h after cessation of restraint. The number of c-Fos–positive cells was measured in stress-related brain areas including the hippocampus, nucleus accumbens (NAcc), dorsal raphe nucleus (DRN), paraventricular nucleus (PVN), ventral tegmental area (VTA), medial prefrontal cortex, and amygdala. The PVN is known to be activated by restraint stress (21). Therefore, to evaluate the validity of our restraint stress paradigm, we first confirmed that it induced c-Fos activation in the PVN of the hypothalamus of WT mice that did not undergo early-life stress (Fig. S5F). All data including statistical analysis are summarized in Table S1.
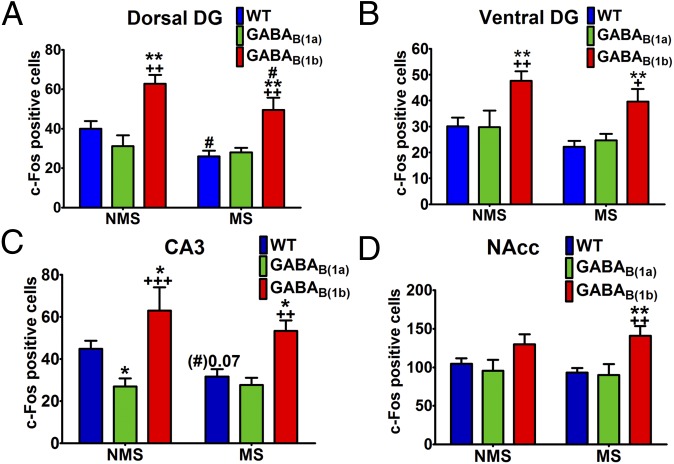
One of the most striking observations was that in several areas of the hippocampus, the number of c-Fos–positive cells in response to acute stress was significantly increased in GABAB(1b)−/− mice compared with WT and GABAB(1a)−/− mice. Importantly, the hippocampus is a key brain area involved in regulation of the stress response (22, 23). The enhanced stress-induced c-Fos activation was most apparent in the dorsal and ventral dentate gyrus and ventral CA3 (Fig. 2 and Table S1), and this effect occurred to the same extent in both NMS and MS GABAB(1b)−/− mice. Interestingly, NMS GABAB(1a)−/− mice exhibited decreased stress-induced c-Fos in the ventral CA3 and this effect was not apparent in MS GABAB(1a)−/− mice (Fig. 2 and Table S1), thus further supporting the hypothesis that GABAB receptors in the hippocampus might be important in the differential response to stress. A similar but weaker pattern of effects was also observed in the PVN and the DRN whereby GABAB(1b)−/− mice exhibited enhanced stress-induced neural activation irrespective of prior MS, although statistical differences were generally restricted to comparisons with GABAB(1a)−/− and not WT mice (Table S1). Of all the regions investigated, the NAcc was the only area where stress-induced c-Fos expression was differentially regulated in GABAB(1b)−/− mice by prior MS (Fig. 2 and Table S1). Specifically, although there were no statistically significant genotype differences under NMS conditions, MS significantly increased stress-induced c-Fos expression in the NAcc of GABAB(1b)−/− mice compared with MS WT (P < 0.01) and MS GABAB(1a)−/− (P < 0.01) mice. Interestingly, NMS GABAB(1a)−/− mice exhibited decreased stress-induced c-Fos activation in the VTA, and this effect was not apparent in MS GABAB(1a)−/− mice (Table S1).
Fig. 2.
Stress-induced c-Fos activation is enhanced in the hippocampus of GABAB(1b)−/− mice (n = 7 females). Stress-induced c-Fos activation was increased in the dorsal dentate gyrus (A), ventral dentate gyrus (B), and ventral CA3 (C) of GABAB(1b)−/− mice irrespective of maternal separation. (D) Stress-induced c-Fos expression in the nucleus accumbens was differentially regulated in GABAB(1b)−/− mice by prior MS. Data are presented as mean ± SEM. *Compared with the respective WT group; *P < 0.05; **P < 0.01. +Compared with the respective GABAB(1a)−/− group; +P < 0.05; ++P < 0.01; +++P < 0.001. #Compared with the respective NMS group; #P < 0.05.
GABAB(1b) mRNA Expression Is Increased in the Hippocampus of a Genetic Model of Depression, the Helpless H/Rouen Mouse.
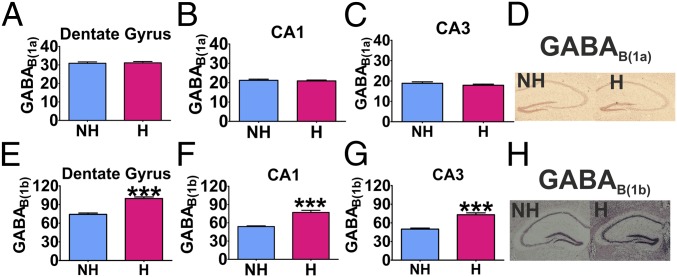
Given our data showing that the GABAB(1b) receptor isoform regulates stress resilience and increased stress-induced neural activation in the hippocampus, it was thus of interest to investigate whether a well-characterized model of depression, helpless H/Rouen mice (24), would exhibit alterations in hippocampal expression of this subunit. GABAB(1b) mRNA was increased in the dentate gyrus (t = 7.591, df = 10, P < 0.001), CA1 (t = 6.377, df = 10, P < 0.001), and CA3 (t = 6.674, df = 10, P < 0.001) of helpless H/Rouen (H) mice compared with nonhelpless (NH) controls (Fig. 3 E–H). GABAB(1a) mRNA expression did not differ between the two groups (Fig. 3 A–D).
Fig. 3.
GABAB(1a) and GABAB(1b) mRNA expression in the hippocampus of helpless and nonhelpless Rouen mice. GABAB(1a) mRNA expression in the dentate gyrus (A), CA1 (B), or CA3 (C) did not differ between H and NH mice. Helpless mice exhibited higher expression of GABAB(1b) mRNA expression in the dentate gyrus (E), CA1 (F), and CA3 (G) compared with NH mice (n = 6). Representative photographs of GABAB(1a) mRNA (D) and GABAB(1b) mRNA (H) expression in the hippocampus. Data are presented as mean ± SEM. ***P < 0.001.
GABAB(1b)−/− Mice Exhibit Increased Proliferation of Newly Born Cells in the Ventral Hippocampus.
Adult hippocampal neurogenesis is thought to play important roles in the stress response, anhedonia, antidepressant action, and regulation of the stress response by antidepressants (25–27). We previously reported that pharmacological inhibition of the GABAB receptor increases hippocampal cell proliferation and induces antidepressant-like behavior (16). This finding along with the present study, which demonstrates increased expression of GABAB(1b) mRNA in the hippocampus of a mouse model of depression and enhanced stress-induced activation of the hippocampus in GABAB(1b)−/− mice, suggest that increased adult hippocampal neurogenesis may be a potential mechanism underlying the stress-resilient and antidepressant-like phenotype of GABAB(1b)−/− mice. Emerging evidence suggests that the effects of stress and antidepressant treatments on adult hippocampal neurogenesis might occur preferentially in the ventral (vHi) rather than the dorsal hippocampus (dHi) (28, 29), and we previously reported that chronic treatment with a GABAB receptor antagonist increases cell proliferation in the vHi but not dHi (16). This is interesting in light of the preferential roles of the dHi in spatial learning and memory and the vHi in the regulation of the stress response and anxiety (30). Therefore, we determined whether the stress-resilient and antidepressant-like phenotype of GABAB(1b)−/− mice was accompanied by increased proliferation and survival of newly born cells and whether such effects occurred preferentially in the vHi rather than in the dHi.
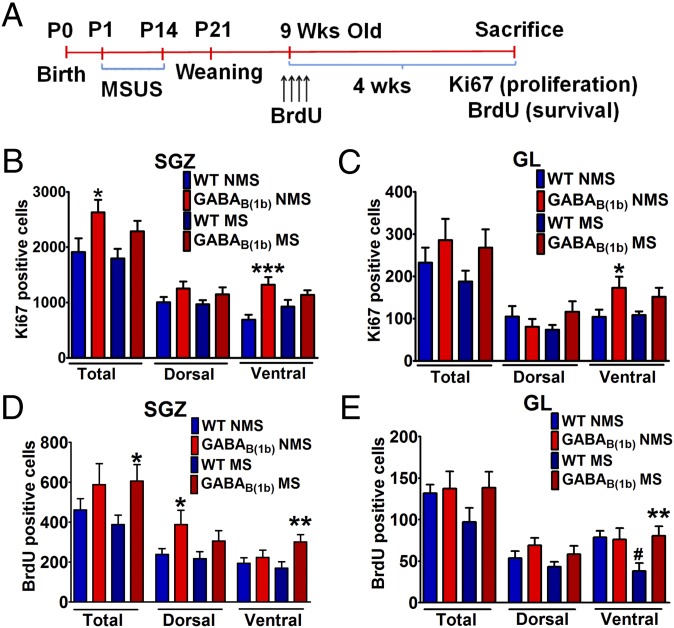
The effects of MS on cell proliferation in the subgranular zone (SGZ) and granule cell layer (GL) are illustrated in Fig. 4 B and C, respectively (representative photographs are in Fig. S6; the experimental design is in Fig. 4A). In the SGZ, there was a significant effect of genotype [F(1,27) = 8.160, P < 0.01]. Specifically, NMS GABAB(1b)−/− mice displayed increased cell proliferation in the SGZ compared with the corresponding WT group (P < 0.05). Cell proliferation in MS GABAB(1b)−/− mice was not significantly different from MS WT mice, although it should also be noted that cell proliferation in MS GABAB(1b)−/− mice was not significantly different from that in NMS GABAB(1b)−/− mice (P = 0.272). Upon segregation of the hippocampus into dorsal and ventral regions, it became apparent that increased hippocampal cell proliferation in NMS GABAB(1b)−/− mice was restricted to the ventral SGZ (genotype [F(1,25) = 15.16, P < 0.001]; genotype–stress interaction [F(1,25) = 3.813, P = 0.062]; post hoc: NMS WT vs. NMS GABAB(1b)−/−, P < 0.001) but that this effect was prevented by MS. Cell proliferation in the SGZ of the dHi did not differ between any of the groups. In the GL, neither genotype, stress, nor stress x genotype affected cell proliferation. However, upon segregation of the GL, there was an effect of genotype [F(1,24) = 7.726, P < 0.05] in the ventral GL. Specifically, NMS but not MS GABAB(1b)−/− mice displayed an increased number of Ki67-positive cells (P < 0.05) compared with their corresponding WT counterparts. Cell proliferation in the GL of the dHi was not different between any of the groups.
Fig. 4.
Increased proliferation and survival of newly born cells in the ventral hippocampus of GABAB(1b)−/− mice. (A) Experimental design. (B and C) Cell proliferation is increased in the subgranular zone and granule cell layer of the vHi of NMS GABAB(1b)−/− mice (n = 8 males). (D) The survival of newly born cells in the SGZ is increased in the dorsal hippocampus of NMS GABAB(1b)−/− mice and the vHi of MS GABAB(1b)−/− mice (n = 10 males). (E) GABAB(1b)−/− mice are resistant to MS-induced decreases in the survival of newly born cells in the GL of the vHi (n = 10 males). Data are presented as mean ± SEM. *Compared with WT of the same stress condition; *P < 0.05; **P < 0.01; ***P < 0.001. #Nonseparated WT vs. maternally separated WT; #P < 0.05.
GABAB(1b)−/− Mice Exhibit Increased Survival of Newly Born Cells and Are Resistant to MS-Induced Decreases in the Survival of Newly Born Cells in the Ventral Hippocampus.
The effects of MS on the survival of newly born cells in the SGZ and GL are illustrated in Fig. 4 D and E, respectively (representative photographs are in Fig. S7). In the SGZ there was a significant effect of genotype [F(1,36) = 5.246, P < 0.05]. Post hoc analysis revealed increased survival of newly born cells in MS GABAB(1b)−/− mice compared with MS WT mice (P < 0.05), although this difference was not observed in NMS mice (P = 0.219). Interestingly, upon segregation of the hippocampus into the dHi and vHi, NMS GABAB(1b)−/− mice exhibited increased survival of newly born cells compared with NMS WT mice specifically in the dorsal but not ventral SGZ. However, under stress conditions, this increased survival of newly born cells in GABAB(1b)−/− mice shifted from the dHi to the vHi [MS WT vs. MS GABAB(1b)−/−, P < 0.01] (two-way ANOVA dorsal SGZ: genotype [F(1,36) = 5.671, P < 0.05]; two-way ANOVA ventral SGZ: genotype [F(1,34) = 5.546, P < 0.05]).
Surviving newly born neurons generated in the SGZ migrate to the GL, where they mature to become integrated into the neural circuitry of the hippocampus. Therefore, we also determined the number of surviving newly born cells in the GL of these mice (Fig. 4E). Whereas the survival of newly born cells in the whole GL was unaffected, segregation of the GL into dorsal and ventral regions revealed interesting effects. In the vHi, MS decreased the survival of newly born cells in the GL of WT mice (P < 0.05) but not in GABAB(1b)−/− mice, thus suggesting that GABAB(1b)−/− mice are resilient to stress-induced reductions in the survival of newly born cells (genotype [F(1,34) = 3.403, P = 0.07]; genotype–stress interaction [F(1,34) = 4.350, P < 0.05]). In contrast, there were no group differences in the survival of newly born cells in the dHi, thus suggesting a preferential effect of both MS and the protective effects of GABAB(1b) deletion in the vHi.
GABAB(1b)−/− but Not GABAB(1a)−/− Mice Exhibit Increased Adult Hippocampal Neurogenesis.
Experiments using doublecortin (DCX) immunohistochemistry in female mice confirmed that increased neurogenesis is restricted to GABAB(1b)−/− mice (P < 0.05) and that GABAB(1a)−/− mice were not different from WT mice (Fig. S8, SI Materials and Methods, and SI Results). Although a similar pattern of effects was observed in the dHi and vHi of NMS and MS mice, statistically significant effects were observed only in the dHi. Variability in these data might be a function of the limited sample number available for analysis (n = 6 vs. n = 10 in the cytogenesis study), estrus cycle regulation of neurogenesis in females (males were used in the cytogenesis study), and variation in the age or maturation state of individual DCX-positive cells, which future studies could investigate using appropriate immunohistochemical analyses.
Discussion
Understanding the molecular mechanisms underlying stress susceptibility and resilience is a key step toward identifying novel targets that could be exploited in the development of new, more effective treatments for stress-related psychiatric disorders, including depression. Here we show that the GABAB receptor may be one such target. Specifically, mice lacking the GABAB(1b) subunit are resilient to both stress-induced anhedonia and psychosocial stress-induced social withdrawal and exhibit antide-pressant-like behavior, whereas GABAB(1a)−/− mice are more susceptible to stress-induced anhedonia and psychosocial stress-induced social withdrawal. These findings have important implications for the pathophysiology and treatment of depression. Furthermore, they support the contention that GABAB receptor subunit isoforms play differential roles in mediating behavioral responses (7–9).
GABAB(1a)−/− mice have previously been shown to exhibit sleep disturbances (6), cognitive impairments (7–9), and a reduced threshold for fear generalization (10). Here we show that GABAB(1a)−/− mice are more susceptible to stress-induced anhedonia. We cannot rule out that the increased maternal care behavior provided by GABAB(1a)−/− dams may have contributed to this phenotype. Future studies examining the phenotype of GABAB(1a)−/− mice following cross-fostering with WT mice would give further insight. Interestingly, a recent small postmortem study reported that GABAB(1a) mRNA expression is decreased in the dentate gyrus of depressed individuals (4), whereas antidepressant-induced increases in GABAB(1a) mRNA expression have been reported in the rat hippocampus (31). Taken together, this suggests that the GABAB(1a) receptor subunit may play a role in depression and antidepressant action.
On the other hand, data from GABAB(1b)−/− mice point to a novel role for this isoform in stress resilience. Previously, these mice have been shown to exhibit impaired fear conditioning (10) and cognitive deficits, including impairments in spatial working memory and extinction of aversive taste memory (7, 8). Here we show that GABAB(1b)−/− mice are stress-resilient and that the hippocampus is a key area that is differentially activated in GABAB(1b)−/− and GABAB(1a)−/− mice in response to stress. Moreover, in another model of depression, helpless H/Rouen mice, hippocampal GABAB(1b) mRNA expression was increased compared with nonhelpless counterparts. This suggests that increased hippocampal GABAB(1b) mRNA expression is associated with a depression-like phenotype and that reducing its expression may have antidepressant-like effects. Indeed, we observed that compared with both WT and GABAB(1a)−/− mice, GABAB(1b)−/− mice were resilient to psychosocial stress-induced social avoidance and the anhedonic effects of early-life stress in the female urine sniffing test. We also observed that GABAB(1b)−/− mice exhibited antidepressant-like behavior in the forced swim test, although this effect should be interpreted with caution given their hyperactive phenotype. Importantly, GABAB(1a) and GABAB(1b) proteins are up-regulated 29% and 15% in CA1 of GABAB(1b)−/− and GABAB(1a)−/− mice, respectively (9), and thus it cannot be ruled out that their differential phenotypes are due to compensatory up-regulation of the other subunit.
Given the focus on the role of adult hippocampal neurogenesis in stress-related disorders (32) and the fact that we recently showed that chronic treatment with a GABAB receptor antagonist increases hippocampal cell proliferation (16), we thus assessed whether the stress-resilient phenotype of GABAB(1b)−/− mice is accompanied by changes in adult hippocampal cytogenesis and neurogenesis. Indeed, GABAB(1b)−/− mice exhibited increased proliferation and survival of newly born cells in the adult hippocampus, and these effects occurred predominantly in the ventral hippocampus, particularly under stress conditions. The vHi is thought to play a preferential role in the modulation of the stress response and anxiety, whereas the dHi is thought to play a preferential role in spatial learning and memory (30). Intriguingly, emerging evidence suggests that stress and antidepressant-like treatments may exert their effects on adult hippocampal neurogenesis preferentially in the vHi rather than in the dHi (28, 29). Moreover, analogous findings have been reported in humans, with antidepressants increasing neurogenesis in the anterior hippocampus (33). In addition to increases in the survival of newly born cells, GABAB(1b)−/− mice were also resistant to stress-induced decreases in the survival of newly born cells. The regulation of adult hippocampal neurogenesis by GABAB receptors has remained largely unexplored, but we previously reported that chronic treatment with a GABAB receptor antagonist that has antidepressant-like behavioral effects increases cell proliferation specifically in the vHi (16). Similarly, it was recently reported that the GABAB receptor is expressed on neural stem cells, and that mice lacking both GABAB isoforms exhibit increased proliferation and accelerated neuronal differentiation (34). Taken together, it is clear that GABAB receptors modulate adult hippocampal neurogenesis and that these effects are coupled with alterations in antidepressant-like behavior and stress resilience, with specific GABAB(1) receptor subunit isoforms playing differential roles in these behavioral effects. Future studies investigating neurogenesis-dependent behaviors such as pattern separation, or antidepressant-induced decreases in anxiety in the novelty-suppressed feeding test (27), will give further insight into the role of the GABAB(1b)–neurogenesis relationship in behavior, although it is also important to note that differences in innate anxiety were not observed in other tests (20).
Although our mechanistic studies focused on the hippocampus, it is important to note that GABAB(1b)−/− mice also exhibited enhanced stress-induced activation of some other brain regions, although these effects were less robust. Interestingly, the nucleus accumbens was the only region that was differentially affected in NMS and MS GABAB(1b)−/− mice by restraint stress. GABAB interactions at the level of the NAcc are well-described in the context of drug addiction (35) and may play a role in stress-induced anhedonia (18). Previous studies have reported serotonin–GABAB receptor interactions (17, 36), and here we show that stress-induced c-Fos activation was increased in the dorsal raphe nucleus in both NMS and MS GABAB(1b)−/− mice. This is interesting in light of recent data showing that the activity of GABAergic neurons in the DRN regulates resilience to SD stress (37) and that the antidepressant fluoxetine suppresses GABAB receptor activity in the DRN (38).
The molecular mechanisms underlying phenotypic differences between GABAB(1a)−/− and GABAB(1b)−/− mice are not yet known, but differences in cellular localization, physiological properties, and ontogenic expression of these subunits have been reported (6). Unlike GABAB(1b), GABAB(1a) can localize to axons via Sushi domains, which also increase surface stability of GABAB(1a,2) receptors (6, 39). In dendrites, GABAB(1a) localizes to glutamatergic terminals for heteroceptor function whereas GABAB(1b) localizes to spines opposing glutamate release sites, thus affecting pre- or postsynaptic inhibition (6). Interestingly, GABAB(1a) and GABAB(1b) are preferentially expressed in the developing and adult brain, respectively (3), and thus GABAB(1b) levels are relatively lower during the plastic period of brain development. In parallel, the present data suggest that inhibiting GABAB(1b) expression facilitates plasticity in the form of neurogenesis. Future studies characterizing the roles of these subunits will give further insight into the mechanisms underlying the differential phenotypes observed here.
In conclusion, GABAB(1) receptor subunit isoforms differentially regulate resilience to stress-induced anhedonia, with reductions in GABAB(1b) receptors associated with resilience whereas reductions in GABAB(1a) receptors are associated with increased susceptibility. These effects were coupled with alterations in stress-induced neural activity of reward pathways and adult hippocampal neurogenesis, and are further supported by alterations in the expression of GABAB(1) subunit isoforms in a genetic mouse model of depression and in response to antidepressant treatment (31) as well as recent postmortem findings in the human hippocampus (4). Taken together, these data further support the concept that the GABAB receptor may play a crucial role in the pathophysiology and treatment of stress-related disorders.
Materials and Methods
Animals.
WT, GABAB(1a)−/−, and GABAB(1b)−/− mice were bred at University College Cork. Helpless H/Rouen and nonhelpless mice were bred at Centre de Recherche en Neurosciences de Lyon INSERM U1028-CNRS 5292, Lyon, France, as previously described (24). Experiments were conducted in accordance with the European Community Council Directive (86/609/EEC) and approved by the Animal Experimentation Ethics Committee of University College Cork. See SI Materials and Methods for details.
Social Defeat Stress.
The effects of a 10-d SD stress (40) on saccharin preference and social interaction in WT, GABAB(1a)−/−, and GABAB(1b)−/− mice were examined. See Fig. 1A and SI Materials and Methods for details.
Maternal Separation Behavioral Experiment.
Pups underwent unpredictable maternal separation combined with unpredictable maternal stress from postnatal day 1 to 14 as well as a battery of behavioral tests conducted in the following order (Fig. 1B): ultrasonic vocalizations, stress-induced hyperthermia (SIH), open field (OF), tail suspension test, elevated plus maze (EPM), saccharin preference test (SPT)/female urine sniffing test (FUST), and forced swim test. Anhedonia was assessed in females using the SPT and in males using the FUST. Maternal care behaviors were assessed 2–3 h after separation. See SI Materials and Methods for details.
In Situ Hybridization.
In situ hybridization was conducted as previously described (41) (probes complementary to GABAB(1a) mRNA [595–636 bp; National Center for Biotechnology Information (NCBI) Nucleotide Database reference no. NM_019439.3] and GABAB(1b) mRNA [39–82 bp; NCBI Nucleotide Database reference no. AF120255]). See SI Materials and Methods for details.
Immunohistochemistry.
c-Fos and doublecortin immunohistochemistry was conducted in female mice as previously described (21). BrdU and Ki67 immunohistochemistry was conducted in male mice as previously described (28). See Fig. 4A and SI Materials and Methods for details.
Statistical Analysis.
Data were analyzed using two-way ANOVA and Fisher’s least significant difference (LSD) post hoc test with the exception of USV (one-way ANOVA and Fisher’s LSD post hoc test), and FUST and in situ hybridization data (unpaired Student t test). P < 0.05 was the criterion for statistical significance.
Supplementary Material
Acknowledgments
Technical assistance from Mr. Patrick Fitzgerald, Dr. Susan Grenham, Riccardo Pizzo, and Elvina Muhaj is gratefully acknowledged. This work was supported by the European Commission [Framework Programme 7 (FP7)-Health-2007-A-201714]. The Alimentary Pharmabiotic Centre is funded by Science Foundation Ireland (SFI) through the Irish Government’s National Development Plan (Grants 02/CE/B124, 07/CE/B1368, and SFI/12/RC/2273). B.B. is supported by the National Center for Competences in Research “Synapsy, Synaptic Bases of Mental Health Disease” and the Swiss National Science Foundation (3100A0-117816).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1404090111/-/DCSupplemental.
References
- 1.Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10(6):446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15(11):1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cryan JF, Kaupmann K. Don’t worry ‘B’ happy!: A role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci. 2005;26(1):36–43. doi: 10.1016/j.tips.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Ghose S, Winter MK, McCarson KE, Tamminga CA, Enna SJ. The GABAB receptor as a target for antidepressant drug action. Br J Pharmacol. 2011;162(1):1–17. doi: 10.1111/j.1476-5381.2010.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pilc A, Nowak G. GABAergic hypotheses of anxiety and depression: Focus on GABA-B receptors. Drugs Today (Barc) 2005;41(11):755–766. doi: 10.1358/dot.2005.41.11.904728. [DOI] [PubMed] [Google Scholar]
- 6.Gassmann M, Bettler B. Regulation of neuronal GABA(B) receptor functions by subunit composition. Nat Rev Neurosci. 2012;13(6):380–394. doi: 10.1038/nrn3249. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson LH, Kelly PH, Bettler B, Kaupmann K, Cryan JF. Specific roles of GABA(B(1)) receptor isoforms in cognition. Behav Brain Res. 2007;181(1):158–162. doi: 10.1016/j.bbr.2007.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobson LH, Kelly PH, Bettler B, Kaupmann K, Cryan JF. GABA(B(1)) receptor isoforms differentially mediate the acquisition and extinction of aversive taste memories. J Neurosci. 2006;26(34):8800–8803. doi: 10.1523/JNEUROSCI.2076-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vigot R, et al. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50(4):589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaban H, et al. Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nat Neurosci. 2006;9(8):1028–1035. doi: 10.1038/nn1732. [DOI] [PubMed] [Google Scholar]
- 11.Klempan TA, et al. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol Psychiatry. 2009;14(2):175–189. doi: 10.1038/sj.mp.4002110. [DOI] [PubMed] [Google Scholar]
- 12.Levinson AJ, et al. Evidence of cortical inhibitory deficits in major depressive disorder. Biol Psychiatry. 2010;67(5):458–464. doi: 10.1016/j.biopsych.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Croarkin PE, et al. Evidence for pretreatment LICI deficits among depressed children and adolescents with nonresponse to fluoxetine. Brain Stimul. 2014;7(2):243–251. doi: 10.1016/j.brs.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Mombereau C, et al. Genetic and pharmacological evidence of a role for GABA(B) receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology. 2004;29(6):1050–1062. doi: 10.1038/sj.npp.1300413. [DOI] [PubMed] [Google Scholar]
- 15.Mombereau C, et al. Altered anxiety and depression-related behaviour in mice lacking GABAB(2) receptor subunits. Neuroreport. 2005;16(3):307–310. doi: 10.1097/00001756-200502280-00021. [DOI] [PubMed] [Google Scholar]
- 16.Felice D, O’Leary OF, Pizzo RC, Cryan JF. Blockade of the GABA(B) receptor increases neurogenesis in the ventral but not dorsal adult hippocampus: Relevance to antidepressant action. Neuropharmacology. 2012;63(8):1380–1388. doi: 10.1016/j.neuropharm.2012.06.066. [DOI] [PubMed] [Google Scholar]
- 17.Slattery DA, Desrayaud S, Cryan JF. GABAB receptor antagonist-mediated antidepressant-like behavior is serotonin-dependent. J Pharmacol Exp Ther. 2005;312(1):290–296. doi: 10.1124/jpet.104.073536. [DOI] [PubMed] [Google Scholar]
- 18.Nowak G, et al. Antidepressant-like activity of CGP 36742 and CGP 51176, selective GABAB receptor antagonists, in rodents. Br J Pharmacol. 2006;149(5):581–590. doi: 10.1038/sj.bjp.0706845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henningsen K, et al. Low maternal care exacerbates adult stress susceptibility in the chronic mild stress rat model of depression. Behav Pharmacol. 2012;23(8):735–743. doi: 10.1097/FBP.0b013e32835a5184. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson LH, Bettler B, Kaupmann K, Cryan JF. Behavioral evaluation of mice deficient in GABA(B(1)) receptor isoforms in tests of unconditioned anxiety. Psychopharmacology (Berl) 2007;190(4):541–553. doi: 10.1007/s00213-006-0631-9. [DOI] [PubMed] [Google Scholar]
- 21.O’Mahony CM, Sweeney FF, Daly E, Dinan TG, Cryan JF. Restraint stress-induced brain activation patterns in two strains of mice differing in their anxiety behaviour. Behav Brain Res. 2010;213(2):148–154. doi: 10.1016/j.bbr.2010.04.038. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12(2):118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 23.Brown ES, Rush AJ, McEwen BS. Hippocampal remodeling and damage by corticosteroids: Implications for mood disorders. Neuropsychopharmacology. 1999;21(4):474–484. doi: 10.1016/S0893-133X(99)00054-8. [DOI] [PubMed] [Google Scholar]
- 24.El Yacoubi M, et al. Behavioral, neurochemical, and electrophysiological characterization of a genetic mouse model of depression. Proc Natl Acad Sci USA. 2003;100(10):6227–6232. doi: 10.1073/pnas.1034823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surget A, et al. Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry. 2011;16(12):1177–1188. doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476(7361):458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10(9):1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 28.O’Leary OF, O’Connor RM, Cryan JF. Lithium-induced effects on adult hippocampal neurogenesis are topographically segregated along the dorso-ventral axis of stressed mice. Neuropharmacology. 2012;62(1):247–255. doi: 10.1016/j.neuropharm.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Tanti A, Belzung C. Neurogenesis along the septo-temporal axis of the hippocampus: Are depression and the action of antidepressants region-specific? Neuroscience. 2013;252:234–252. doi: 10.1016/j.neuroscience.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Bannerman DM, et al. Regional dissociations within the hippocampus—Memory and anxiety. Neurosci Biobehav Rev. 2004;28(3):273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Sands SA, Reisman SA, Enna SJ. Effect of antidepressants on GABA(B) receptor function and subunit expression in rat hippocampus. Biochem Pharmacol. 2004;68(8):1489–1495. doi: 10.1016/j.bcp.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 32.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: Opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16(3):239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 33.Boldrini M, et al. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34(11):2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giachino C, et al. GABA suppresses neurogenesis in the adult hippocampus through GABAB receptors. Development. 2014;141(1):83–90. doi: 10.1242/dev.102608. [DOI] [PubMed] [Google Scholar]
- 35.Vlachou S, Markou A. GABAB receptors in reward processes. Adv Pharmacol. 2010;58:315–371. doi: 10.1016/S1054-3589(10)58013-X. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi A, Shimamoto A, Boyson CO, DeBold JF, Miczek KA. GABA(B) receptor modulation of serotonin neurons in the dorsal raphé nucleus and escalation of aggression in mice. J Neurosci. 2010;30(35):11771–11780. doi: 10.1523/JNEUROSCI.1814-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Challis C, et al. Raphe GABAergic neurons mediate the acquisition of avoidance after social defeat. J Neurosci. 2013;33(35):13978–13988, 13988a. doi: 10.1523/JNEUROSCI.2383-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cornelisse LN, et al. Reduced 5-HT1A- and GABAB receptor function in dorsal raphé neurons upon chronic fluoxetine treatment of socially stressed rats. J Neurophysiol. 2007;98(1):196–204. doi: 10.1152/jn.00109.2007. [DOI] [PubMed] [Google Scholar]
- 39.Hannan S, Wilkins ME, Smart TG. Sushi domains confer distinct trafficking profiles on GABAB receptors. Proc Natl Acad Sci USA. 2012;109(30):12171–12176. doi: 10.1073/pnas.1201660109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savignac HM, et al. Increased sensitivity to the effects of chronic social defeat stress in an innately anxious mouse strain. Neuroscience. 2011;192:524–536. doi: 10.1016/j.neuroscience.2011.04.054. [DOI] [PubMed] [Google Scholar]
- 41.Bravo JA, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.