Abstract
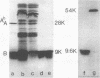
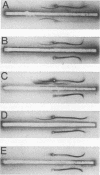
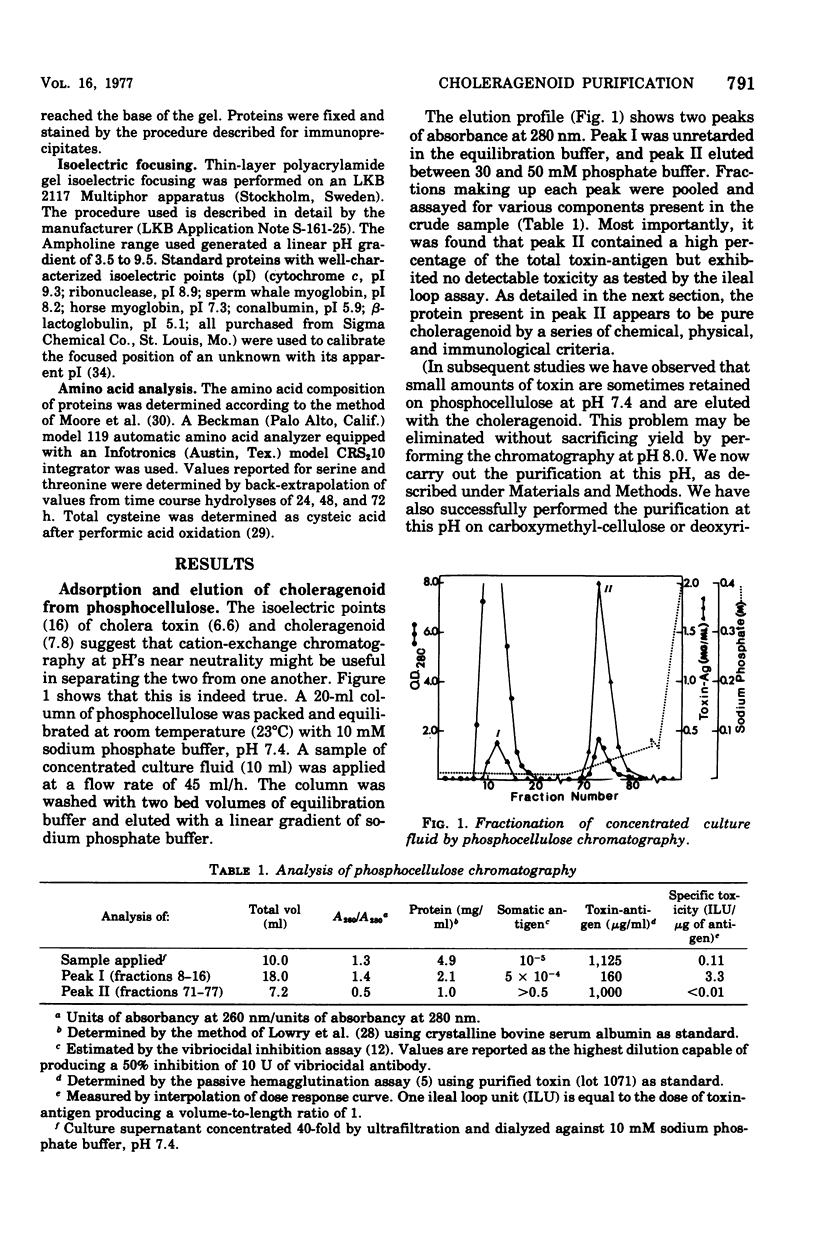
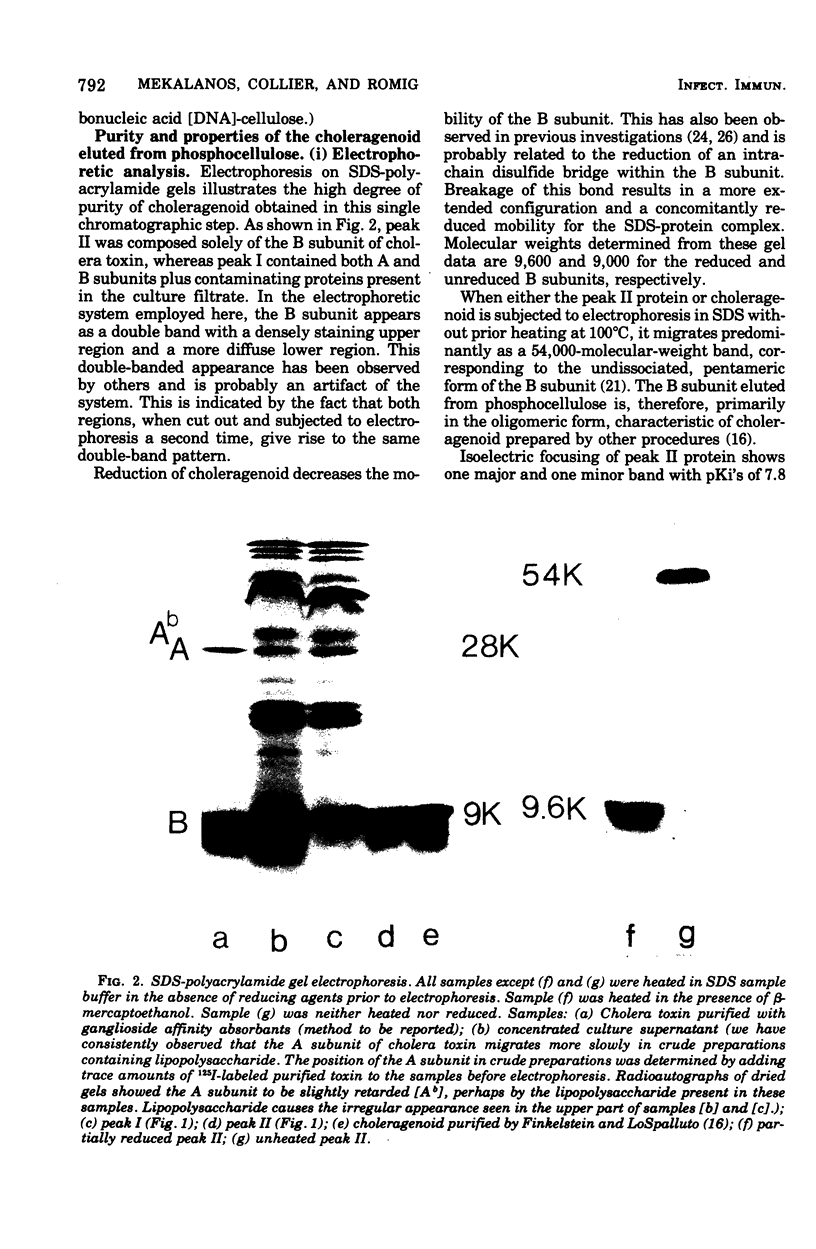
Choleragenoid, a nontoxic aggregate of the B subunit of cholera toxin, has been purified from concentrated culture filtrates in a single step by ion-exchange chromatography on phosphocellulose or other cation-exchange resins. This procedure is far simpler than others currently used to isolate choleragenoid and yields a preparation essentially free from nucleic acid, lipopolysaccharide, toxin, and other proteins present in the crude culture filtrates. The purified choleragenoid retained the specific receptor-binding capacity of the toxin but exhibited no enterotoxic activity by either the ileal loop assay or the skin permeability assay. This purification methods may therefore be superior to others currently used for obtaining choleragenoid for immunization or other purposes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Blachman U., Clark W. R., Pickett M. J. Radiobacteriolysis: a new technique using chromium-51 for assaying anti-Vibrio cholerae antibodies. Infect Immun. 1973 Jan;7(1):53–61. doi: 10.1128/iai.7.1.53-61.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachman U., Graboff S. R., Haag G. E., Gottfeld E., Pickett M. J. Experimental Cholera in Chinchillas: the Immune Response in Serum and Intestinal Secretions to Vibrio cholerae and Cholera Toxin. Infect Immun. 1974 Nov;10(5):1098–1104. doi: 10.1128/iai.10.5.1098-1104.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows W., Musteikis G. M. Cholera infection and toxin in the rabbit ileal loop. J Infect Dis. 1966 Apr;116(2):183–190. doi: 10.1093/infdis/116.2.183. [DOI] [PubMed] [Google Scholar]
- Callahan L. T., 3rd, Ryder R. C., Richardson S. H. Biochemistry of vibrio cholerae virulence. II. Skin permeability factor-cholera enterotoxin production in a chemically defined medium. Infect Immun. 1971 Nov;4(5):611–618. doi: 10.1128/iai.4.5.611-618.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash R. A., Music S. I., Libonati J. P., Craig J. P., Pierce N. F., Hornick R. B. Response of man to infection with Vibrio cholerae. II. Protection from illness afforded by previous disease and vaccine. J Infect Dis. 1974 Oct;130(4):325–333. doi: 10.1093/infdis/130.4.325. [DOI] [PubMed] [Google Scholar]
- Coleman W. H., Kaur J., Iwert M. E., Kasai G. J., Burrows W. Cholera toxins: purification and preliminary characterization of ileal loop reactive type 2 toxin. J Bacteriol. 1968 Oct;96(4):1137–1143. doi: 10.1128/jb.96.4.1137-1143.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. P. A permeability factor (toxin) found in cholera stools and culture filtrates and its neutralization by convalescent cholera sera. Nature. 1965 Aug 7;207(997):614–616. doi: 10.1038/207614a0. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Vibrio cholerae choleragenoid. Mechanism of inhibition of cholera toxin action. Biochemistry. 1973 Aug 28;12(18):3577–3581. doi: 10.1021/bi00742a034. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Fujita K., LoSpalluto J. J. Procholeragenoid: an aggregated intermediate in the formation of choleragenoid. J Immunol. 1971 Oct;107(4):1043–1051. [PubMed] [Google Scholar]
- Finkelstein R. A., Peterson J. W., Lospalluto J. J. Conversion of cholera exo-enterotoxin (choleragen) to natural toxoid (choleragenoid). J Immunol. 1971 Mar;106(3):868–871. [PubMed] [Google Scholar]
- Finkelstein R. A. Properties of cholera exo-enterotoxin (choleragen) and its natural toxoid (choleragenoid). Toxicon. 1972 Aug;10(5):441–450. doi: 10.1016/0041-0101(72)90168-7. [DOI] [PubMed] [Google Scholar]
- Fujita K., Finkelstein R. A. Antitoxic immunity in experimental cholera: comparison of immunity induced perorally and parenterally in mice. J Infect Dis. 1972 Jun;125(6):647–655. doi: 10.1093/infdis/125.6.647. [DOI] [PubMed] [Google Scholar]
- Germanier R., Fürer E., Varallyay S., Inderbitzin T. M. Preparation of a purified antigenic cholera toxoid. Infect Immun. 1976 Jun;13(6):1692–1698. doi: 10.1128/iai.13.6.1692-1698.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M. The arrangement of subunits in cholera toxin. Biochemistry. 1976 Mar 23;15(6):1242–1248. doi: 10.1021/bi00651a011. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Lonnroth I. Oligomeric structure of cholera toxin: characteristics of the H and L subunits. J Gen Microbiol. 1975 Jan;86(1):49–65. doi: 10.1099/00221287-86-1-49. [DOI] [PubMed] [Google Scholar]
- Kaur J., Burrows W., Cercavski L. Cholera toxins: immunogenicity of the rabbit ileal loop toxin and related antigens. J Bacteriol. 1969 Nov;100(2):985–993. doi: 10.1128/jb.100.2.985-993.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis A. C., Freeman B. A. Separation of type 2 toxins of Vibrio cholerae. Science. 1969 Aug 22;165(3895):808–809. doi: 10.1126/science.165.3895.808. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Reynolds H. Y. Immunity to experimental cholera. II. Secretory and humoral antitoxin response to local and systemic toxoid administration. J Infect Dis. 1975 Apr;131(4):383–389. doi: 10.1093/infdis/131.4.383. [DOI] [PubMed] [Google Scholar]
- Radola B. J. Isoelectric focusing in layers of granulated gels. I. Thin-layer isoelectric focusing of proteins. Biochim Biophys Acta. 1973 Feb 21;295(2):412–428. doi: 10.1016/0005-2795(73)90037-8. [DOI] [PubMed] [Google Scholar]
- Rappaport R. S., Bonde G., McCann T., Rubin B. A., Tint H. Development of a purified cholera toxoid. II. Preparation of a stable, antigenic toxoid by reaction of purified toxin with glutaraldehyde. Infect Immun. 1974 Feb;9(2):304–317. doi: 10.1128/iai.9.2.304-317.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid M. S., Bieleski R. L. A simple apparatus for vertical flat-sheet polyacrylamide gel electrophoresis. Anal Biochem. 1968 Mar;22(3):374–381. doi: 10.1016/0003-2697(68)90278-9. [DOI] [PubMed] [Google Scholar]
- Van Heyningen S., King C. A. Short communications. Subunit A from cholera toxin is an activator of adenylate cyclase in pigeon erythrocytes. Biochem J. 1975 Jan;146(1):269–271. doi: 10.1042/bj1460269. [DOI] [PMC free article] [PubMed] [Google Scholar]