Significance
The environmental control of neuronal wiring is one of the most intriguing issues in neuroscience. However, the molecular mechanisms are largely unknown. Here, we demonstrate that the expression of the netrin family member netrin-4 (NTN4) is activity-dependent in the developing cortex and promotes terminal branching of thalamocortical axons. Evidence further shows that unc-5 homolog B (Unc5B), a putative receptor of NTN4, is expressed in the developing thalamus and mediates NTN4 signaling. These results suggest that NTN4 is the key molecule that underlies activity-dependent axon branch formation in neocortical circuits.
Keywords: development, neocortex, axon guidance, neuronal wiring
Abstract
Axon branching is remodeled by sensory-evoked and spontaneous neuronal activity. However, the underlying molecular mechanism is largely unknown. Here, we demonstrate that the netrin family member netrin-4 (NTN4) contributes to activity-dependent thalamocortical (TC) axon branching. In the postnatal developmental stages of rodents, ntn4 expression was abundant in and around the TC recipient layers of sensory cortices. Neuronal activity dramatically altered the ntn4 expression level in the cortex in vitro and in vivo. TC axon branching was promoted by exogenous NTN4 and suppressed by depletion of the endogenous protein. Moreover, unc-5 homolog B (Unc5B), which strongly bound to NTN4, was expressed in the sensory thalamus, and knockdown of Unc5B in thalamic cells markedly reduced TC axon branching. These results suggest that NTN4 acts as a positive regulator for TC axon branching through activity-dependent expression.
Axon branching is an essential process to determine the final pattern of neuronal connections. Previous studies have demonstrated that axon branching is controlled not only by axon guidance-related molecules (1–6) but also by neuronal activity, such as firing and synaptic activity (7–10). However, how neuronal activity is converted into the molecular signals that underlie axon branching is still largely unknown.
The thalamocortical (TC) projection is a well-characterized system in which to address this issue. TC axons originating from sensory thalamic nuclei form elaborate arbors, primarily in layer 4 of the neocortex (11). Lamina-specific axon branching occurs from the onset of development and is universal in the mammalian cortex (12–17), indicating that a rigid developmental program is predominant for laminar specificity. TC axon branching is also known to be modified by neuronal activity. In the visual system, geniculocortical axon arbors can be remodeled drastically by manipulating visual experience and cortical-cell activity (18–22). A similar feature has been demonstrated in the somatosensory system. In mutant mice in which synaptic transmission or downstream signaling mechanisms are disrupted, TC axon arbors are affected primarily along the tangential axis whereas their laminar pattern is not obviously influenced (23–26). We have also shown in vitro that the loss of firing and synaptic activities substantially suppresses TC axon branching in the target layer (27). All of these findings imply the existence of a target-derived, branch-promoting molecule whose expression is regulated by neuronal activity. In this study, we attempted to identify this hypothetical molecule and to reveal the molecular mechanism of its action.
Results
Identification of a Target-Derived Molecule Whose Expression Is Regulated by Neural Activity.
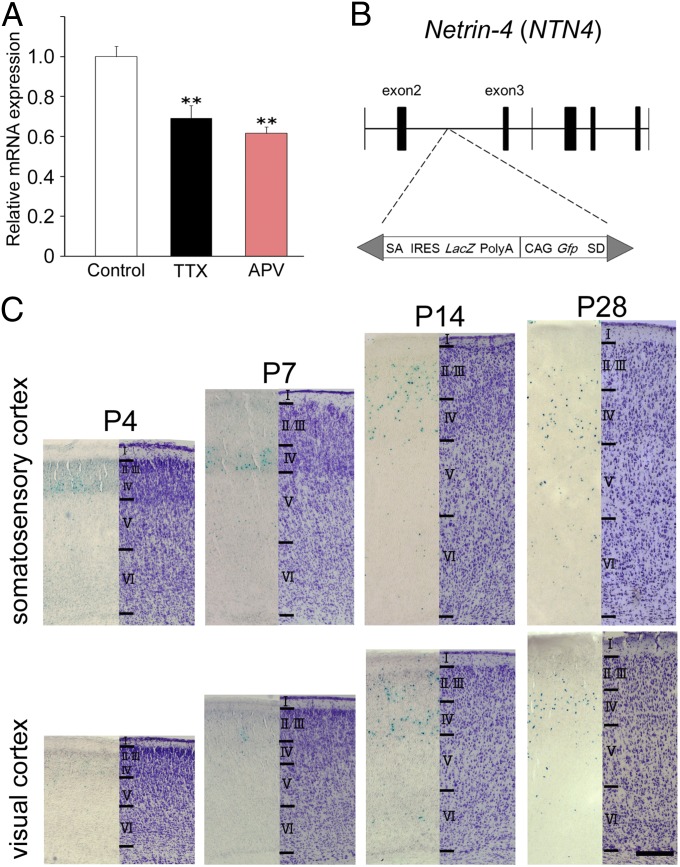
Because we have shown previously that spontaneous activity promotes TC axon branching in cocultures of the thalamus and cortex (27, 28), we attempted to identify gene(s) whose expression is down-regulated in cortical-slice cultures by blocking firing and synaptic activity. Based on prior molecular screening and gene-expression analyses in the developing cortex, cadherin-6 (cdh6), ephrin-A5 (efna5), netrin-4 (ntn4), semaphorin7a (sema7a) and kit ligand (kitlg) were selected as candidate genes that are expressed in and around the TC recipient layer and may directly affect axon behavior (29–33). Their expression in cortical explants was examined using quantitative PCR analysis after 2 wk in culture, when TC axons form extensive branches (27, 34, 35). ntn4 expression was significantly reduced by chronic application of tetrodotoxin (TTX, 100 nM), a voltage-gated sodium channel blocker (relative mRNA expression compared with the untreated control; 0.69 ± 0.06, n = 4, P < 0.05, Dunnett test) (Fig. 1A and Fig. S1). DL-2-amino-5-phosphonovalerate (APV), an antagonist of the NMDA-type glutamate receptor (NMDAR), also decreased ntn4 expression (relative mRNA expression; 0.62 ± 0.03, n = 3, P < 0.05, Dunnett test) (Fig. 1A). These results suggest that firing and synaptic activity are required for ntn4 expression in the cortex. In contrast, transcripts of the other candidate genes were not reduced by activity blockade (Fig. S1); indeed, the expression levels of three genes increased with one or the other of the pharmacological treatments.
Fig. 1.
Expression of ntn4 in the cortex during development. (A) Ntn4 expression was examined by quantitative PCR in organotypic cocultures of the thalamus and cortex in normal culture medium (white) and in the presence of TTX (black) or APV (red). Histograms represent relative mRNA expression of ntn4 in cortical slices. Ntn4 expression was reduced by TTX as well as by APV application. Error bars show SEMs. **P < 0.01, Dunnett test. (B) Mutagenesis by insertion of the LacZ-containing cassette in ntn4 gene locus. SA, splice acceptor; IRES, internal ribosome entry site; SD, splice donor. (C) β-gal (Left) and Nissl staining (Right) in the somatosensory and visual cortex at P4, P7, P14, and P28. (Scale bar: C, 0.2 mm.)
Developmental Expression of ntn4.
As a netrin family member, NTN4 is thought to be involved in cellular survival in the CNS, but its expression is not well-characterized in the developing brain (33, 36, 37). We investigated the expression pattern at postnatal developmental stages, using transgenic rats in which the β-galactosidase (β-gal) coding region was inserted into the ntn4 gene locus by a transposon-based technique (Fig. 1B) (38). Staining for β-gal showed that ntn4-expressing cells began to appear in the dense cortical plate of the somatosensory cortex at postnatal day 4 (P4), when TC axons are forming branches, but were undetectable in the visual cortex at this stage (Fig. 1C). At P7, when cortical lamination is complete, the β-gal–positive (β-gal+) cells were mostly found in the upper layers (layers 2/3 and 4) of both the somatosensory and visual cortices (Fig. 1 B and C), although weakly stained cells were also found in the deep layers (layers 5 and 6). At P14, stronger expression was observed in the sensory cortex, albeit diffused to other layers (Fig. 1C). The expression level was maintained until P28 (Fig. 1C) but decreased thereafter. Quantitative analysis showed that the number of β-gal+ cells in the unit area was larger in somatosensory than visual cortex at P4 and P7 but was not significantly different between these two cortical areas at later stages (Table S1).
Sensory Activity Induces ntn4 Expression in the Visual Cortex.
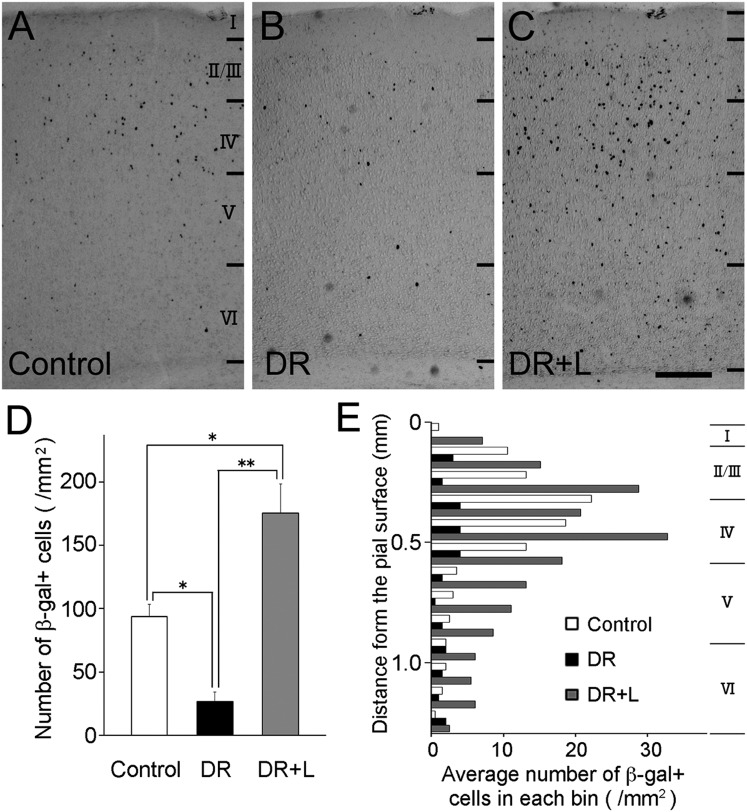
The observed elevation of ntn4 expression in the sensory cortex during postnatal developmental stages raised the possibility that sensory input modulates ntn4 expression. To test this possibility, the expression of ntn4 in the visual cortex of transgenic animals (heterozygotes) was investigated by manipulating visual experience. Dark rearing throughout the first 3 wk after birth resulted in a considerable reduction in the number of β-gal+ cells, compared with their number in animals reared normally (for control, 93.8 ± 9.7 per mm2, n = 3; for dark-reared animals, 26.7 ± 7.3, n = 3) (Fig. 2 A and B). Furthermore, a 1-d light exposure after the dark-rearing period restored, and even increased, the number of β-gal+ cells (175.5 ± 23.2, n = 3) (Fig. 2C). Quantitative analysis clearly showed that the number of β-gal+ cells was significantly different among the three groups (Fig. 2D). As for laminar specificity, β-gal+ cell distribution was maximal at 200–500 μm (layers 2/3 and 4) from the pial surface in every case although the distribution was broader in animals whose visual experience was manipulated (Fig. 2E).
Fig. 2.
Sensory activity-dependent expression of ntn4 in the visual cortex. (A) The distribution of β-gal+ cells was investigated in the visual cortex of ntn4-heterozygous rats at P23. Similarly, the distribution was examined in 3-wk-old heterozygous animals subjected to dark rearing after birth (B) and to a 1-d light exposure after the dark rearing (C). (Scale bar: 0.2 mm.) (D) The number of β-gal+ cells was counted in a unit area (layer I to VI) of cortical sections in the three conditions. Error bars show SEMs. *P < 0.05, **P < 0.01, Dunnett test. (E) Laminar distribution of β-gal+ cells. Histograms show the mean number of β-gal+ cells in each bin (0.1 mm) as a function of cortical depth. Average laminar boundaries are indicated to the right.
Activity-dependent distribution of ntn4-expressing cells was further studied in vitro. For this analysis, cortical slices from the heterozygous animals were subjected to either TTX or KCl treatment, and β-gal staining was performed after 2 wk in vitro. Consistent with the in vivo result, β-gal+ cells were distributed in the upper layers in the normal culture medium, but TTX treatment completely suppressed β-gal expression (Fig. S2). In contrast, KCl treatment increased the number of β-gal+ cells considerably (Fig. S2). These in vivo and in vitro results suggest that ntn4 expression in the cortex is modified by sensory-evoked and spontaneous activity.
TC Axon Branching Is Increased by NTN4 in Vitro.
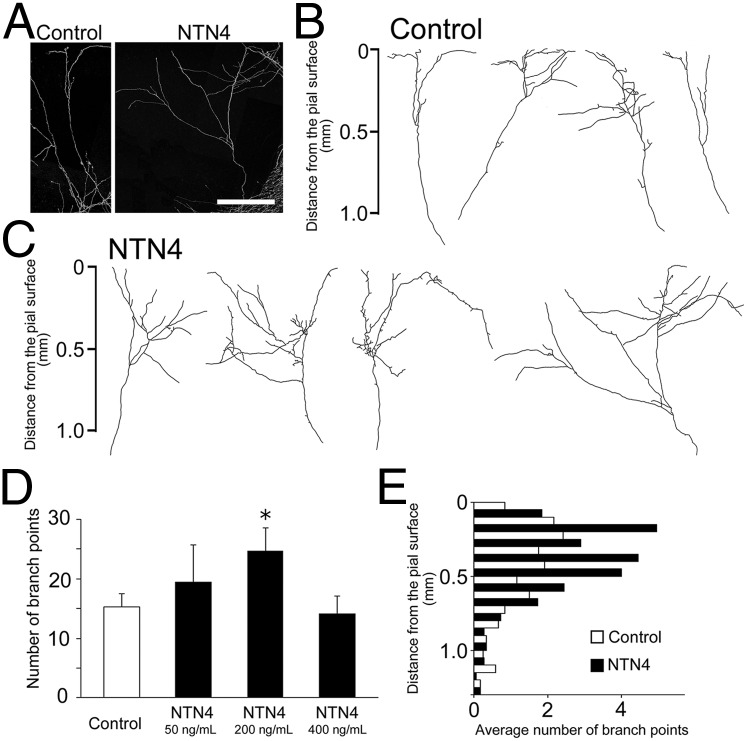
Next, we investigated whether NTN4 has branch-promoting activity. TC axon branching was examined in cocultures of the thalamus and cortex by adding the recombinant NTN4 protein to the culture medium. The morphology of individual TC axons was examined by transfecting a small number of thalamic cells with a plasmid containing the coding region of enhanced yellow fluorescent protein (EYFP) (27). After 2 wk in vitro, significantly more elaborate branching was evident in the presence of NTN4 than in its absence (number of branch points per individual axon, 15.3 ± 2.2, n = 22 for control; 24.7 ± 3.8, n = 18 for 200 ng/mL NTN4) although the high concentration (400 ng/mL) was not significantly different from the control (Fig. 3 A–D). The addition of NTN4 (200 ng/mL) increased the total branch length (3.4 ± 0.3 mm for control; 4.9 ± 0.6 mm for NTN4) and branch width (0.54 ± 0.06 mm for control; for 1.1 ± 0.19 mm for NTN4). The majority of branching points were found in the upper layers, including the presumed layer 4, irrespective of the presence or absence of exogenous NTN4 (Fig. 3E).
Fig. 3.
TC axon branching is promoted by exogenous NTN4. (A) Representative individual TC axons after 2 wk in vitro in the presence or absence of NTN4. Axon branching increased after NTN4 application (200 ng/mL) (C), compared with control (B). (Scale bar: 0.5 mm.) (D) Quantification of branch numbers in control (n = 22) and NTN4-treated (n = 12 for 50 ng/mL, n = 18 for 200 ng/mL, and n = 14 for 400 ng/mL) cultures. *P < 0.05, Dunnett test. (E) Laminar distribution of branching points in untreated and treated cultures. Histograms show the mean number of branching points in each bin (0.1 mm) as a function of cortical depth (22 axons for control and 18 axons for NTN4 application).
To examine whether NTN4 directly affects TC axon branching, we applied NTN4 to dissociated thalamic cell cultures (Fig. S3 A and B). As a result, the number of branches from the longest processes, which were shown to be axons in the previous study (39), was significantly increased by NTN4 application (200 ng/mL) (Fig. S3C). The total branch length was also increased (Fig. S3D). This result strongly suggests that NTN4 acts on TC axons directly as a branch-promoting factor.
An intriguing possibility is that NTN4 acts as a mediator of activity-dependent branching. To test this possibility, the effect of exogenous NTN4 was studied in the presence of TTX (Fig. S4). As shown previously, the activity blockade inhibited axon branching in culture (27), but the addition of NTN4 rescued the phenotype considerably (Fig. S4). Therefore, NTN4 can promote axon branching downstream of neuronal activity.
Endogenous NTN4 in the Cortex Is Required for TC Axon Branching.
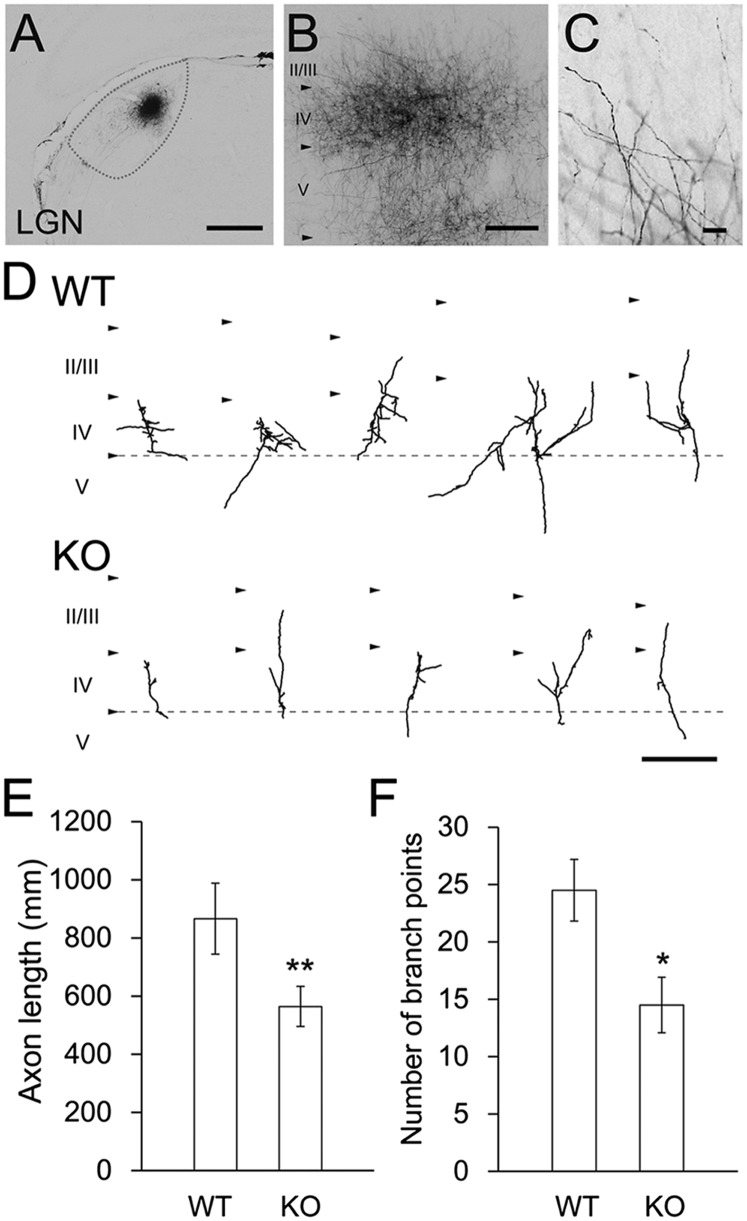
To investigate the role of endogenous NTN4 in TC axon branching, geniculocortical axon arbors were studied in 3-wk-old wild-type and ntn4 mutant rats by injecting an axon tracer, biotinylated dextran amine (BDA), into the dorsal part of the lateral geniculate nucleus (Fig. 4A). Although numerous geniculocortical axons were labeled by this method, we were able to trace individual axonal fragments across layer 4 of the primary visual cortex (Fig. 4 B and C). As shown in Fig. 4D, many branches had emerged from individual axon fragments in layer 4 of the wild-type cortex, but branching was less prominent in the KO. Quantitative analysis demonstrated that the number of branches emerging from each axon fragment across layer 4 was significantly smaller in the KO (Fig. 4E) (24.5 ± 8.5 for wild-type, n = 10; 14.5 ± 7.6 for ntn4 KO, n = 12, P < 0.01, Student t test). Likewise, the total branch length of each axon fragment was also reduced in the KO (Fig. 4F) (866 ± 386 µm for wild-type, n = 10; 564 ± 217 µm for ntn4 KO, n = 12, P < 0.05, Student t test). Thus, arbor formation in geniculate axons was suppressed in ntn4 KO.
Fig. 4.
Endogenous NTN4 regulates TC axon branching in vivo. In vivo labeling of single TC axons. (A) BDA injection site in the LGN at P19. (B) Labeled TC axons in visual cortex. (C) Higher-magnification image of TC axon terminals. (Scale bars: A and B, 0.5 mm; C, 0.1 mm.) (D) Typical examples of reconstructed TC axon arbors of wild-type (Upper, WT) and ntn4 KO animals (Lower, KO). All examples represent single arbors in layer 4 of the V1 cortex in 50-μm-thick coronal sections. Quantitative analysis of TC axon length (E) and the number of axon branching point (F). *P < 0.05, **P < 0.01, Student t test.
The TC projection in the somatosensory cortex in wild-type and ntn4 KO was also studied by immunohistochemistry with an antibody against the serotonin transporter (5-HTT), a specific marker of developing TC axons. Because 5-HTT expression in thalamic axons is negligible in 2-wk-old animals, this analysis was performed at P10. At this stage, 5-HTT staining clearly showed barrel structures in the wild-type (Fig. S5A) (40, 41). Although similar 5-HTT staining was observed in the ntn4 KO, the intensity was much weaker (Fig. S5B). Quantitative analysis of 5-HTT staining confirmed that the intensity of 5-HTT in layer 4 was significantly lower in the ntn4 KO than in wild-type (ratio of peak value in KO to wild-type; 0.70 ± 0.12, n = 3, P < 0.05, Student t test) (Fig. S5 C and D). This result also suggested that TC axon branching is less complex in the KO in vivo.
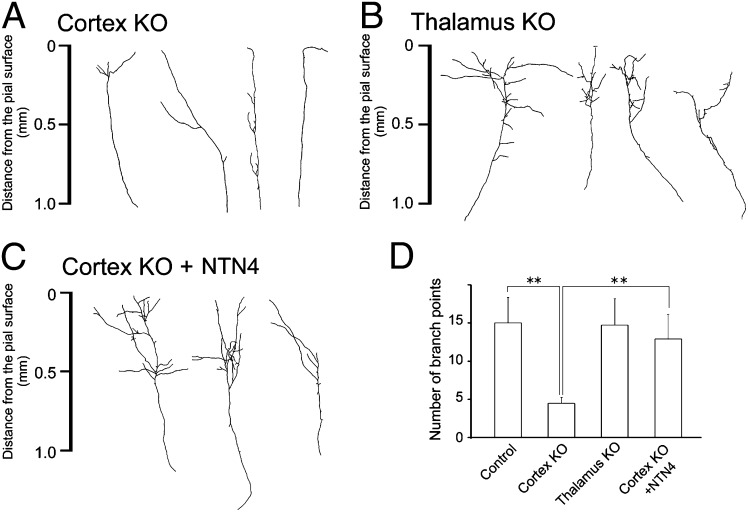
To specify the effect of NTN4 knockdown in the cortex, the action of endogenous NTN4 was further investigated by examining individual TC axon branching in chimeric cocultures of wild-type thalamus and ntn4-deficient cortex (Fig. 5A). After 2 wk in culture, EYFP-labeled TC axons were found to have simpler branching in the ntn4-deficient cortical explants (Fig. 5A). The number of branch points in the KO cortex was reduced considerably, compared with that in the wild-type cortex (15.3 ± 2.2, n = 22 for wild-type; 4.5 ± 0.8, n = 47 for KO; P < 0.01, Dunnett test) (Fig. 5B and Table S1). Likewise, the total branch length (3.4 ± 0.3 mm for wild-type; 1. 6 ± 0.1 mm for KO) and branch width (0.54 ± 0.06 mm for wild-type; 0.19 ± 0:03 mm for KO) were significantly reduced in the ntn4-deficient cortex (Fig. 5 A and B and Table S2). This decrease of branch complexity is very similar to that observed in ntn4 KO rats in vivo (Fig. 4F). In contrast, branch complexity was unaffected in cocultures of ntn4-deficient thalamus and wild-type cortex (number of branch points, 14.7 ± 3.4, n = 22) (Fig. 5 B and D and Table S2). Moreover, addition of NTN4 recombinant protein to the culture medium rescued the phenotype of ntn4 KO (number of branch points, 11.7 ± 3.1; n = 11) (Fig. 5 C and D and Table S2). These in vivo and in vitro results clearly indicated that endogenous NTN4 in the cortex contributes to normal TC axon branching.
Fig. 5.
NTN4 is required in cortical explants for TC axon branching. (A–C) Typical examples of individual TC axons are shown in chimeric cocultures of wild-type and ntn4 KO. (A) Wild-type thalamus was cocultured with ntn4 KO cortex. (B) Ntn4 KO thalamus was cocultured with wild-type cortex. (C) NTN4 recombinant protein (200 ng/mL) was added to the culture medium in cocultures of wild-type thalamus and the KO cortex. (D) Quantification of branching points in each case. **P < 0.01, Dunnett test.
Unc5B Acts as a Putative Receptor of NTN4.
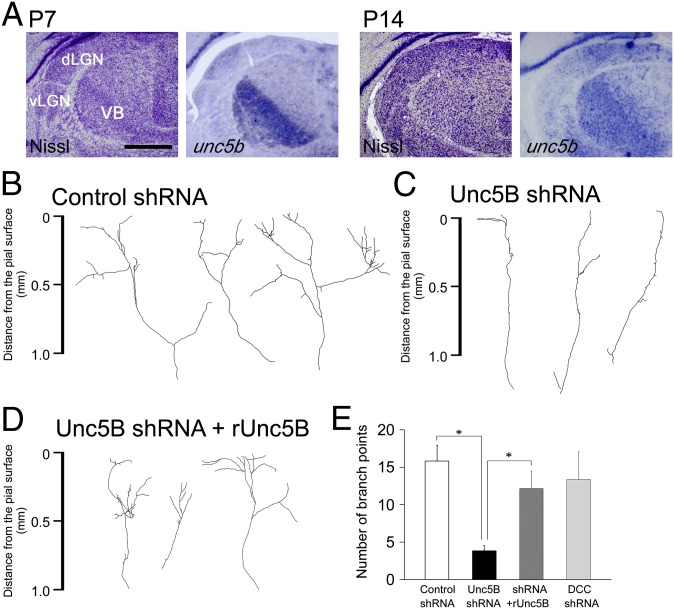
We studied the expression in the thalamus of putative receptors that reportedly bind to NTN4: deleted in colorectal carcinoma (dcc), neogenin-1 (neo1), and unc5 family (36, 42). At P7, dcc, neo1, and unc5b were strongly expressed in the ventrobasal (VB) thalamus and the dorsal part of the lateral geniculate nucleus (dLGN) (Fig. 6A and Fig. S6A, Upper). unc5b expression was sustained at P14 although the expression of dcc and neo1 became weaker and more diffuse (Fig. 6A and Fig. S6A, Lower). Thus, it is likely that developing sensory thalamic cells express receptor molecules that can respond to NTN4.
Fig. 6.
Unc5B is involved in TC axon branching by being expressed in the thalamus. (A) Expression pattern of unc5b was examined at P7 and P14 by in situ hybridization and compared with the distribution of thalamic nuclei as shown by Nissl staining. (Scale bar: 1 mm.) (B and C) Typical examples of TC axons transfected with control shRNA (B) and with unc5b shRNA (C). (D) TC axons cotransfected with unc5b shRNA and shRNA-resistant unc5b (runc5b). (E) Quantification of branching points in B–D and TC axons transfected with dcc shRNA. vLGN, the ventral part of the LGN. *P < 0.01, Dunnett test.
Receptor binding to NTN4 was examined by a cell surface binding assay, in which HEK293T cells expressed one of the above three candidate receptors. His-tag NTN4 was found to bind strongly to Unc5B-expressing cells, but to neither DCC- nor Neo1-expressing cells (Fig. S6C).
The role of endogenous Unc5B in TC axon branching was further studied using organotypic coculture preparations. Thalamic cells were cotransfected with an unc5b shRNA-expressing plasmid and the eyfp plasmid, and TC axon branching was observed after 14 d in culture. Knockdown of endogenous Unc5B significantly suppressed branch number, compared with scramble shRNA (Fig. 6 B, C, and E) (3.8 ± 0.6, n = 24 for shRNA; 15.8 ± 4.8, n = 16 for the scrambled shRNA, P < 0.05, Dunnett test). Moreover, cotransfection with an shRNA-resistant unc5b rescued the phenotype (Fig. 6 D and E) (12.2 ± 3.1, n = 18 for resistant unc5b with shRNA, P < 0.05, Dunnett test). In contrast, transfection of dcc shRNA did not decrease TC axon branching (13.4 ± 3.7, n = 8) (Fig. 6E). Thus, it is likely that Unc5B is expressed in sensory thalamic cells and contributes to axon branching as a NTN4 receptor.
Discussion
The present study indicates that NTN4 is a branch-promoting molecule that is expressed in the sensory cortex with activity dependence. Previous observations led us to hypothesize that branch-promoting activity for TC axons is distributed in the TC recipient layer and is up-regulated by neuronal activity (27, 43). Although it had previously shown that guidance molecules and neuronal activity work together to pattern precise connections in the retinotectal projection (44–47), the molecular basis of this cooperation was unresolved. The present findings clearly demonstrate the existence of a molecule, NTN4, that combines both elements.
NTN4 has been shown to play an important role in angiogenesis (42, 48), lung branching (49), and salivary gland formation (50). In these systems, NTN4 contributes to morphogenesis via mechanisms that also pertain to axon guidance (36, 37, 42) although its action can be both stimulatory and inhibitory. The present study has added a previously unidentified aspect, neuronal activity dependence, to the conventional function of NTN4. What neuronal activity is required for ntn4 expression? As shown by mRNA expression in the pharmacological blockade, both firing and synaptic activity are likely to be necessary for ntn4 expression. Consistent with this notion, our previous work has shown that the same pharmacological treatments suppress TC axon branching in coculture preparations (27).
To date, several molecular mechanisms in pre- and post-synaptic cells have been demonstrated to regulate axon branching (1–6, 51–54). Our results show that NTN4 synthesized by cortical cells has branch-promoting activity for TC axons in the mammalian brain. The fact that exogenous NTN4 slightly but significantly promoted axon branching in the upper layers (Fig. 3) implies that the effect of NTN4 may be more prominent for the formation of terminal axon branches.
Our present result further shows that Unc5B is expressed in sensory thalamic cells and is involved in TC axon branching. In retinotectal axon branching, DCC mediates the netrin-1–induced branch-promoting signal (55). Similarly, growth of TC and callosal axons at the embryonic stages is promoted by netrin-DCC signaling (56–59). However, the present data show that DCC is not strongly expressed in the thalamus at P14, and its binding to NTN4 is substantially weaker than that of Unc5B (Fig. 6). Thus, it is likely that Unc5B acts as a mediator for NTN4-induced branch-promoting activity, perhaps by activating of RhoA (60, 61). The expression of unc5b is also found in upper layer cells of the developing cortex (Fig. S6), suggesting the possibility that neurite growth of these neurons can be promoted by NTN4. Such growth might strengthen synaptic connections and increase neuronal activity in local circuits, which can in turn further enhance TC axon branching (10). However, we emphasize that NTN4 promotes branch formation via direct interaction with TC axons, due to the increase in branch formation of dissociated thalamic cells after NTN4 application (Fig. S3). This view is further supported by the inhibition of axon branching in unc5b knockdown cells (Fig. 6).
The developmental characteristics of ntn4 expression imply a specific role in TC axon branching. The fact that its expression in rodents is stronger during postnatal 2–4 wk suggests that NTN4 does not contribute to initial branch formation of TC axons because fundamental branching is established by the end of the first postnatal week (12, 16, 17, 23, 40). This temporal expression is considered to depend on not only neuronal activity but also a developmental program, considering that spontaneous firing and sensory-evoked activity are present in the cortex even during the first postnatal week (62–65). Similarly, the expression of brain-derived neurotrophic factor (BDNF), which can affect axon branching (7, 66), is also known to depend on neuronal activity (67) but is regulated developmentally (68, 69). Indeed, bdnf expression begins to increase in 2- to 3-wk-old rat cortex (68). Thus, it is likely that NTN4 is involved in activity-dependent TC axon branching in the juvenile stages of cortical development.
A series of evidence has demonstrated that activity-independent mechanisms are also responsible for TC axon branching (70–72). It has been reported that TC axons begin to form branches in snap-25 KO in which synaptic transmission is disrupted (73). In accordance with this finding, SEMA7A acts as an early branch-promoting factor through activity-independent expression (Fig. S1) (32). EFNA may also work together for initial TC axon branching regardless of neuronal activity (74). Taken together, multiple branch-promoting molecules including NTN4 are expressed during different developmental phases and are likely to contribute to axon branching in distinct contexts (39).
In summary, NTN4 is expressed in a target- and activity-dependent fashion to contribute to TC axon branching in the developing sensory cortex. We propose that NTN4 is a key molecule that underlies activity-dependent neural circuit formation.
Materials and Methods
Animals.
Sprague–Dawley (SD) rats were used for organotypic cocultures of the thalamus and cortex. ntn4 mutant rats generated by transposon-tagged mutagenesis were used for gene expression and loss-of-function analyses (38). All experiments were performed according to the guidelines laid down by the animal welfare committees of Osaka University, Tottori University, and the Japan Neuroscience Society.
Organotypic Coculture of Thalamus and Cortex.
Cocultures of the cortex with thalamic explants were prepared as described previously (34). In brief, the dorsal thalamic region was dissected from embryonic day (E) 15 rat embryos, and cortical slices were dissected from the visual and somatosensory cortex of P1 to -2 rats. A thalamic block and a cortical slice were placed on a membrane filter and cultured for 2 wk.
Axon Labeling by Electroporation and Image Analysis.
To label individual thalamic axons in the cortical explants, a small number of thalamic cells were transfected with an eyfp plasmid as described previously (27). See SI Materials and Methods for details.
β-Galactosidase Staining.
Whole brains or cultured slices were fixed with 4% (wt/vol) paraformaldehyde in 0.1 M phosphate buffer for 3–4 h, followed by cryoprotection with 15% and 30% (wt/vol) sucrose in 0.01 M phosphate buffer with saline. The frozen tissues were then cut into 50-µm coronal sections with a cryostat. After two washes with 0.01 M phosphate buffer containing 2 mM MgCl2, the sections were incubated with a staining solution (1 mg/mL X-gal, 5 mM potassium ferricyanide crystalline, 5 mM potassium ferricyanide trihydrate, 2 mM MgCl2 in 0.01 M phosphate buffer with saline) at 37 °C for a few days. The sections were then treated in 50%, 70%, 95%, and 100% (vol/vol) ethanol, and xylene, and finally embedded. Adjacent sections were subjected to Nissl staining with cresyl violet.
Dark-Rearing Conditioning.
NTN4 heterozygotes (Tp/+) were reared in darkness from birth. Exchange of food or cages was performed using night scope. At P21, animals were either deeply anesthetized in darkness and killed as dark-reared animals or exposed to the light for the next 24 h, and then killed as the light-reared group. As a control, animals were reared under 12 h:12 h light-dark cycles.
Tracer Injection and Morphological Analysis of Labeled Axons.
Tracer injection and morphological analysis of labeled axons were as described previously (18). In brief, a glass microelectrode filled with a solution containing BDA (Molecular Probe) was positioned stereotaxically to locate a clear visual response from the dorsal part of the LGN, and BDA was injected iontophoretically. After several days, the animals were euthanized with an overdose of Nembutal and perfused transcardially with cold saline and 4% paraformaldehyde in 0.1 M phosphate buffer. The brain was removed and postfixed overnight. The brains were cut into 50-μm-thick sections and subjected to the standard ABC method. All sections were mounted on gelatinized slides. All of the injection sites were confirmed to be localized in the LGN. As many BDA-filled axonal fragments as possible that could be followed individually across layer 4 of the primary visual cortex were selected and traced with the aid of a computer graphic system (Neurolucida; Microbrightfield). The number of branch points and total branch lengths were measured for each axon fragment.
Knockdown of Endogenous Unc5B Expression.
To knock down endogenous Unc5B expression, short hairpin-type RNA (shRNA)-expressing plasmids were constructed. Three target sequences were designed for generating shRNAs and were inserted into the piGEMEmU6 vector (Clontech). A plasmid that contains the target sequence corresponding to nucleotides 486–506 of rat unc5b mRNA was used in this study. shRNA-resistant unc5b (rUnc5B) sequence was generated by using PCR with KOD-plus DNA polymerase (Toyobo) and was inserted into the pcDNA3.1 vector.
Supplementary Material
Acknowledgments
We thank Drs. Edward Ruthazer, Pierre Vanderhaeghen, Satoshi Kawamura, and Goichi Miyoshi for helpful and important suggestions. We also thank Dr. Ian Smith for critical reading. This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas “Mesoscopic Neurocircuitry” (23115102) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT), by Grants-in-Aid for Scientific Research 203001100 and 20021018 from MEXT, and by the Uehara Memorial Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. N.S. is a Guest Editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1402095111/-/DCSupplemental.
References
- 1.Dent EW, Barnes AM, Tang F, Kalil K. Netrin-1 and semaphorin 3A promote or inhibit cortical axon branching, respectively, by reorganization of the cytoskeleton. J Neurosci. 2004;24(12):3002–3012. doi: 10.1523/JNEUROSCI.4963-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell DS, et al. Semaphorin 3A elicits stage-dependent collapse, turning, and branching in Xenopus retinal growth cones. J Neurosci. 2001;21(21):8538–8547. doi: 10.1523/JNEUROSCI.21-21-08538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang KH, et al. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell. 1999;96(6):771–784. doi: 10.1016/s0092-8674(00)80588-7. [DOI] [PubMed] [Google Scholar]
- 4.Krylova O, et al. WNT-3, expressed by motoneurons, regulates terminal arborization of neurotrophin-3-responsive spinal sensory neurons. Neuron. 2002;35(6):1043–1056. doi: 10.1016/s0896-6273(02)00860-7. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Halloran MC. Central and peripheral axon branches from one neuron are guided differentially by Semaphorin3D and transient axonal glycoprotein-1. J Neurosci. 2005;25(45):10556–10563. doi: 10.1523/JNEUROSCI.2710-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yates PA, Roskies AL, McLaughlin T, O’Leary DD. Topographic-specific axon branching controlled by ephrin-As is the critical event in retinotectal map development. J Neurosci. 2001;21(21):8548–8563. doi: 10.1523/JNEUROSCI.21-21-08548.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen-Cory S. BDNF modulates, but does not mediate, activity-dependent branching and remodeling of optic axon arbors in vivo. J Neurosci. 1999;19(22):9996–10003. doi: 10.1523/JNEUROSCI.19-22-09996.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruthazer ES, Akerman CJ, Cline HT. Control of axon branch dynamics by correlated activity in vivo. Science. 2003;301(5629):66–70. doi: 10.1126/science.1082545. [DOI] [PubMed] [Google Scholar]
- 9.Sretavan DW, Shatz CJ, Stryker MP. Modification of retinal ganglion cell axon morphology by prenatal infusion of tetrodotoxin. Nature. 1988;336(6198):468–471. doi: 10.1038/336468a0. [DOI] [PubMed] [Google Scholar]
- 10.Dantzker JL, Callaway EM. The development of local, layer-specific visual cortical axons in the absence of extrinsic influences and intrinsic activity. J Neurosci. 1998;18(11):4145–4154. doi: 10.1523/JNEUROSCI.18-11-04145.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López-Bendito G, Molnár Z. Thalamocortical development: How are we going to get there? Nat Rev Neurosci. 2003;4(4):276–289. doi: 10.1038/nrn1075. [DOI] [PubMed] [Google Scholar]
- 12.Kageyama GH, Robertson RT. Development of geniculocortical projections to visual cortex in rat: Evidence early ingrowth and synaptogenesis. J Comp Neurol. 1993;335(1):123–148. doi: 10.1002/cne.903350109. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh A, Shatz CJ. Pathfinding and target selection by developing geniculocortical axons. J Neurosci. 1992;12(1):39–55. doi: 10.1523/JNEUROSCI.12-01-00039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agmon A, Yang LT, O’Dowd DK, Jones EG. Organized growth of thalamocortical axons from the deep tier of terminations into layer IV of developing mouse barrel cortex. J Neurosci. 1993;13(12):5365–5382. doi: 10.1523/JNEUROSCI.13-12-05365.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonini A, Fagiolini M, Stryker MP. Anatomical correlates of functional plasticity in mouse visual cortex. J Neurosci. 1999;19(11):4388–4406. doi: 10.1523/JNEUROSCI.19-11-04388.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catalano SM, Robertson RT, Killackey HP. Individual axon morphology and thalamocortical topography in developing rat somatosensory cortex. J Comp Neurol. 1996;367(1):36–53. doi: 10.1002/(SICI)1096-9861(19960325)367:1<36::AID-CNE4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 17.Catalano SM, Robertson RT, Killackey HP. Rapid alteration of thalamocortical axon morphology follows peripheral damage in the neonatal rat. Proc Natl Acad Sci USA. 1995;92(7):2549–2552. doi: 10.1073/pnas.92.7.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haruta M, Hata Y. Experience-driven axon retraction without binocular imbalance in developing visual cortex. Curr Biol. 2007;17(1):37–42. doi: 10.1016/j.cub.2006.10.064. [DOI] [PubMed] [Google Scholar]
- 19.Antonini A, Stryker MP. Rapid remodeling of axonal arbors in the visual cortex. Science. 1993;260(5115):1819–1821. doi: 10.1126/science.8511592. [DOI] [PubMed] [Google Scholar]
- 20.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6(11):877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 21.Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274(5290):1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 23.Lee LJ, Iwasato T, Itohara S, Erzurumlu RS. Exuberant thalamocortical axon arborization in cortex-specific NMDAR1 knockout mice. J Comp Neurol. 2005;485(4):280–292. doi: 10.1002/cne.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, et al. Laminar and columnar development of barrel cortex relies on thalamocortical neurotransmission. Neuron. 2013;79(5):970–986. doi: 10.1016/j.neuron.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narboux-Nême N, et al. Neurotransmitter release at the thalamocortical synapse instructs barrel formation but not axon patterning in the somatosensory cortex. J Neurosci. 2012;32(18):6183–6196. doi: 10.1523/JNEUROSCI.0343-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gheorghita F, Kraftsik R, Dubois R, Welker E. Structural basis for map formation in the thalamocortical pathway of the barrelless mouse. J Neurosci. 2006;26(39):10057–10067. doi: 10.1523/JNEUROSCI.1263-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uesaka N, Hayano Y, Yamada A, Yamamoto N. Interplay between laminar specificity and activity-dependent mechanisms of thalamocortical axon branching. J Neurosci. 2007;27(19):5215–5223. doi: 10.1523/JNEUROSCI.4685-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada A, et al. Role of pre- and postsynaptic activity in thalamocortical axon branching. Proc Natl Acad Sci USA. 2010;107(16):7562–7567. doi: 10.1073/pnas.0900613107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yabuta NH, Butler AK, Callaway EM. Laminar specificity of local circuits in barrel cortex of ephrin-A5 knockout mice. J Neurosci. 2000;20(15):RC88. doi: 10.1523/JNEUROSCI.20-15-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong Y, et al. Identification of the genes that are expressed in the upper layers of the neocortex. Cereb Cortex. 2004;14(10):1144–1152. doi: 10.1093/cercor/bhh074. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki SC, Inoue T, Kimura Y, Tanaka T, Takeichi M. Neuronal circuits are subdivided by differential expression of type-II classic cadherins in postnatal mouse brains. Mol Cell Neurosci. 1997;9(5-6):433–447. doi: 10.1006/mcne.1997.0626. [DOI] [PubMed] [Google Scholar]
- 32.Fukunishi A, et al. The action of Semaphorin7A on thalamocortical axon branching. J Neurochem. 2011;118(6):1008–1015. doi: 10.1111/j.1471-4159.2011.07390.x. [DOI] [PubMed] [Google Scholar]
- 33.Takemoto M, et al. Laminar and areal expression of unc5d and its role in cortical cell survival. Cereb Cortex. 2011;21(8):1925–1934. doi: 10.1093/cercor/bhq265. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto N, Yamada K, Kurotani T, Toyama K. Laminar specificity of extrinsic cortical connections studied in coculture preparations. Neuron. 1992;9(2):217–228. doi: 10.1016/0896-6273(92)90161-6. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto N, Kurotani T, Toyama K. Neural connections between the lateral geniculate nucleus and visual cortex in vitro. Science. 1989;245(4914):192–194. doi: 10.1126/science.2749258. [DOI] [PubMed] [Google Scholar]
- 36.Qin S, Yu L, Gao Y, Zhou R, Zhang C. Characterization of the receptors for axon guidance factor netrin-4 and identification of the binding domains. Mol Cell Neurosci. 2007;34(2):243–250. doi: 10.1016/j.mcn.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Yin Y, Sanes JR, Miner JH. Identification and expression of mouse netrin-4. Mech Dev. 2000;96(1):115–119. doi: 10.1016/s0925-4773(00)00369-5. [DOI] [PubMed] [Google Scholar]
- 38.Kitada K, et al. Transposon-tagged mutagenesis in the rat. Nat Methods. 2007;4(2):131–133. doi: 10.1038/nmeth1002. [DOI] [PubMed] [Google Scholar]
- 39.Maruyama T, Matsuura M, Suzuki K, Yamamoto N. Cooperative activity of multiple upper layer proteins for thalamocortical axon growth. Dev Neurobiol. 2008;68(3):317–331. doi: 10.1002/dneu.20592. [DOI] [PubMed] [Google Scholar]
- 40.Rebsam A, Seif I, Gaspar P. Refinement of thalamocortical arbors and emergence of barrel domains in the primary somatosensory cortex: A study of normal and monoamine oxidase a knock-out mice. J Neurosci. 2002;22(19):8541–8552. doi: 10.1523/JNEUROSCI.22-19-08541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lebrand C, et al. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17(5):823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- 42.Lejmi E, et al. Netrin-4 inhibits angiogenesis via binding to neogenin and recruitment of Unc5B. Proc Natl Acad Sci USA. 2008;105(34):12491–12496. doi: 10.1073/pnas.0804008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto N, et al. Inhibitory mechanism by polysialic acid for lamina-specific branch formation of thalamocortical axons. J Neurosci. 2000;20(24):9145–9151. doi: 10.1523/JNEUROSCI.20-24-09145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chandrasekaran AR, Plas DT, Gonzalez E, Crair MC. Evidence for an instructive role of retinal activity in retinotopic map refinement in the superior colliculus of the mouse. J Neurosci. 2005;25(29):6929–6938. doi: 10.1523/JNEUROSCI.1470-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cang J, Wang L, Stryker MP, Feldheim DA. Roles of ephrin-as and structured activity in the development of functional maps in the superior colliculus. J Neurosci. 2008;28(43):11015–11023. doi: 10.1523/JNEUROSCI.2478-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicol X, et al. cAMP oscillations and retinal activity are permissive for ephrin signaling during the establishment of the retinotopic map. Nat Neurosci. 2007;10(3):340–347. doi: 10.1038/nn1842. [DOI] [PubMed] [Google Scholar]
- 47.McLaughlin T, Torborg CL, Feller MB, O’Leary DD. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40(6):1147–1160. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- 48.Wilson BD, et al. Netrins promote developmental and therapeutic angiogenesis. Science. 2006;313(5787):640–644. doi: 10.1126/science.1124704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, et al. Novel role for Netrins in regulating epithelial behavior during lung branching morphogenesis. Curr Biol. 2004;14(10):897–905. doi: 10.1016/j.cub.2004.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneiders FI, et al. Binding of netrin-4 to laminin short arms regulates basement membrane assembly. J Biol Chem. 2007;282(33):23750–23758. doi: 10.1074/jbc.M703137200. [DOI] [PubMed] [Google Scholar]
- 51.Gallo G. The cytoskeletal and signaling mechanisms of axon collateral branching. Develop Neurobiol. 2011;71(3):201–220. doi: 10.1002/dneu.20852. [DOI] [PubMed] [Google Scholar]
- 52.Gibson DA, Ma L. Developmental regulation of axon branching in the vertebrate nervous system. Development. 2011;138(2):183–195. doi: 10.1242/dev.046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewis TL, Jr., Courchet J, Polleux F. Cell biology in neuroscience: Cellular and molecular mechanisms underlying axon formation, growth, and branching. J Cell Biol. 2013;202(6):837–848. doi: 10.1083/jcb.201305098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalil K, Dent EW. Branch management: mechanisms of axon branching in the developing vertebrate CNS. Nature Review Neurosci. 2014;15(1):7–18. doi: 10.1038/nrn3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manitt C, Nikolakopoulou AM, Almario DR, Nguyen SA, Cohen-Cory S. Netrin participates in the development of retinotectal synaptic connectivity by modulating axon arborization and synapse formation in the developing brain. J Neurosci. 2009;29(36):11065–11077. doi: 10.1523/JNEUROSCI.0947-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Braisted JE, et al. Netrin-1 promotes thalamic axon growth and is required for proper development of the thalamocortical projection. J Neurosci. 2000;20(15):5792–5801. doi: 10.1523/JNEUROSCI.20-15-05792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fothergill T, et al. Netrin-DCC signaling regulates corpus callosum formation through attraction of pioneering axons and by modulating Slit2-mediated repulsion. Cereb Cortex. 2013;24(5):1138–51. doi: 10.1093/cercor/bhs395. [DOI] [PubMed] [Google Scholar]
- 58.Bonnin A, Torii M, Wang L, Rakic P, Levitt P. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci. 2007;10(5):588–597. doi: 10.1038/nn1896. [DOI] [PubMed] [Google Scholar]
- 59.Braisted JE, Tuttle R, O’leary DD. Thalamocortical axons are influenced by chemorepellent and chemoattractant activities localized to decision points along their path. Dev Biol. 1999;208(2):430–440. doi: 10.1006/dbio.1999.9216. [DOI] [PubMed] [Google Scholar]
- 60.Hata K, Kaibuchi K, Inagaki S, Yamashita T. Unc5B associates with LARG to mediate the action of repulsive guidance molecule. J Cell Biol. 2009;184(5):737–750. doi: 10.1083/jcb.200807029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ohnami S, et al. Role of RhoA in activity-dependent cortical axon branching. J Neurosci. 2008;28(37):9117–9121. doi: 10.1523/JNEUROSCI.1731-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rochefort NL, et al. Sparsification of neuronal activity in the visual cortex at eye-opening. Proc Natl Acad Sci USA. 2009;106(35):15049–15054. doi: 10.1073/pnas.0907660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mirmiran M, Corner M. Neuronal discharge patterns in the occipital cortex of developing rats during active and quiet sleep. Brain Res. 1982;255(1):37–48. doi: 10.1016/0165-3806(82)90074-8. [DOI] [PubMed] [Google Scholar]
- 64.Flint AC, Maisch US, Kriegstein AR. Postnatal development of low [Mg2+] oscillations in neocortex. J Neurophysiol. 1997;78(4):1990–1996. doi: 10.1152/jn.1997.78.4.1990. [DOI] [PubMed] [Google Scholar]
- 65.Weliky M, Katz LC. Correlational structure of spontaneous neuronal activity in the developing lateral geniculate nucleus in vivo. Science. 1999;285(5427):599–604. doi: 10.1126/science.285.5427.599. [DOI] [PubMed] [Google Scholar]
- 66.Granseth B, Fukushima Y, Sugo N, Lagnado L, Yamamoto N. Regulation of thalamocortical axon branching by BDNF and synaptic vesicle cycling. Front Neural Circuits. 2013;7:202. doi: 10.3389/fncir.2013.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron. 2008;60(4):610–624. doi: 10.1016/j.neuron.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hanamura K, Harada A, Katoh-Semba R, Murakami F, Yamamoto N. BDNF and NT-3 promote thalamocortical axon growth with distinct substrate and temporal dependency. Eur J Neurosci. 2004;19(6):1485–1493. doi: 10.1111/j.1460-9568.2004.03228.x. [DOI] [PubMed] [Google Scholar]
- 69.Lein ES, Hohn A, Shatz CJ. Dynamic regulation of BDNF and NT-3 expression during visual system development. J Comp Neurol. 2000;420(1):1–18. doi: 10.1002/(sici)1096-9861(20000424)420:1<1::aid-cne1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 70.Molnár Z, Blakemore C. Lack of regional specificity for connections formed between thalamus and cortex in coculture. Nature. 1991;351(6326):475–477. doi: 10.1038/351475a0. [DOI] [PubMed] [Google Scholar]
- 71.Molnár Z, Blakemore C. Development of signals influencing the growth and termination of thalamocortical axons in organotypic culture. Exp Neurol. 1999;156(2):363–393. doi: 10.1006/exnr.1999.7032. [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto N, Higashi S, Toyama K. Stop and branch behaviors of geniculocortical axons: a time-lapse study in organotypic cocultures. J Neurosci. 1997;17(10):3653–3663. doi: 10.1523/JNEUROSCI.17-10-03653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blakey D, Wilson MC, Molnár Z. Termination and initial branch formation of SNAP-25-deficient thalamocortical fibres in heterochronic organotypic co-cultures. Eur J Neurosci. 2012;35(10):1586–1594. doi: 10.1111/j.1460-9568.2012.08120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mann F, Peuckert C, Dehner F, Zhou R, Bolz J. Ephrins regulate the formation of terminal axonal arbors during the development of thalamocortical projections. Development. 2002;129(16):3945–3955. doi: 10.1242/dev.129.16.3945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.