Significance
The skin develops antigen-specific immune response, called “allergic contact dermatitis” in humans and “contact hypersensitivity” (CHS) in mice. IL-1 family member IL-37 expression in transgenic mice (IL-37tg) inhibits innate immune responses. In this study we show that DCs from IL-37tg mice exhibit diminished CHS response to an antigen-specific exposure. Although DCs from IL-37tg mice maintain migratory capacity to lymph nodes, they fail to activate T cells effectively; instead, they promote generation of T regulatory cells, a characteristic of semimature DCs that dampen adaptive immune response. Thus, IL-37 emerges as an inhibitor of adaptive immunity.
Keywords: tolerance, skin inflammation, allergic contact dermatitis
Abstract
IL-1 family member IL-37 limits innate inflammation in models of colitis and LPS-induced shock, but a role in adaptive immunity remains unknown. Here, we studied mice expressing human IL-37b isoform (IL-37tg) subjected to skin contact hypersensitivity (CHS) to dinitrofluorobenzene. CHS challenge to the hapten was significantly decreased in IL-37tg mice compared with wild-type (WT) mice (−61%; P < 0.001 at 48 h). Skin dendritic cells (DCs) were present and migrated to lymph nodes after antigen uptake in IL-37tg mice. When hapten-sensitized DCs were adoptively transferred to WT mice, antigen challenge was greatly impaired in mice receiving DCs from IL-37tg mice compared with those receiving DCs from WT mice (−60%; P < 0.01 at 48 h). In DCs isolated from IL-37tg mice, LPS-induced increase of MHC II and costimulatory molecule CD40 was reduced by 51 and 31%, respectively. In these DCs, release of IL-1β, IL-6, and IL-12 was reduced whereas IL-10 secretion increased (37%). Consistent with these findings, DCs from IL-37tg mice exhibited a lower ability to stimulate syngeneic and allogeneic naive T cells as well as antigen-specific T cells and displayed enhanced induction of T regulatory (Treg) cells (86%; P < 0.001) in vitro. Histological analysis of CHS skin in mice receiving hapten-sensitized DCs from IL-37tg mice revealed a marked reduction in CD8+ T cells (−74%) but an increase in Treg cells (2.6-fold). Together, these findings reveal that DCs expressing IL-37 are tolerogenic, thereby impairing activation of effector T-cell responses and inducing Treg cells. IL-37 thus emerges as an inhibitor of adaptive immunity.
Interleukin-37 (IL-37; formerly IL-1 family member 7) isoform b inhibits innate inflammation (1–3). In human peripheral blood from healthy subjects, low levels of steady-state IL-37 mRNA and protein are expressed in monocytes, dendritic cells (DCs), and plasma cells perhaps due to instability elements within the coding region (4, 5); however, stimulation with proinflammatory cytokines or Toll-like receptor (TLR) ligands induces IL-37 levels, which in turn suppress the proinflammatory cytokines IL-1α, IL-1β, IL-6, M-CSF, and GM-CSF but not anti-inflammatory cytokines IL-10 and IL-1Ra (4–7). Although an ORF for the murine homolog of IL37 is absent in various mouse databases, human IL-37 expression in a variety of human and murine cells inhibits innate immunity and suppresses production of proinflammatory cytokines and chemokines (2, 3, 8), indicating that human IL-37 is functional in murine cells. Compared with WT mice, mice expressing human IL-37b (IL-37tg mice) produce lower amounts of proinflammatory cytokines after lipopolysaccharide (LPS) administration and are protected from LPS-induced septic shock (3) and dextran sulfate sodium-induced colitis (8). Administration of recombinant human IL-37 in mice suppresses cytokine and chemokine production, neutrophil infiltration, and cell death, thereby ameliorating hepatic and myocardial ischemia/reperfusion injury (9, 10). Treatment of mice with human IL-37 plasmid-DNA reduces local and systemic inflammation in Con A-induced hepatitis and psoriasis (11, 12).
Despite IL-37’s suppressive effects on inflammation, the role of IL-37 in specific immune responses such as DC functions has remained elusive. Innate immunity increases the production of cytokines and chemokines and alters the activity and function of DCs (13–15). Indeed, cytokines derived from DCs and T cells such as IL-12, IFNγ, IL-4, and IL-10 play a pivotal role in the induction and initiation of adaptive immunity. DCs isolated from the spleen of IL-37tg mice were reported to display reduced expression of CD86 and major histocompatibility complex (MHC) class II (MHC II) after LPS challenge in vivo (3). We considered that the expression of IL-37 in DCs might modulate their antigen-presenting capacity, thereby regulating adaptive immunity.
The skin immune system relies on a rich network of DCs that populate the epidermis and the dermis (16). Murine contact hypersensitivity (CHS) is a DC-dependent inflammation mediated by hapten-specific T cells and a useful model to investigate delayed-type hypersensitivity (17–19). In the present study, we investigated the role of IL-37 in DCs and subsequent development of antigen-specific CHS responses using IL-37tg mice.
Results
Transgenic Expression of IL-37 Suppresses Skin Inflammation in Response to Specific Antigen.
We compared CHS responses to the hapten 2,4-dinitrofluorobenzene (DNFB) between WT mice and IL-37tg mice. Minimally expressed at baseline, IL-37 was induced in the abdominal skin of IL-37tg mice 6 h following topical application of DNFB and reached a 15-fold maximal increase by 48 h (Fig. 1A). Five days after sensitization, mice were challenged by local application of hapten to the ear. Whereas WT mice exhibited increased ear swelling at 24 and 48 h after challenge, the response was significantly reduced by 34% in heterozygous IL-37tg mice and further reduced by 61% in homozygous IL-37tg mice at 48 h (Fig. 1B). These differences paralleled the expression levels of IL-37 (7.1-fold higher in homozygous IL-37tg mice compared with heterozygous IL-37tg mice) (Fig. 1C).
Fig. 1.
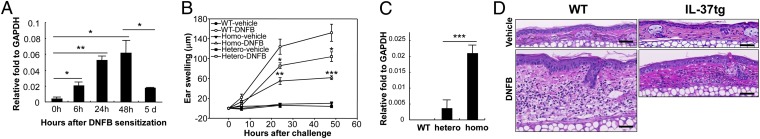
CHS responses in WT and IL-37tg mice. (A) qRT-PCR analysis of IL-37 in the skin from homozygous IL-37tg mice. Skin samples were obtained at 0, 6, 24, and 48 h and 5 d after sensitization with DNFB. GAPDH served as internal control. Data represent mean ± SEM (n = 3) and are representative of three independent experiments. *P < 0.05; **P < 0.01. (B) CHS responses measured by ear thickness at 0, 6, 24, and 48 h after vehicle or DNFB challenge in WT, homozygous-tg (Homo), and heterozygous-tg (Hetero) mice. Data represent mean ± SEM (n = 5) and are representative of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, compared with WT-DNFB. (C) qRT-PCR analysis of IL-37 in the skin from WT, hetero-tg, and homo-tg mice. GAPDH served as internal control. Data represent mean ± SEM (n = 3) and are representative of two independent experiments. ***P < 0.001. (D) Representative H&E staining of ear specimens from WT and IL-37tg mice obtained at 48 h after vehicle or DNFB challenge. (Scale bar, 100 µm.)
Consistent with the increased ear swelling, histological analysis revealed epidermal hyperplasia, dermal edema, vasodilatation, and dermal inflammatory infiltrates in DNFB-challenged WT mouse skin, whereas these changes were markedly reduced in IL-37tg mice (Fig. 1D). Because of the higher IL-37 expression in homozygous IL-37tg mice compared with heterozygous IL-37tg mice, homozygous IL-37tg mice were used in subsequent experiments.
Skin DCs Are Present in IL-37tg Mice and Migrate to Draining Lymph Nodes After Antigen Uptake.
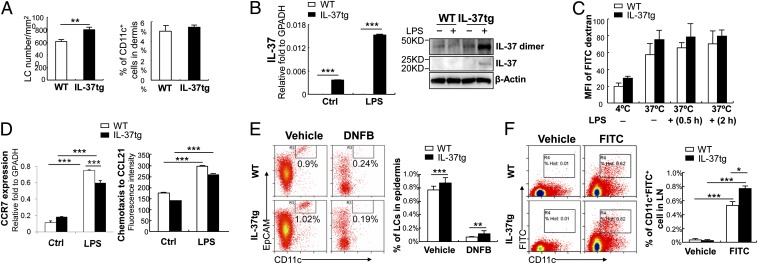
Skin DCs initiate CHS by capturing antigens, migrating to draining lymph nodes (dLNs), and priming naive T cells (17–19). Because cytokines and chemokines can alter development and localization of DCs (20, 21), we examined the distribution of skin DCs in IL-37tg mice. Staining of epidermal sheets revealed that the morphology of Langerhans cells (LCs), MHC II+ DCs in epidermis, was comparable between WT and IL-37tg mice (Fig. S1), but their density was 31% higher in IL-37tg mice compared with WT mice (Fig. 2A, Left). Flow cytometric analysis of dermal sheets showed no significant difference in the density of dermal DCs between WT and IL-37tg mice (Fig. 2A, Right), indicating that IL-37 does not impair the development and localization of DCs in the skin.
Fig. 2.
Distribution, phagocytotic activity, and migratory ability of DCs from WT and IL-37tg mice. (A, Left) Density of epidermal LCs, determined by MHC II staining of epidermal sheets from untreated ear skin. Data represent mean ± SEM (n = 5) and are representative of three independent experiments. (Right) percentage of dermal CD11c+ cells determined by flow cytometric analyses of single-cell suspension of dermal sheets from untreated ear skin. Data represent mean ± SEM (n = 3) and are representative of three independent experiments. (B, Left) qRT-PCR analysis of IL-37 in BMDCs cultured in the absence (Ctrl) or presence of 100 ng/mL LPS for 24 h. GAPDH served as internal control. Data represent mean ± SEM (n = 3) and are representative of three independent experiments. (Right) IL-37 Western blot in BMDCs cultured with (+) or without (−) LPS for 24 h. β-Actin used as internal control. Data are representative of two independent experiments. (C) Phagocytotic activity of BMDCs in vitro. BMDCs from WT or IL-37tg mice were cultured with (+) or without (−) LPS for 0.5 or 2 h and incubated with FITC–dextran at 4 °C or 37 °C. MFI of FITC was calculated by flow cytometry. Data represent mean ± SEM (n = 3) and are representative of two independent experiments. (D, Left) qRT-PCR analysis of CCR7 in BMDCs from WT or IL-37tg mice cultured in the absence (Ctrl) or presence of LPS for 24 h. GAPDH served as internal control. Data represent mean ± SEM (n = 3) and are representative of two independent experiments. (Right) Chemotaxis of BMDCs (24 h after LPS stimulation) toward CCL21. Data represent mean ± SEM (n = 3) and are representative of two independent experiments. (E) Flow cytometric analysis of epidermal LCs (CD11c+EpCAM+ cells) in WT and IL-37tg mice at 24 h after vehicle or DNFB sensitization. (Left) Representative flow cytometric analysis. (Right) Percentage of LCs in the epidermis. Data represent mean ± SEM (n = 3) and are representative of two independent experiments. (F) Flow cytometric analysis of FITC+CD11c+ cells in dLNs at 24 h after vehicle or FITC sensitization on abdominal skin. (Left) Representative flow cytometric analysis. (Right) Percentage of FITC+CD11c+ cells in dLNs. Data represent mean ± SEM (n = 3) and are representative of two independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
Because DCs mature and migrate to dLNs following exposure to antigen, we examined the phagocytotic activity and migratory ability of DCs using bone marrow-derived DCs (BMDCs). Without stimulation, steady-state levels of IL-37 mRNA were low in BMDCs from IL-37tg mice. However, the levels increased 3.4-fold upon LPS treatment (Fig. 2B, Left). Western blot confirmed that LPS enhanced expression of IL-37 protein and its dimer in BMDCs isolated from IL-37tg mice (Fig. 2B, Right). In these BMDCs, phagocytotic activity measured by FITC–dextran uptake did not show significant difference compared with BMDCs from WT mice (Fig. 2C), suggesting no apparent defect in the phagocytic abilities of DCs by IL-37.
Upon antigen uptake, DCs increase the expression of the chemokine receptor CCR7, which directs DCs to CCR7 ligands CCL19 and CCL21 expressed in dLNs (17–19). Similar to those from WT mice, BMDCs from IL-37tg mice upregulated CCR7 expression (Fig. 2D, Left) and migrated toward CCL21 in vitro (Fig. 2D, Right). Although the migratory capacity was slightly reduced in BMDCs isolated from IL-37tg mice compared with BMDCs from WT mice, there was no significant difference. These findings were also observed during in vivo experiments (Fig. 2 E and F). The reduction in epidermal LC numbers after DNFB sensitization suggests normal emigration activity of LCs from the skin (Fig. 2E) whereas the increase of FITC+ cells in dLNs after FITC painting indicates the ability of skin DCs to migrate from the skin to dLNs (Fig. 2F) in IL-37tg mice. Taken together, these data suggest that, similar to those in WT mice, skin DCs in IL-37tg mice uptake antigens and migrate to dLNs in the sensitization phase.
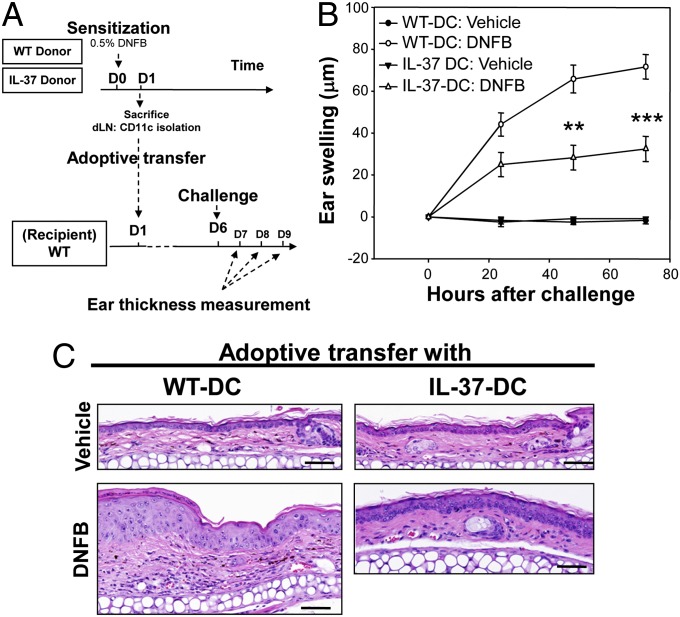
Mice Adoptively Transferred with Hapten-Sensitized IL-37–Expressing DCs Exhibit Suppressed CHS Response After Challenge.
Due to the CMV promoter in the pIRES vector, IL-37 expression can be present in any cell in IL-37tg mice after inflammation, which might alter CHS responses irrespective of DC function. Therefore, we adoptively transferred hapten-sensitized DCs into naive recipient WT mice that were subsequently challenged with DNFB applied to the ears (Fig. 3A). Specifically, DCs isolated from dLNs of hapten-sensitized donor mice (either WT or IL-37tg mice) were injected subcutaneously. As expected, CHS responses developed at 24 h after hapten challenge in mice receiving sensitized DCs from WT mice (Fig. 3B). However, ear swelling was significantly reduced in mice receiving sensitized DCs from IL-37tg mice (60 and 54% reduction at 48 and 72 h, respectively, compared with mice receiving sensitized DCs from WT mice) (Fig. 3B). Consistent with these findings, histological analysis revealed a marked reduction of epidermal hyperplasia, dermal edema, vasodilation, and dermal inflammatory infiltrates in mice receiving sensitized DCs from IL-37tg mice (Fig. 3C). These data prove that IL-37 expression in DCs in the sensitization phase alters their ability to initiate CHS responses.
Fig. 3.
CHS responses after adoptive transfer with hapten-sensitized DCs. (A) Scheme of adoptive transfer of hapten-sensitized DCs from WT or IL-37tg mice (donor) to WT mice (recipient). (B) CHS responses measured by ear thickness at 0, 24, 48, and 72 h after vehicle or DNFB challenge in WT mice adoptively transferred with hapten-sensitized DCs. Results are representative of two independent experiments. Data represent mean ± SEM (n = 5). **P < 0.01 and ***P < 0.001, compared with mice adoptively transferred with hapten-sensitized DCs from WT mice. (C) Representative H&E staining of ear specimens (72 h after vehicle or DNFB challenge) from mice adoptively transferred with DCs from WT mice (WT-DCs) or IL-37tg mice (IL-37-DCs). (Scale bar, 100 µm.)
IL-37 Impairs Upregulation of Maturation-Induced Molecules of DCs.
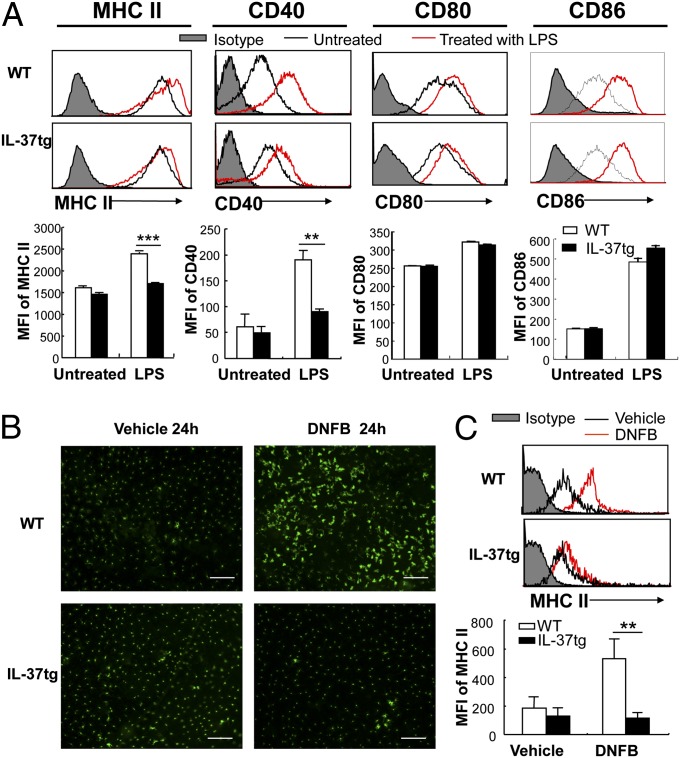
To investigate the effect of IL-37 on DC maturation, we stimulated BMDCs with LPS in vitro and analyzed their phenotypes. Whereas LPS induced an increase in MHC II and CD40 expression in BMDCs from WT mice, mean fluorescence intensity (MFI) of MHC II and CD40 was reduced by 31 and 52%, respectively, with LPS stimulation in BMDCs isolated from IL-37tg mice (Fig. 4A). Levels of CD80 and CD86 were comparable between WT and IL-37tg mice.
Fig. 4.
Expression of costimulatory molecules in DCs from WT and IL-37tg mice. (A) Representative histogram (Upper) and MFI (Lower) of MHC II, CD40, CD80, and CD86 in BMDCs from WT and IL-37tg mice. Cells were cultured in the presence or absence of 100 ng/mL LPS for 24 h. Data represent mean ± SEM (n = 3) and are representative of two independent experiments. (B) Immunofluorescent staining of epidermal sheets with MHC II. WT and IL-37tg mouse ears were sensitized with vehicle or DNFB for 24 h. (Scale bar, 100 µm.) (C) Flow cytometric analysis of MHC II expression of epidermal cells. Representative histogram (Upper) and MFI of MHC II (Lower) at 24 h after vehicle or DNFB sensitization. Cells were gated on allophycocyanin-labeled CD11c. Data represent mean ± SEM (n = 5) and are representative of two independent experiments. **P < 0.01; ***P < 0.001.
To determine whether IL-37 expression also affects DC maturation in vivo, we analyzed MHC II expression on skin DCs after DNFB sensitization. Whereas DNFB sensitization induced elongated dendritic morphology and increased MHC II expression in DCs from WT skin, these effects were markedly suppressed in DCs from IL-37tg skin (Fig. 4B). Consistent with these findings, flow cytometric analysis of epidermal cells displayed a 1.8-fold increase in MHC II expression in DCs from WT mice after DNFB sensitization compared with vehicle application, whereas this increase was not observed in DNFB-sensitized skin DCs from IL-37tg mice (Fig. 4C), suggesting that IL-37 expression in DCs impairs their maturation.
IL-37 Suppresses IL-1β, IL-6, and IL-12 Secretion but Enhances IL-10 in DCs.
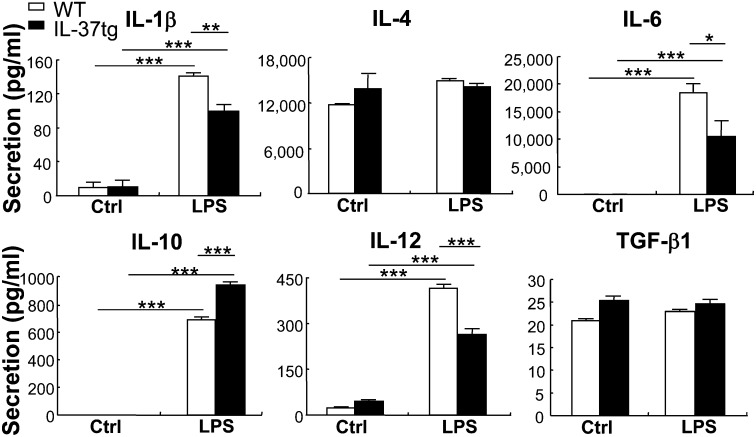
In addition to the effects on costimulatory molecules, cytokines from DCs play a critical role in determining the activation and polarization of T cells. We compared cytokines released from BMDCs following LPS stimulation and found that secretion of IL-1β, IL-6, and IL-12 was significantly less in BMDCs isolated from IL-37tg mice (30, 42, and 38% reduction, respectively) compared with that from WT mice (Fig. 5). On the other hand, the secretion of IL-10 was 37% enhanced in BMDCs from IL-37tg mice. The secretion of IL-4 and TGF-β1 in BMDCs was comparable between WT and IL-37tg mice. Downregulation of IL-1β and upregulation of IL-10 was also detected by qRT-PCR in BMDCs isolated from IL-37tg mice (25% reduction and 40% increase, respectively, under LPS stimulation) (Fig. S2). Consistent with its protein level, the mRNA level of TGF-β1 in BMDCs did not show significant difference between WT and IL-37tg mice (Fig. S2). The combined effects of IL-37 on DC maturation and cytokine profiles suggest that IL-37 modulates DC function to activate and polarize T cells.
Fig. 5.
Levels of cytokine secretion in DCs from WT and IL-37tg mice. Twenty-four-hour levels of secreted IL-1β, IL-4, IL-6, IL-10, IL-12, and TGF-β1 in BMDCs from WT and IL-37tg mice cultured in the absence (Ctrl) or presence of 100 ng/mL LPS. Data represent mean ± SEM (n = 3) and are representative of two independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
IL-37 Impairs Ability of DCs to Activate Naive T Cells and Antigen-Specific T Cells.
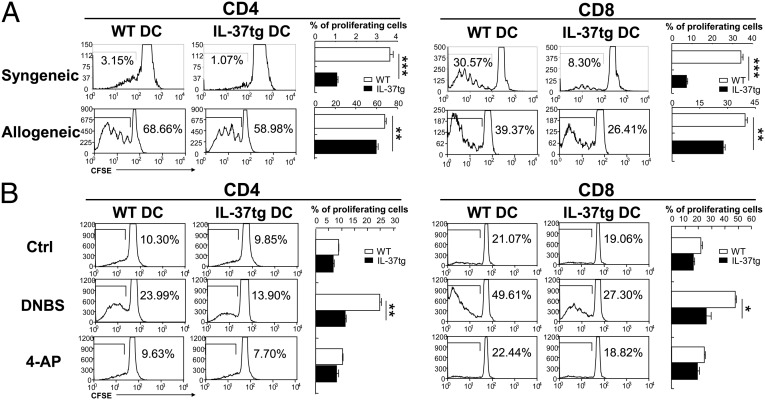
To address whether IL-37 expression in DCs modulate DC function in the sensitization phase, we conducted T-cell activation and proliferation assays. 5,6-Carboxyfluorescein diacetate, succinimidy1 ester (CFSE)-labeled CD4+ and CD8+ T cells from splenocytes of WT mice or FVB/N mice were used as responders for syngeneic or allogeneic responses, respectively. Coculture of syngeneic T cells with BMDCs from WT mice induced proliferation of CD4+ and CD8+ T cells, whereas this proliferation was reduced by 70% in CD4+ T cells and 76% in CD8+ T cells when T cells were cocultured with BMDCs from IL-37tg mice (Fig. 6A, Upper). Likewise, coculture of allogeneic T cells with BMDCs from WT mice induced proliferation of CD4+ and CD8+ T cells; however, this proliferation was decreased by 14% in CD4+ T cells and by 31% in CD8+ T cells when FVB/N T cells were cocultured with BMDCs from IL-37tg mice (Fig. 6A, Lower).
Fig. 6.
T-cell proliferation. (A) T-cell proliferation stimulated by syngeneic (Upper) and allogeneic (Lower) DCs. Naive CD3+ T cells from WT (syngeneic) or FVB/N (allogeneic) splenocytes were labeled with CFSE and incubated with BMDCs from WT or IL-37tg (both from C57BL/6) mice for 72 h. CD4+ and CD8+ T cells were gated and analyzed. Representative flow cytometric analysis and percentage of proliferating cells are shown. Results are representative of three independent experiments. Data are shown as the mean ± SEM (n = 3). (B) Antigen-specific T-cell proliferation. BMDCs from WT or IL-37tg mice were pulsed with control, DNBS, or 4-AP. CD3+ T cells were isolated from dLNs of DNFB-sensitized WT mice 5 d after sensitization, labeled with CFSE, and incubated for 72 h with BMDCs. CD4+ and CD8+ T cells were gated and analyzed. Representative flow cytometric analysis and percentage of proliferating cells are shown. Results are representative of three independent experiments. Data represent mean ± SEM (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001.
Furthermore, we examined the effect of IL-37 on antigen-specific T-cell proliferation. WT mice were sensitized with DNFB, and 5 d later T cells were harvested from dLNs, labeled with CFSE, and used as responders. BMDCs from WT or IL-37tg mice were pulsed with 4,6-dinitrobenzothiadiazole (DNBS), the water soluble analog of DNFB, and used as stimulators for CFSE-labeled CD4+ and CD8+ T cells. Unhaptenated BMDCs and BMDCs pulsed with irrelevant compound 4-aminophenol (4-AP) were used as controls. As expected, DNBS-pulsed BMDCs from WT mice primed proliferation of sensitized CD4+ and CD8+ T cells more efficiently than unhaptenated or 4-AP–pulsed BMDCs (Fig. 6B). In contrast, the antigen-specific T-cell proliferation was decreased by 53% in CD4+ T cells and by 46% in CD8+ T cells when sensitized T cells were cultured with DNBS-pulsed BMDCs from IL-37tg mice. Taken together, the data reveal that IL-37 expression in DCs impairs their function to activate naive T cells and antigen-specific T cells.
IL-37 Expression in DCs Promotes Induction of T Regulatory Cells.
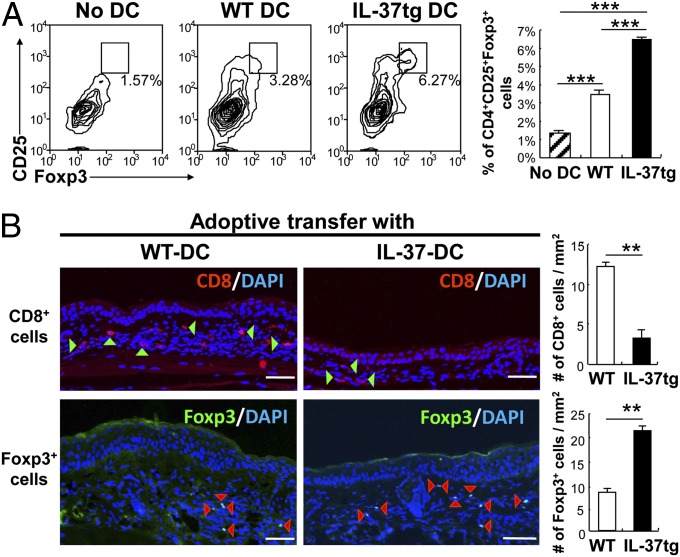
We considered that IL-37 may also regulate T-cell differentiation and polarization in the sensitization phase. To investigate whether IL-37 promotes induction of T regulatory (Treg) cells, we cultured naive T cells with LPS-stimulated BMDCs from WT or IL-37tg mice. Coculture of splenic T cells with BMDCs from WT mice induced CD4+CD25+Foxp3+ Treg cells; however, when T cells were cocultured with BMDCs from IL-37tg mice, Treg cell induction was further enhanced by 86% (Fig. 7A).
Fig. 7.
Induction of Treg cells in vitro and in vivo. (A) Flow cytometric analysis of splenic CD3+ T cells after coculture with BMDCs from WT or IL-37tg mice for 3 d. (Left) Dot plots showing CD4+CD25+Foxp3+ cells in the absence (no DC) or presence of BMDCs from WT or IL-37tg mice. (Right) Percentage of CD4+CD25+Foxp3+ cells in CD4+ cells. Results are representative of three independent experiments. Data represent mean ± SEM (n = 3). (B, Left) Immunofluorescent staining of ear specimens from WT mice adoptively transferred with haptensensitized DCs from WT or IL-37tg mice. Specimens were stained with Alexa Fluor 594-labeled CD8 (red) or Alexa Fluor 488-labeled Foxp3 (green) and counterstained with DAPI (blue). Arrowheads indicate Foxp3/DAPI or CD8/DAPI double-positive cells. (Scale bar, 100 µm.) (Right) Number of CD8+ or Foxp3+ cells determined by cell counting. Data represent mean ± SEM (n = 4). **P < 0.01; ***P < 0.001.
The ability of IL-37 to impair DC priming of T cells and induce Treg cells in the sensitization phase was confirmed in vivo by immunofluorescence staining of CHS skin after adoptive transfer of hapten-sensitized DCs. Histological analysis of an ear specimen revealed a marked decrease in CD8+ T cells (74% reduction) and an increase in Treg cells (2.6-fold increase) in mice receiving DNFB-sensitized DCs from IL-37tg mice compared with those receiving DNFB-sensitized DCs from WT mice (Fig. 7B). These data indicate that IL-37 expression in DCs enhances Treg cell development in the sensitization phase.
Discussion
The inhibitory effects of IL-37 on innate immune response have been studied using cells transfected or silenced with IL-37, mice treated with recombinant or plasmid-DNA IL-37, and IL-37tg mice (2, 3, 8–12). In the present study, we not only demonstrated the inhibitory effects of IL-37 on adaptive immune response, but also provided evidence that IL-37 promotes generation of semimature tolerogenic DCs.
DCs have the potential to induce both immunity and tolerance: Whereas fully mature DCs are immunogenic, immature DCs and partially or semimature DCs are tolerogenic (22, 23). In contrast to immunogenic mature DCs, tolerogenic DCs express reduced levels of MHC II and costimulatory molecules, release less IL-12, and secrete more immunosuppressive mediators such as IL-10, thereby delivering inadequate signals for effector T-cell activation and promoting expansion of Treg cells (22–25). The difference between immature DCs and semimature DCs is the presence of migratory capacity and intermediate levels of MHC II and costimulatory molecules on the surface of semimature DCs. Therefore, semimature DCs migrate to dLNs or inflamed tissues to actively induce tolerance (22–25) whereas immature DCs reside in tissues and lead to T-cell anergy (26). In the present study, we found that contact hypersensitivity to hapten was markedly diminished in IL-37tg mice or mice receiving hapten-sensitized DCs from IL-37tg mice. These IL-37–expressing DCs maintained inducible CCR7 expression after stimulation and thereby migrated to its ligand CCL21 in vitro and dLNs where CCL19 and 21 were expressed. However, IL-37tg DCs not only displayed reduced MHC II and costimulatory molecule CD40, but also secreted higher levels of immunosuppressive IL-10 and reduced levels of proinflammatory cytokines such as IL-1β, IL-6, and IL-12. Most strikingly, the presence of IL-37 in DCs impaired their function in prime T cells (naive T cells, allogeneic T cells, and antigen-specific T cells) and promoted their ability to induce Treg cells in vitro and in vivo. These findings provide evidence that IL-37 suppresses adaptive immune response of skin by generating semimature tolerogenic DCs.
Because tolerogenic DCs play critical roles in autoimmunity, cancer immunology, and transplant medicine, the conditions that induce tolerogenic DCs have been the subject of several investigations during the past decade. These include properties of IL-10 (27, 28), TNFα (29), IL-21 (30), corticosteroids (31), vitamin D3 (32), rapamycin (33), α-1 antitrypsin (34), and neuropeptides such as VIP (35). We demonstrate in the present study that the expression of IL-37 in DCs generates a unique type of tolerogenic DC. Stimulation of DCs under IL-37–mediated diminished inflammatory conditions appears to impede the maturation process of DCs and to arrest DCs at a semimature or partially mature stage.
Skin DCs originate in the BM and migrate to the skin where they are differentiated and populated (36). During this process, cytokines and chemokines play a critical role in the development and differentiation of skin DCs (20). For example, the absence of TGF-β1 during embryogenesis leads to the lack of epidermal DCs (LCs) (21). Because the expression of IL-37 is affected by a variety of inflammatory mediators including TGF-β1 (3), we examined the effect of IL-37 on the distribution of skin DCs. IL-37tg mice did not show an altered DC compartment in the skin, and the skin DCs from both WT and IL-37tg mice remained in an immature phenotype. In agreement with this phenotype, IL-37 expression in DCs had little impact on their capacity to phagocytose antigens, a major functional characteristic of immature DCs (37). The effect of IL-37 on DC development and differentiation may depend upon the expression levels of IL-37. Although IL-37tg mice were generated under a CMV promoter (3), baseline expression of IL-37 mRNA and protein was low due to instability elements within the IL-37–coding region. Only with stimulation by proinflammatory cytokines or TLR ligands are the levels of IL-37 upregulated (3, 4). Therefore, it is reasonable to speculate that in the absence of infection or inflammation IL-37 levels are low or absent, resulting in IL-37’s minimal effects on immature DCs. Alternatively, IL-37 is dispensable for the development and distribution of DCs.
CHS is mediated by a consequence of both innate and adaptive responses to haptens, which may have important clinical implications for the development of anti-inflammatory or immunosuppressive therapies. In this study, expression of IL-37 inhibits CHS by inducing semimature tolerogenic DCs. A recent study shows that IL-37 is a top downregulated gene in the human skin transcriptome during a delayed-type hypersensitivity to a contact allergen diphencyprone (38), suggesting that reduced IL-37 levels are responsible for skin inflammation of contact dermatitis. Considering IL-37’s broad anti-inflammatory effects on innate immunity and adaptive immunity, this cytokine could be used as a therapeutic mediator for a variety of inflammatory diseases, including contact dermatitis, eczema, psoriasis, graft-vs.-host disease, and autoimmune diseases. Conversely, this cytokine could be upregulated in a variety of immunosuppressed conditions such as HIV and cancer and contributes to these diseases.
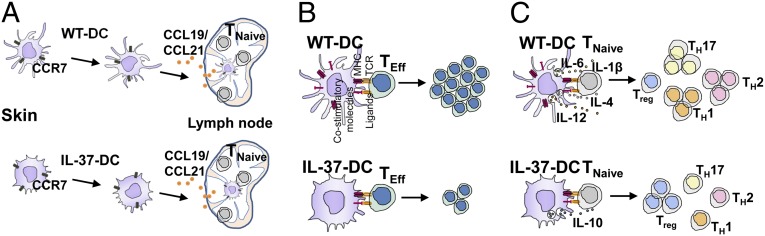
In conclusion, IL-37 expression in DCs promotes generation of semimature tolerogenic DCs and suppresses antigen-specific immune response of skin. The hypothetical role of IL-37 in DCs is summarized in Fig. 8. Under stimulation, IL-37–expressing DCs upregulate CCR7 and migrate to its ligands (Fig. 8A). However, IL-37–expressing DCs show reduced expression of costimulatory molecules, which impairs T-cell–proliferating capacity (Fig. 8B). IL-37–expressing DCs also display cytokine profiles favoring generation of Treg cells (Fig. 8C). Our findings broaden the horizon in understanding the development of tolerogenic DCs by a newly defined IL-1 family member: IL-37. IL-37 thus emerges as an inhibitor of adaptive immune response.
Fig. 8.
Hypothetical role of IL-37 in DCs. (A) Migratory capacity of DCs from WT or IL-37tg mice. (B) Proliferation of effector T cells (TEff) by DCs from WT or IL-37tg mice. (C) Polarization of naive T cells (TNaive) to TH1, TH2, TH17, and Treg cells by DCs from WT or IL-37tg mice.
Materials and Methods
For mice and CHS sensitization, see SI Materials and Methods. Information on histology, skin cell preparation, immunostaining of epidermal sheets, flow cytometry, generation of bone marrow-derived DCs, RNA extraction and qRT-PCR analysis, Western blot, phagocytotic assay, chemotaxis assay, DC migration assay in vivo, DC adoptive transfer, cytokine measurements, T-cell proliferation assay, analysis of DC-induced Treg cells, and immunofluorescent staining are provided in SI Materials and Methods. For statistical analysis, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Alistaire S. Acosta and Karen Helm [supported by National Institutes of Health (NIH) Grants P30CA046934 and P30AR057212], Laura Hoaglin (supported by NIH Grant P30AR057212), and Tania Azam for assisting with experiments, and Kasey Couts, PhD, and Zili Zhai, PhD, for critically reviewing the manuscript. This study was supported in part by research grants from the Veterans Affairs Merit Review Award (to M.F.), Interleukin Foundation (to M.F.), Cancer League of Colorado (to M.F.), Tadamitsu Research Fund (to M.F.), and NIH Pilot Grants P30AR057212 (to M.F.) and AI-15614 (to C.A.D.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1416714111/-/DCSupplemental.
References
- 1.Kumar S, et al. Identification and initial characterization of four novel members of the interleukin-1 family. J Biol Chem. 2000;275(14):10308–10314. doi: 10.1074/jbc.275.14.10308. [DOI] [PubMed] [Google Scholar]
- 2.Sharma S, et al. The IL-1 family member 7b translocates to the nucleus and down-regulates proinflammatory cytokines. J Immunol. 2008;180(8):5477–5482. doi: 10.4049/jimmunol.180.8.5477. [DOI] [PubMed] [Google Scholar]
- 3.Nold MF, et al. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11(11):1014–1022. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bufler P, Gamboni-Robertson F, Azam T, Kim SH, Dinarello CA. Interleukin-1 homologues IL-1F7b and IL-18 contain functional mRNA instability elements within the coding region responsive to lipopolysaccharide. Biochem J. 2004;381(Pt 2):503–510. doi: 10.1042/BJ20040217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan G, et al. IL-1H, an interleukin 1-related protein that binds IL-18 receptor/IL-1Rrp. Cytokine. 2001;13(1):1–7. doi: 10.1006/cyto.2000.0799. [DOI] [PubMed] [Google Scholar]
- 6.Smith DE, et al. Four new members expand the interleukin-1 superfamily. J Biol Chem. 2000;275(2):1169–1175. doi: 10.1074/jbc.275.2.1169. [DOI] [PubMed] [Google Scholar]
- 7.Bufler P, et al. A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity. Proc Natl Acad Sci USA. 2002;99(21):13723–13728. doi: 10.1073/pnas.212519099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNamee EN, et al. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci USA. 2011;108(40):16711–16716. doi: 10.1073/pnas.1111982108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakai N, et al. Interleukin-37 reduces liver inflammatory injury via effects on hepatocytes and non-parenchymal cells. J Gastroenterol Hepatol. 2012;27(10):1609–1616. doi: 10.1111/j.1440-1746.2012.07187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu B, et al. Interleukin-37 ameliorates myocardial ischemia/reperfusion injury in mice. Clin Exp Immunol. 2014;176(3):438–451. doi: 10.1111/cei.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulau AM, et al. In vivo expression of interleukin-37 reduces local and systemic inflammation in concanavalin A-induced hepatitis. ScientificWorldJournal. 2011;11:2480–2490. doi: 10.1100/2011/968479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teng X, et al. IL-37 ameliorates the inflammatory process in psoriasis by suppressing proinflammatory cytokine production. J Immunol. 2014;192(4):1815–1823. doi: 10.4049/jimmunol.1300047. [DOI] [PubMed] [Google Scholar]
- 13.Hoebe K, Janssen E, Beutler B. The interface between innate and adaptive immunity. Nat Immunol. 2004;5(10):971–974. doi: 10.1038/ni1004-971. [DOI] [PubMed] [Google Scholar]
- 14.Granucci F, Zanoni I, Feau S, Ricciardi-Castagnoli P. Dendritic cell regulation of immune responses: A new role for interleukin 2 at the intersection of innate and adaptive immunity. EMBO J. 2003;22(11):2546–2551. doi: 10.1093/emboj/cdg261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: Linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8(3):193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 16.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8(12):935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe H, Unger M, Tuvel B, Wang B, Sauder DN. Contact hypersensitivity: The mechanism of immune responses and T cell balance. J Interferon Cytokine Res. 2002;22(4):407–412. doi: 10.1089/10799900252952181. [DOI] [PubMed] [Google Scholar]
- 18.Christensen AD, Haase C. Immunological mechanisms of contact hypersensitivity in mice. APMIS. 2012;120(1):1–27. doi: 10.1111/j.1600-0463.2011.02832.x. [DOI] [PubMed] [Google Scholar]
- 19.Honda T, Egawa G, Grabbe S, Kabashima K. Update of immune events in the murine contact hypersensitivity model: toward the understanding of allergic contact dermatitis. J Invest Dermatol. 2013;133(2):303–315. doi: 10.1038/jid.2012.284. [DOI] [PubMed] [Google Scholar]
- 20.Wang B, et al. Cytokine knockouts in contact hypersensitivity research. Cytokine Growth Factor Rev. 2003;14(5):381–389. doi: 10.1016/s1359-6101(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 21.Borkowski TA, Letterio JJ, Farr AG, Udey MC. A role for endogenous transforming growth factor beta 1 in Langerhans cell biology: The skin of transforming growth factor beta 1 null mice is devoid of epidermal Langerhans cells. J Exp Med. 1996;184(6):2417–2422. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: Which signals induce tolerance or immunity? Trends Immunol. 2002;23(9):445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 23.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 24.Gordon JR, Ma Y, Churchman L, Gordon SA, Dawicki W. Regulatory dendritic cells for immunotherapy in immunologic diseases. Front Immunol. 2014;5:7. doi: 10.3389/fimmu.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7(8):610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz RH, Mueller DL, Jenkins MK, Quill H. T-cell clonal anergy. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 2):605–610. doi: 10.1101/sqb.1989.054.01.072. [DOI] [PubMed] [Google Scholar]
- 27.Enk AH, Angeloni VL, Udey MC, Katz SI. Inhibition of Langerhans cell antigen-presenting function by IL-10. A role for IL-10 in induction of tolerance. J Immunol. 1993;151(5):2390–2398. [PubMed] [Google Scholar]
- 28.Gregori S, et al. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 2010;116(6):935–944. doi: 10.1182/blood-2009-07-234872. [DOI] [PubMed] [Google Scholar]
- 29.Menges M, et al. Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J Exp Med. 2002;195(1):15–21. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brandt K, Bulfone-Paus S, Foster DC, Rückert R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood. 2003;102(12):4090–4098. doi: 10.1182/blood-2003-03-0669. [DOI] [PubMed] [Google Scholar]
- 31.Piemonti L, et al. Glucocorticoids increase the endocytic activity of human dendritic cells. Int Immunol. 1999;11(9):1519–1526. doi: 10.1093/intimm/11.9.1519. [DOI] [PubMed] [Google Scholar]
- 32.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164(5):2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 33.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105(12):4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 34.Ozeri E, Mizrahi M, Shahaf G, Lewis EC. α-1 antitrypsin promotes semimature, IL-10-producing and readily migrating tolerogenic dendritic cells. J Immunol. 2012;189(1):146–153. doi: 10.4049/jimmunol.1101340. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez-Rey E, Chorny A, Fernandez-Martin A, Ganea D, Delgado M. Vasoactive intestinal peptide generates human tolerogenic dendritic cells that induce CD4 and CD8 regulatory T cells. Blood. 2006;107(9):3632–3638. doi: 10.1182/blood-2005-11-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ginhoux F, et al. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7(3):265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banchereau J, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 38.Gulati N, et al. Molecular characterization of human skin response to diphencyprone at peak and resolution phases: Therapeutic insights. J Invest Dermatol. 2014;134(10):2531–2540. doi: 10.1038/jid.2014.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.