Significance
Bats are dying in unprecedented numbers at wind turbines, but causes of their susceptibility are unknown. Fatalities peak during low-wind conditions in late summer and autumn and primarily involve species that evolved to roost in trees. Common behaviors of “tree bats” might put them at risk, yet the difficulty of observing high-flying nocturnal animals has limited our understanding of their behaviors around tall structures. We used thermal surveillance cameras for, to our knowledge, the first time to observe behaviors of bats at experimentally manipulated wind turbines over several months. We discovered previously undescribed patterns in the ways bats approach and interact with turbines, suggesting behaviors that evolved at tall trees might be the reason why many bats die at wind turbines.
Keywords: energy development, sensory perception, video surveillance, wildlife, wind energy
Abstract
Wind turbines are causing unprecedented numbers of bat fatalities. Many fatalities involve tree-roosting bats, but reasons for this higher susceptibility remain unknown. To better understand behaviors associated with risk, we monitored bats at three experimentally manipulated wind turbines in Indiana, United States, from July 29 to October 1, 2012, using thermal cameras and other methods. We observed bats on 993 occasions and saw many behaviors, including close approaches, flight loops and dives, hovering, and chases. Most bats altered course toward turbines during observation. Based on these new observations, we tested the hypotheses that wind speed and blade rotation speed influenced the way that bats interacted with turbines. We found that bats were detected more frequently at lower wind speeds and typically approached turbines on the leeward (downwind) side. The proportion of leeward approaches increased with wind speed when blades were prevented from turning, yet decreased when blades could turn. Bats were observed more frequently at turbines on moonlit nights. Taken together, these observations suggest that bats may orient toward turbines by sensing air currents and using vision, and that air turbulence caused by fast-moving blades creates conditions that are less attractive to bats passing in close proximity. Tree bats may respond to streams of air flowing downwind from trees at night while searching for roosts, conspecifics, and nocturnal insect prey that could accumulate in such flows. Fatalities of tree bats at turbines may be the consequence of behaviors that evolved to provide selective advantages when elicited by tall trees, but are now maladaptive when elicited by wind turbines.
Bats are long-lived mammals with low reproductive potential and require high adult survivorship to maintain populations (1, 2). The recent phenomenon of widespread fatalities of bats at utility scale wind turbines represents a new hazard with the potential to detrimentally affect entire populations (3, 4). Bat fatalities have been found at wind turbines on several continents (3–6), with hypothesized estimates of fatalities in some regions ranging into the tens to hundreds of thousands of bats per year (4, 6). Before recent observations of dead bats beneath wind turbines, fatal collisions of bats with tall structures had been rarely recorded (7). Most fatalities reported from turbines in the United States, Canada, and Europe are of species that evolved to roost primarily in trees during much of the year (“tree bats”), some of which migrate long distances in spring and late summer to autumn (8). In North America, tree bats compose more than three-quarters of the reported bat fatalities found at wind-energy sites (6, 9), although there is a paucity of information from the southwestern United States and Mexico. Similar patterns occur in Europe (4). Another prominent pattern in bat fatality data from northern temperate zones is that most fatalities are found during late summer and autumn, sometimes with a much smaller peak of fatality in spring (4, 6). Concurrent involvement of species with shared behaviors suggests that behavior plays a key role in the susceptibility of bats to wind turbines, and that tree bats might somehow be attracted to wind turbines (8).
The causes of bat collisions with wind turbines are unknown, and many explanations for this phenomenon remain unexplored (8). Proposed causes of susceptibility range from bats randomly being struck by turbine blades while migrating past in large numbers to bats being attracted to wind turbines while searching for important resources, such as food, shelter, and social opportunities (8). Although causes of susceptibility remain unknown, altering turbine operations under certain conditions during periods of high risk can reduce bat deaths. Fatalities during late summer and autumn tend to occur when average wind speeds are lower than about 5–6 m/s (4, 9, 10), and studies in Canada (11), the United States (12), and Germany (4) demonstrated that bat deaths can be substantially reduced by preventing turbine blades from turning until winds reach such speeds. Such operational modifications at wind facilities bring logistical and financial costs but may prove to be effective at reducing bat fatalities in many areas (11, 12). Discovering the underlying reasons why bats are susceptible to wind turbines could help improve the efficiency of existing strategies and potentially uncover new ways of further reducing fatalities while maximizing power production.
In late summer and autumn of 2012, we observed the behaviors of bats at a wind facility in northwest Indiana using thermal video-surveillance cameras, supplemented with near-infrared video, acoustic detectors, and radar. Our aim was to better understand how wind and turbine blade movement influence behaviors of bats around turbines, and thus fatality risk. Turbine operation was manipulated so that we could observe if bat behaviors and activity patterns differed around rotating versus stationary blades, and how bats interact with turbines under various operating and environmental conditions. Specifically, we tested the hypotheses that wind and blade rotation speed influenced the way that bats approached turbines.
Results
We recorded bat activity in the rotor-swept zones of three turbines on 163 camera-nights (one camera deployed for one night at a turbine) during July 29 to October 1, 2012, for a total of 1,304 h of thermal imagery. Video detections of bats were treated as the same event when detected within 1 min or less of other bat observations (Fig. 1). Bats were detected at turbines throughout the study period (Fig. S1) and throughout the night without any apparent trend toward later or earlier activity over the study period (Fig. S1).
Fig. 1.
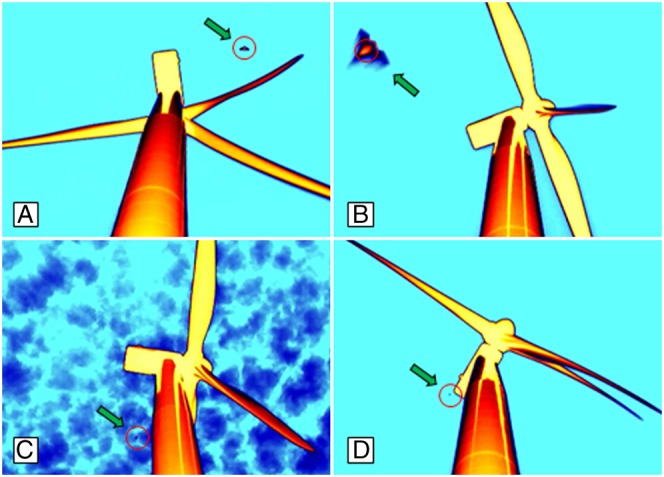
Still images of night-flying bats (green arrows) at wind turbines that were detected in thermal-infrared video footage. Cameras were positioned 12 m from the base of the turbine, looking up the 80-m monopole toward the nacelle (rectangular machinery enclosure) and rotor, to which three 40-m blades attach. Red circles represent the object identified as a bat by the automated software used for finding their presence in nightly (∼10 h) video recordings. A variety of detection conditions are illustrated, including a bat approaching fast-rotating (14 rpm) turbine blades at about midtower height (A), a bat flying low (<10 m) above the camera (B), a bat approaching the leeward side of a turbine monopole in cloudy conditions (C), and a bat flying at about nacelle height in the leeward airspace on the far side of a turbine with blades rotating at full speed (D).
Approximately 3–4 million animals were detected by radar flying through the monitored portions of the wind facility at or below about 200 m above ground level during this study (SI Results). Of this number, about a quarter were vertebrates occurring within the range of heights swept by turbine blades (≤200 m) (SI Results). Among a total of 1,261 video detections of flying animals, a large proportion were identified as bats (79%), with fewer detections of bat-like targets (15%), birds (2%), likely insects (3%), and unidentified objects (1%); only the bat detections (n = 993) were included in this analysis. Adjusting for the number of thermal cameras operating per night, the average number of animal detections on video per turbine-night was 7.8 (min: 0; first quartile: 2; third quartile: 12; max: 31) and the average number of bat detections per turbine-night was 6.2 (min: 0; first quartile: 1; third quartile: 12; max: 26).
Most (88%) video detections of bats involved flight trajectories indicating the individual was moving toward the turbine, hereafter referred to as “focal” behavior. We observed multiple focal behaviors of bats at turbines, several of which have not been previously reported (Table S1 and Movies S1–S9) (13). Behaviors included close approaches to the monopole and nacelle (enclosure of machinery on top of monopole to which rotor and blades are attached), close approaches to slowly moving blades, flight loops and dives centered on the turbine, distant hovering, and chasing other bats toward or near the turbines. Focal behaviors often involved bats closely (<2 m) approaching the turbine monopole (13%), nacelle (30%), and occasionally blades (6%;) (Table S1). Most bats exhibiting focal behavior made single approaches and then moved away (72%), but many (27%) approached turbines multiple times during a detection (Table S1) and such interactions at times lasted several minutes. Bat detections within a night at a turbine were found to be significantly clustered in time for 23 of the 163 camera-nights (14%), as measured by an index (14) applied to bat counts in sequential 10-min periods. Bats were more frequently detected during periods when the moon phase was more than half full and visible above the horizon (Kolmogorov–Smirnov test, D = 0.0822, P < 0.0001) (Fig. S2). Because the thermal cameras do not rely on reflected light, we assume this relationship with moonlight was attributable to biological causes rather than detection bias.
Twelve fresh bat fatalities were found under turbines after nights when video imagery was recorded (SI Results). Possible strikes or bats being moved by air around turbine blades were observed on video during two of the nights after which fatalities were found, and during only 18 of the 993 bat video detections (2%). Because of this low frequency of video-observed strikes and other rarely observed interactions and behaviors (<1% prevalence in Table S1), we were unable to adequately test the effects of wind and turbine blade speed on these phenomena. Based on the species composition of fatalities and acoustic calls recorded on the turbines (SI Results), it is likely that most of our video detections involved tree bats.
Bats were detected more frequently at lower relative to higher wind speeds, and this pattern was evident regardless of whether the turbine blades were spinning (Kolmogorov–Smirnov test, D = 0.2365, P < 0.0001) or not (D = 0.1937, P < 0.0001) (Fig. S3). When the wind was blowing > 1 m/s (96% of the time), bats exhibiting focal behaviors were observed significantly more often (∼80% of detections) on the leeward (downwind) compared with the windward side of the turbine (χ2 test = 329.3, df = 1, P < 0.0001), regardless of turbine nacelle orientation. When the wind was blowing ≤1 m/s, observed activity between leeward and windward areas (gauged relative to nacelle orientation) was approximately equal and the strong prevalence of leeward bat activity was not evident (Fig. 2A). However, the propensity for leeward activity at higher wind speeds was also influenced by the rotation of turbine blades. Similar to the general trend observed in video detections, logistic regression revealed a significant interaction between wind speed and blade rotation that resulted in opposite patterns of leeward activity (P = 0.0196). For example, when turbine blades were prevented from rotating, the observed frequency of leeward approaches to the nacelle increased from 65% to >90% as wind speeds increased from 0 to >8 m/s, whereas the proportion of leeward activity declined from >85% to <70% with a similar increase in wind speed when the turbine blades were spinning (Fig. 2B).
Fig. 2.
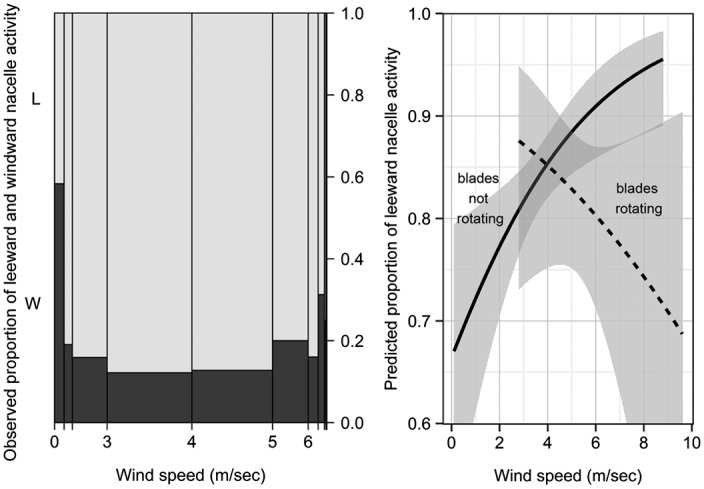
Spineplot (Left) depicts the proportion of observed bat activity as a function of wind speed (m/s) in leeward (L; light gray) and windward (W; dark gray) areas near the turbine nacelle. Spineplot bar widths are proportional to the number of observations within each wind-speed interval, with wider bars representing more observations (n = 208 in the 3–4 m/s category) and narrower bars representing fewer observations (n = 6 in the >8 m/s category). Predicted proportion of leeward activity (mean and 95% confidence interval) derived from logistic regression (Right) illustrates the significant interaction of wind speed and blade rotation (where curtailment prevented turbine blades from rotating on some nights).
Focal bat behaviors, including close approaches to the monopole, blades, and nacelle, were observed across a range of wind speeds (0–9.6 m/s), but were detected more frequently at low blade-rotation speeds and less frequently at intermediate and high speeds (classed as 0 to <1 rpm, 1–10 rpm, >10 rpm). For example, of the 55 detections that involved apparent investigations of turbine blades, 31% occurred when the blades were stationary, 69% occurred when blades were spinning very slowly (<1 rpm, a speed typical of near-windless conditions or when the blade edges were pointed into the wind), and none were detected when blades were spinning at ≥1 rpm (χ2 test = 27.5, df = 2, P < 0.0001). Similarly, about 41% of the 110 monopole approaches occurred when the blades were stationary, 51% occurred when blades were spinning very slowly, and only 8% of detections were noted at higher rotation speeds (χ2 test = 31.6, df = 2, P < 0.0001). Nacelle approaches demonstrated a similar pattern, with 42%, 40%, and 18% of the 258 detections in the stationary, very slow, and higher rotation-speed categories, respectively (χ2 test = 28.6, df = 2, P < 0.0001). These findings are all consistent with the hypotheses that wind speed and blade rotation speed influenced the way that bats approached turbines.
Most video detections of bats involved single individuals, although a small proportion (3%; n = 29) included pairs of bats. Bats were seen chasing or following each other during 48% (n = 14) of the observations involving pairs. On a few occasions, bats chasing each other near turbines appear to touch in flight. One video event revealed two hoary bats (Lasiurus cinereus; identified from concurrent acoustic recordings) hovering next to each other in the airspace near the turbine nacelle for over 10 s after a prior sequence in which they interacted in the lee of the turbine tower (Movie S10).
Discussion
Our video observations indicate that many bats passing close (<50 m) to wind turbines with stationary or slow-moving blades during late summer and autumn are attracted to and actively approach them using minimally turbulent air currents, vision, and to some degree echolocation for orientation. In contrast, radar observations indicate that nocturnally migrating vertebrates, presumed to be mostly birds, likely far outnumbered bats in the airspace, yet their near absence from video observations suggests that birds did not interact with turbines in the same way as bats, possibly avoiding them. Furthermore, acoustic detectors pointing upward from the tops of the turbine nacelles regularly detected the calls of bats not observed by video cameras, indicating that some bats passed high in the airspace above the turbines without closely approaching. It remains to be determined what proportion of passing bats approach turbines and whether they might respond to the presence of turbines over greater distances than those we observed with video cameras.
Bats likely can sense and respond to air currents. We saw no preference in the directions from which they approached turbines when the wind was not blowing or was blowing very gently, but bats consistently approached from leeward at wind speeds >1 m/s. The downwind direction of activity only when air was moving suggests that bats know which way the wind blows and approach tall structures in a patterned way that is independent of cardinal direction. As do many animals that move through air (15), bats orient by sensing and responding to flows through which they fly. Bats sometimes commute and forage on the leeward sides of windbreaks, such as tree rows and cliffs, with the postulated benefits of leeward activity including lower risk of predation, favorable conditions for energy-efficient flight, and greater availability of insect prey, particularly during high winds (16, 17). Being able to follow flows can provide substantial selective advantages to animals, particularly when other sensory cues are limited and when important resources can be predictably found within flows (15). It was once believed that bats made their way through darkness with the help of highly sensitive touch receptors in their wings and ears (18), but this concept of landscape orientation received little subsequent attention after the discovery of echolocation (19). Highly evolved hair-cell receptors on the skin surfaces of bat wings recently have been studied in detail; hair receptors in bat wings are now known to play an important role in flight control by sensing minute changes in airflow across the wing surfaces (20). Whether wing receptors help bats to sense subtle patterns of airflow at larger spatial scales is unclear, but Brazilian free-tailed bats (Tadarida brasiliensis) show evidence of orienting through wind currents and exploiting migrating insects concentrated in airflows in the absence of other visual or acoustic cues at high altitudes [up to 3,000 m above ground level (21, 22)]. In light of previous general observations of bat activity in the lee of windbreaks and our observations of consistent leeward bat activity at turbines, we suspect that bats are well adapted for sensing and orienting by airflows at landscape scales and that going with the flow, or against in the case of bats at turbines, may be an underappreciated sensory modality that evolved in these night-flying mammals.
Our thermal video cameras detected bats at turbines more often during periods of night with bright moon illumination and less often during periods with lower levels of moonlight, suggesting that vision plays a role in bats perceiving and approaching wind turbines. Bats rely on vision for long-distance orientation (23–25), are known or suspected to orient through landscapes using light cues, such as stars and postsunset glow (26, 27), and use visual cues to help them find roosts in trees (28). The effects of moonlight on bat activity and fatality at turbines are not well understood, but a study in Alberta, Canada, reported higher fatality rates of silver-haired bats (Lasionycteris noctivagans) at wind turbines on nights when the moon was fully illuminated (29). There is no evidence that tree bat activity in the absence of, or distant from, turbines varies with lunar cycles or illumination (30). Acoustic data gathered on the turbines we monitored, which included many calls from bats passing higher in the airspace than our cameras could image, did not show a trend toward proportionally more activity under moonlit conditions (SI Results), further indicating general activity levels are not influenced by moonlight. The patterns we observed on video could be attributable to the visual conspicuousness of the wind turbines waxing and waning with the moon, affecting the probability of passing bats seeing and moving closer to them to investigate.
Despite our observations that suggest bats orient toward wind turbines using flow and visual cues, the reasons why they do so remain unknown. Although we could not determine why bats behaved the way they did around turbines, we suspect that such behaviors evolved in association with trees. At a fundamental level, tree bats may not be able to discriminate wind turbines from trees (3). Both trees and turbines have tall and cylindrical “trunks” (monopoles), visually conspicuous “crowns” (nacelles), and radially extending “limbs” (blades). Bats are rarely reported interacting or colliding with other tall structures (7), as might be expected if the behaviors we observed were a general response to structural stimuli. However, a recent study revealed higher activity of tree bats during late summer and autumn at tall communication towers compared with surrounding habitats (31). Bats may not have the cognitive ability to differentiate wind turbines or other tree-like structures from real trees either at a distance or at close range, particularly if visual cues, such as similar silhouettes against the night sky, are accompanied, reinforced, or overwhelmed by other perceptual cues, such as similar downwind airflow patterns. For example, the predatory beetle (Rhizophagus grandis) responds to disturbance of airflow around a simulated tree more than the tree’s visual silhouette (32).We do not know if the patterns of behavior we observed apply to cave-roosting species of bats that die at wind turbines [e.g., genera Myotis and Tadarida (6)], but even cave-roosting bats may occasionally visit trees for the reasons discussed below.
Key findings of our study were that wind speed and blade rotation speed influenced the way that bats approached turbines. Bats approached turbines less frequently when their blades were spinning fast and the prevalence of leeward approaches to the nacelle increased with wind speed at turbines with slow-moving or stationary blades. A plausible explanation for these patterns (see SI Discussion for others) is that airflow profiles around tall trees and turbines with stationary blades may be very similar to each other (e.g., oscillating swirling patterns, called a Kármán vortex street), whereas the spinning blades of turbines cause chaotic downwind turbulence (33) that is unlikely to resemble any natural airflow patterns that bats might associate with trees. If tree bats find and orient toward trees by sensing and moving into upstream airflows, turbines may resemble trees only when the blades are moving slowly or are stationary. In other words, airflow paths that bats potentially follow may not be present downwind of turbines with fast-spinning blades. Nighttime flight behaviors of bats around tall trees during late summer and autumn have not been reported, but finding and observing such behaviors if they exist might help explain why tree bats are susceptible to wind turbines.
Compounding the potential for bats to mistake wind turbines for trees is the possibility that they expect important resources when they arrive at the “trees.” Such possible expectations may not apply to concurrently migrating birds, which radar detected in apparently high abundance in the surrounding airspace yet were infrequently observed on video near turbines. Bats may exploit streams of air flowing downwind from trees, turbines, and perhaps other tree-like structures [e.g., communication towers (31)] at night while searching for roosts, conspecifics, and possibly feeding on nocturnal insects that could accumulate in such flows. Many of the hypothesized causes of tree bat susceptibility to turbines involve attraction (8). Our observations are consistent with the possibility that bats are attracted at close distances (<50 m) to turbines with stationary or slow-moving blades, but the potential source of attraction remains unknown. We did not see evidence of close-scale attraction based solely on physical phenomena, such as heat, electromagnetic fields, or sounds generated by specific parts of the turbines, because focal behaviors were dispersed across many different parts of the turbine structure, often involving motionless blades and inert monopoles. A prior study also reported bats focusing attention on monopoles, nacelles, and blades of wind turbines, but no part stood out as attracting disproportionately more bats than others (13). The variety of turbine parts toward which bats focus their attention suggests a general close-range attraction, but the strong leeward component to these varied focal behaviors may offer clues as to what bats might be trying to find.
Resource-based hypotheses of attraction include bats seeking shelter, social opportunities, or food at turbines (3, 8, 9, 13), all of which may occur more often on the leeward sides of tall, tree-like structures. The simplest explanation for bats closely approaching turbines may be that they are seeking places to roost in what they perceive as trees while migrating. We regularly observed hoary bats and eastern red bats (Lasiurus borealis) flying in under the bottom of the leeward nacelle and making close approaches to the recessed exhaust port (Movie S4). Although we did not see clear evidence of bats consistently trying to land on turbines, we frequently observed bats approaching the monopoles very closely, as previously reported (4, 13, 34). The high proportions of close approaches focused on nacelles and monopoles (Table S1) are consistent with bats trying to find places to land. After not finding suitable places to alight upon (e.g., close investigations reveal turbine surfaces too smooth), bats may simply move on.
Bats might also closely approach turbines while looking for social opportunities. Similarities in the social behaviors of tree bats in North America and Europe led to speculation that bats might use the tallest trees in landscapes as flocking or gathering places (35). Tree bats tend to begin mating during the time when most mortality is documented at turbines (36), and bats seeking mates at trees may be drawn toward turbines (37) and other tall structures (31). We observed pairs of bats in 3% of our observations, and in about half of those cases they appeared to be following or chasing each other. In one case we observed two hoary bats in the lee and recorded social calls (Movie S10), but did not see evidence of larger social aggregations that were hypothesized for this species (37). Many species of tree bats in Europe exhibit mating flight displays centered on trees during late summer and autumn, but such flight behaviors have not been reported for any temperate North American bats (37). We speculate that some of the sustained leeward focal behaviors that we observed at turbines in our study, such as repeated looping returns and dives (Movie S2), might be associated with mating displays that could occur at trees. The “upstream orientation” we frequently observed is common in other types of flying and swimming organisms during foraging and mate-searching movements (15).
Bats may be drawn in by insects whose distribution is concentrated around wind turbines. Empirical data demonstrating the consistent presence and aggregation of insects at turbines during the night are lacking, but insects are known to foul turbine blades (38), be attracted to certain turbine paint colors (39), and migrate in large numbers during periods of bat fatality at turbines (40). In addition, bats have been observed foraging near turbines (4, 34) or found dead beneath them with full stomachs (41, 42), highlighting the plausibility of the feeding hypothesis. Although we regularly observed insects in the video imagery, we did not observe the frequent presence of insects with bat detections or record any unambiguous feeding calls of bats at turbines (SI Results), nor did we regularly observe what we would consider typical foraging behaviors of bats during our study. However, this observed lack of insects and typical foraging patterns does not preclude the possibility that bats expected to find insects at the turbines they approached.
There are several general patterns of insect behavior and distribution that give us reason to suspect the leeward behaviors we observed at turbines might be associated with bats expecting insects at the structures as they approached, irrespective of the actual presence of insects. Insects often accumulate on the leeward sides of artificial and natural structures, and behind windbreaks insects tend to increase in number and density with wind speed (43, 44). Many diurnal and crepuscular insects swarm above prominent high points in landscapes during calm conditions and such aggregations often blow leeward in windy conditions (45, 46). Certain nocturnal insects, such as gypsy (Lymantria dispar) and spruce-budworm (Choristoneura spp.) moths, lay eggs in and disperse from the tops of tall trees during late summer and autumn (47, 48). Bats sometimes feed on insects dispersing from trees. For example, Lloyd et al. (49) reported bats feeding on emerging spruce-budworm moths, and Lewis (50) reported eastern red bats feeding on moths in the airflows leeward of human-made structures: “When a moderate, steady wind is blowing over a moth-infested [corn] crib I have seen bats strung out in a narrow belt to a distance of 200 yards or more, catching moths that were carried by the wind.” Given the likelihood of insects accumulating at night above and in the lee of tall trees in natural environments, the leeward focus of bat behaviors at tree-like structures may not be coincidental.
Resource-based attraction hypotheses involving shelter, social opportunities, and food all seem plausible in the light of our results, but gathering direct evidence of such resource use by bats may not be possible at wind turbines or other anthropogenic structures. The roosts, conspecifics, and insect prey that bats might expect at turbines or other tree-like artificial structures would not necessarily have to occur there to draw them in and put them at risk. Bats may be acting upon the expectation of resources rather than the actual presence of resources. Fatalities of bats at turbines may be the consequence of behaviors that evolved to provide selective advantages when elicited by tall trees, but are now maladaptive when elicited by wind turbines. Paradoxically, direct evidence of the causes of tree bat susceptibility to wind turbines may not be observable at wind turbines, but instead at the trees and their associated resources where potentially causal behaviors evolved.
Our observations have practical implications. Although our scope of inference is limited to certain tree bats (L. borealis, L. cinereus, and L. noctivagans), areas of turbines from the rotor-swept zone around the nacelle to near the ground (different behaviors may occur higher in the airspace), and are based on observations from just three turbines in midwestern North America, efforts to monitor bat activity near turbines (e.g., acoustic detectors and video cameras), or deter bats from turbines [e.g., devices producing startling sounds (51)] may benefit by aiming instruments from the back of the nacelle into the leeward airspace, an area where we consistently observed higher bat activity regardless of changing wind directions. Strategies for minimizing fatalities of bats at turbines currently focus on preventing blades from spinning during low wind periods (4, 11, 12). Our observations that tree bats show a tendency to closely investigate inert turbines and sometimes linger for minutes to perhaps hours (in the cases of clustered observations) highlight the plausibility of a scenario in which bats are drawn toward turbines in low winds, but sometimes remain long enough to be put at risk when wind picks up and blades reach higher speeds. Therefore, the frequency of intermittent, blade-spinning wind gusts within such low-wind periods might be an important predictor of fatality risk; fatalities may occur more often when turbine blades are transitioning from potentially attractive (stationary or slow) to lethal (fast) speeds. Efforts to minimize bat fatalities at wind facilities might benefit by averaging wind-speed curtailment thresholds over longer periods of time (e.g., >10 min) to prevent gusts from intermittently pushing blades to lethal speed during low-wind periods. Finally, fatalities may be reducible by altering the appearance of turbines. Fewer fatalities of eastern red bats were found under turbines with flashing red aviation lights at a large wind facility in Texas (52), hinting at the possibility that supplemental lighting of turbines might make some bats less likely to mistake them for trees.
Methods
Study Area and Experimental Design.
We conducted this study at a wind turbine facility (Fowler Ridge Wind Farm, BP Alternative Energy, Oakland, CA and Dominion Resources Inc., Richmond, VA) in Benton County, IN, which consisted of 355 wind turbines with a total nameplate capacity of 600 megawatts (MW). The 20,234-ha site is dominated by agricultural lands (mostly soybean and corn fields) with buildings and forested areas composing <5% of the total area. The topography is mostly flat with elevations ranging from about 210–225 m. The three turbines monitored (Model V82, Vestas Wind Systems) each had a nameplate capacity of 1.65 MW, 82 m rotor diameter, and 80-m-high monopole.
To observe bat interactions with turbines across a range of weather and operating conditions, turbines were run under three different scenarios: (i) blades never rotating, (ii) blades not rotating (curtailed) until wind speeds reached 6.5 m/s, and (iii) blades rotating under normal operating conditions (begin rotating at about 2 m/s wind speed). We randomly assigned operation treatments each night so that on any given night, one of the three turbines was randomly assigned to be never rotating, curtailed, or fully operational.
Recording Video Imagery.
We monitored the three turbines using video surveillance cameras with sensors that operate in the “thermal” spectrum of infrared light (∼9,000–14,000 nm; Model Q1921-E with a 19-mm lens, Axis Communications) and which require no supplemental illumination. The effective sensor-array size of the cameras was 384 × 288 pixels, and we recorded digital video at a rate of 30 frames per second using netbook computers (Model 1104 A7K67UT, Hewlett-Packard) equipped with external hard drives. We positioned these cameras 12 m from the base of each turbine so that they imaged about two-thirds of the rotor-swept zone. Video recording began within 1 h of sunset and continued until ∼1 h after sunrise. In addition to the thermal cameras, we simultaneously recorded supplemental near-infrared (NIR) video imagery (SI Methods).
Review of Video Imagery.
We manually reviewed video imagery at high speed (scan speed ∼1 min/h of recorded imagery) with viewing software (VirtualDub, www.virtualdub.org; VLC, www.videolan.org) and then a second time using custom-written code (Dataset S1) and matrix-based statistical software (Matlab with Image Processing Toolbox, Mathworks) that automatically detected events in which animals flew through the thermal video scenes. Automatic processing algorithms identified frames with motion of small objects not associated with the moving turbine blades. Although video was recorded at 30 frames per second, only every 30th video frame was analyzed because of time constraints on automated processing, resulting in detection of events mostly lasting ≥1 s. However, because bats usually took several seconds to traverse the tens of meters of airspace around the turbine, we saw no evidence that this sampling rate consistently missed bats when they were present. Species of bats we observed likely fly at speeds ≤7 m/s (53). The size of the field of view was about 55 × 40 m given the ∼110-m resolution range of the cameras. We estimate that a bat at that height would require at least 5–6 s to traverse the imaged area and would be detected in as many video frames. Therefore, any bias associated with missing bats passing through the video scenes in <1 s would involve those passing relatively close to the camera and not affect the detection of bats at nacelle height.
All potential flying objects detected by high-speed scanning or software algorithms were visually reviewed and characterized by at least two observers (P.M.C. and P.M.G.). These detections included insects flying close to the cameras (which were ignored and not tabulated), as well as bats and small birds flying around the turbines up to the airspace above the nacelle, larger birds flying higher above the rotor-swept zone, and airplanes and clouds much higher. Based on the pixel resolution of the thermal cameras and the distance at which a bat could be resolved with more than 1 pixel, we estimate our range of detecting bats with the cameras was upwards of 110 m. With the thermal cameras situated 12 m from the base of the turbine and the nacelles sitting atop 80-m towers, the distance from camera to nacelle was 81 m. Our video observations from the thermal cameras and supplementary imagery from the NIR cameras (SI Methods) revealed that smaller bats (for example, eastern red bats, identified acoustically) (SI Methods) were easily detectable up to nacelle height but tended to become much less detectable as they moved higher than the nacelle, whereas larger bats (for example, hoary bats, identified acoustically) were detectable in the airspace 20–40 m above the nacelle. Although spatial positions of objects are sometimes difficult to determine in 2D video imagery, we were typically able to judge locations of bats in the airspace using reflections in the thermal imagery (e.g., during close approaches thermal reflections of bats could be seen on the turbine tower), shadows in the corresponding NIR imagery (e.g., bat passing close under bottom of nacelle), and by visually observing the parallax of the bat from the different view angles of the thermal and NIR cameras.
For each detection of a bat in the thermal imagery we recorded the following information: number of individuals present, orientation of the nosecone on the turbine nacelle, predominant area of bat activity relative to direction the turbine nose was pointing (leeward, windward), rotor speed (rpm), whether the bat altered course in response to the presence of the turbine (focal behavior), whether the bat made close (<2 m) approaches to the turbine monopole, nacelle, or blades during the event, whether the bat appeared to be struck or displaced by a moving turbine blade, as well as descriptive comments about the event. Turbine orientation was characterized from video, but we also analyzed meteorological and operational data gathered at the turbine nacelle. These data included wind speed (m/s) and rotor speed. Moon illumination was recorded as the proportion of lunar disk illuminated given that it was visible above the horizon. Moon illumination data were obtained from the Astronomical Applications Department of the US Naval Observatory (aa.usno.navy.mil/index.php).
Analysis of Bat Behavior from Video Detections.
Patterns of bat detection in relation to behavior, wind speed, and turbine operation were examined with Kolmogorov–Smirnov and χ2 tests and logistic regression. Kolmogorov–Smirnov tests of bat behavior compared conditions (e.g., moon illumination) during bat detections relative to that recorded throughout the study period for all nighttime 10-min intervals. All statistical analyses were performed in program R (v2.15.1; R Development Core Team, R: A Language and Environment for Statistical Computing, 2011). The temporal clustering of bat detections within a night at each turbine was evaluated with an index developed for use with temporal sequences where the data are grouped into equally-spaced intervals (14). Detections were grouped by 10-min intervals, and a conservative measure of closeness was specified by limiting the identification of a cluster of detections to those occurring in adjacent time intervals. An α-level of 0.05 was used to determine statistical significance. Temporal cluster analysis was performed with the R script “TangoT.index” (accessed 10/11/13 from www.niph.go.jp/soshiki/gijutsu/download/Rfunctions).
Supplemental Monitoring with Other Techniques.
In addition to monitoring the three wind turbines with thermal and near-infrared video surveillance cameras, we concurrently monitored them with acoustic detectors mounted on the turbine nacelles and radar, as described in SI Methods. See Movies S11 and S12 for examples of bats flying close to turbines with fast-moving blades.
Supplementary Material
Acknowledgments
We thank C. Willis and two anonymous reviewers for helpful input on earlier drafts of the manuscript; B. Gunderman, B. Gibson, S. Tompkins, C. Newton, C. Wehkamp, R. Linzner, K. Sowers, K. Owens, J. Richards, and the Remote Operations Center Staff for giving us the opportunity to do this research at the Fowler Ridge Wind Farm and for helping with the daily logistics of working at the site; S. Pruitt, L. Pruitt, and T. J. Miller of the US Fish and Wildlife Service provided helpful guidance when we were designing the study; and the field crew and analysis technicians, without whom none of this would have been possible: J. Foster, E. Anstedt, C. Wilkins, K. Scherr, J. Wagner, L. Smith, R. Price, and J. Johnson. This study was supported in part by the Fowler Ridge Wind Farm; the US Geological Survey; Bat Conservation International; and a David H. Smith Fellowship from the Cedar Tree Foundation and Society of Conservation Biology (to D.T.S.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1406672111/-/DCSupplemental.
References
- 1.Barclay RMR, Harder LD. Life histories of bats: Life in the slow lane. In: Kunz TH, Parsons S, editors. Bat Ecology. Univ of Chicago Press; Chicago: 2003. pp. 209–253. [Google Scholar]
- 2.Racey PA, Entwistle AC. Life-history and reproductive strategies of bats. In: Crichton EG, Krutzsch PH, editors. Reproductive Biology of Bats. Academic; New York: 2000. pp. 363–414. [Google Scholar]
- 3.Kunz TH, et al. Ecological impacts of wind energy development on bats: Questions, research needs, and hypotheses. Front Ecol Environ. 2007;5(6):315–324. [Google Scholar]
- 4.Rydell J, et al. Bat mortality at wind turbines in northwestern Europe. Acta Chiropt. 2010;12(2):261–274. [Google Scholar]
- 5.Parsons S, Battley P. Impacts of energy developments on wildlife: A southern hemisphere perspective. NZ J Zool. 2013;40(1):1–4. [Google Scholar]
- 6.Arnett EB, Baerwald EF. Impacts of wind energy development on bats: Implications for conservation. In: Adams RA, Pedersen SC, editors. Bat Evolution, Ecology, and Conservation. Springer; New York: 2013. pp. 435–456. [Google Scholar]
- 7.Cryan PM. Wind turbines as landscape impediments to the migratory connectivity of bats. Environ Law. 2011;41(2):355–370. [Google Scholar]
- 8.Cryan PM, Barclay RMR. Causes of bat fatalities at wind turbines: Hypotheses and predictions. J Mammal. 2009;90(6):1330–1340. [Google Scholar]
- 9.Arnett EB, et al. Patterns of bat fatalities at wind energy facilities in North America. J Wildl Manage. 2008;71(1):61–78. [Google Scholar]
- 10.Amorim F, Rebelo H, Rodrigues L. Factors influencing bat activity and mortality at a wind farm in the Mediterranean region. Acta Chiropt. 2012;14(2):439–457. [Google Scholar]
- 11.Baerwald EF, Edworthy J, Holder M, Barclay RMR. A large-scale mitigation experiment to reduce bat fatalities at wind energy facilities. J Wildl Manage. 2009;73(7):1077–1081. [Google Scholar]
- 12.Arnett EB, Huso MMP, Schirmacher MR, Hayes JP. Altering turbine speed reduces bat mortality at wind-energy facilities. Front Ecol Environ. 2011;9(4):209–214. [Google Scholar]
- 13.Horn JW, Arnett EB, Kunz TH. Behavioral responses of bats to working wind turbines. J Wildl Manage. 2008;72(1):123–132. [Google Scholar]
- 14.Tango T. Statistical Methods for Disease Clustering. Springer; New York: 2010. [Google Scholar]
- 15.Chapman JW, et al. Animal orientation strategies for movement in flows. Curr Biol. 2011;21(20):R861–R870. doi: 10.1016/j.cub.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Limpens HJGA, Kapteyn K. Bats, their behaviour and linear landscape elements. Myotis. 1991;29:39–48. [Google Scholar]
- 17.Verboom B, Spoelstra K. Effects of food abundance and wind on the use of tree lines by an insectivorous bat, Pipistrellus pipistrellus. Can J Zool. 1999;77(9):1393–1401. [Google Scholar]
- 18.Allen GM. Bats: Biology, Behavior and Folklore. Dover; Mineola, New York: 1939. [Google Scholar]
- 19.Griffin DR. Listening in the Dark: The Acoustic Orientation of Bats and Men. Yale Univ Press; New Haven, CT: 1958. [Google Scholar]
- 20.Sterbing-D’Angelo S, et al. Bat wing sensors support flight control. Proc Natl Acad Sci USA. 2011;108(27):11291–11296. doi: 10.1073/pnas.1018740108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams TC, Ireland LC, Williams JM. High altitude flights of the free-tailed bat, Tadarida brasiliensis, observed with radar. J Mammal. 1973;54(4):807–821. [Google Scholar]
- 22.McCracken GF, et al. Brazilian free-tailed bats (Tadarida brasiliensis: Molossidae, Chiroptera) at high altitude: Links to migratory insect populations. Integr Comp Biol. 2008;48(1):107–118. doi: 10.1093/icb/icn033. [DOI] [PubMed] [Google Scholar]
- 23.Griffin DR. Migration and homing of bats. In: Wimsatt WA, editor. Biology of Bats. Vol 1. Academic; New York: 1970. pp. 233–264. [Google Scholar]
- 24.Boonman A, Bar-On Y, Cvikel N, Yovel Y. It’s not black or white-on the range of vision and echolocation in echolocating bats. Front Physiol. 2013;4:248. doi: 10.3389/fphys.2013.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altringham J, Fenton MB. Sensory ecology and communication in the Chiroptera. In: Kunz TH, Fenton MB, editors. Bat Ecology. Univ of Chicago Press; Chicago: 2003. pp. 90–127. [Google Scholar]
- 26.Holland RA, Wikelski M. Studying the migratory behaviour of individual bats: Current techniques and future directions. J Mammal. 2009;90(6):1324–1329. [Google Scholar]
- 27.Cryan PM, Diehl RH. Analyzing bat migration. In: Kunz TH, Parsons S, editors. Ecological and Behavioral Methods for the Study of Bats. 2nd Ed. Johns Hopkins Univ Press; Baltimore: 2009. pp. 477–488. [Google Scholar]
- 28.Ruczyński I, Szarlik A, Siemers BM. Conspicuous visual cues can help bats to find tree cavities. Acta Chiropt. 2011;13(2):385–389. [Google Scholar]
- 29.Baerwald EF, Barclay RMR. Patterns of activity and fatality of migratory bats at a wind energy facility in Alberta, Canada. J Wildl Manage. 2011;75(5):1003–1114. [Google Scholar]
- 30.Lima SL, O’Keefe JM. Do predators influence the behaviour of bats? Biol Rev Camb Philos Soc. 2013;88(3):626–644. doi: 10.1111/brv.12021. [DOI] [PubMed] [Google Scholar]
- 31.Jameson JW, Willis CKR. Activity of tree bats at anthropogenic tall structures: Implications for mortality of bats at wind turbines. Anim Behav. 2014;97:145–152. [Google Scholar]
- 32.Wyatt TD, Phillips ADG, Grégoire JC. Turbulence, trees and semiochemicals: Wind-tunnel orientation of the predator, Rhizophagus grandis, to its barkbeetle prey, Dendroctonus micans. Physiol Entomol. 1993;18(2):204–210. [Google Scholar]
- 33.Porté-Agel F, Lu H, Wu YT. Interaction between large wind farms and the atmospheric boundary layer. Procedia IUTAM. 2014;10:307–318. [Google Scholar]
- 34.Ahlén I, Baagøe HJ, Bach L. Behavior of Scandinavian bats during migration and foraging at sea. J Mammal. 2009;90(6):1318–1323. [Google Scholar]
- 35.Cryan PM, Brown AC. Migration of bats past a remote island offers clues toward the problem of bat fatalities at wind turbines. Biol Conserv. 2007;139(1):1–11. [Google Scholar]
- 36.Cryan PM, et al. Evidence of late-summer mating readiness and early sexual maturation in migratory tree-roosting bats found dead at wind turbines. PLoS ONE. 2012;7(10):e47586. doi: 10.1371/journal.pone.0047586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cryan PM. Mating behavior as a possible cause of bat fatalities at wind turbines. J Wildl Manage. 2008;72(3):845–849. [Google Scholar]
- 38.Corten GP, Veldkamp HF. Aerodynamics. Insects can halve wind-turbine power. Nature. 2001;412(6842):41–42. doi: 10.1038/35083698. [DOI] [PubMed] [Google Scholar]
- 39.Long CV, Flint JA, Lepper PA. Insect attraction to wind turbines: Does colour play a role? Eur J Wildl Res. 2011;57(2):323–331. [Google Scholar]
- 40.Rydell J, et al. Mortality of bats at wind turbines linked to nocturnal insect migration? Eur J Wildl Res. 2010;56(6):823–827. [Google Scholar]
- 41.Reimer JP, Baerwald EF, Barclay RMR. Diet of hoary (Lasiurus cinereus) and silver-haired (Lasionycteris noctivagans) bats while migrating through southwestern Alberta in late summer and autumn. Am Midl Nat. 2010;164(2):230–237. [Google Scholar]
- 42.Valdez EW, Cryan PM. Insect prey eaten by hoary bats (Lasiurus cinereus) prior to fatal collisions with wind turbines. West N Am Nat. 2013;73(4):516–524. [Google Scholar]
- 43.Lewis T. The effects of an artificial windbreak on the aerial distribution of flying insects. Ann Appl Biol. 1965;55(3):503–512. [Google Scholar]
- 44.Lewis T. The distribution of flying insects near a low hedgerow. J Appl Ecol. 1969;6(3):443–452. [Google Scholar]
- 45.Downes JA. The swarming and mating flight of diptera. Annu Rev Entomol. 1969;14:271–298. [Google Scholar]
- 46.Wickman PO, Rutowski RL. The evolution of mating dispersion in insects. Oikos. 1999;84(3):463–472. [Google Scholar]
- 47.Greenbank DO, Schaefer GW, Rainey RC. Spruce budworm (Lepidoptera: Tortricidae) moth flight and dispersal: New understanding from canopy observations, radar, and aircraft. Mem Entomol Soc Can. 1980;110:1–45. [Google Scholar]
- 48.Weseloh RM. Spatial distribution of the gypsy moth (Lepidoptera: Lymantriidae) and some of its parasitoids within a forest environment. Entomophaga. 1972;17(3):339–351. [Google Scholar]
- 49.LLoyd N, Wilson JA, Barclay RMR. Behaviors of western spruce budworm moths (Choristoneura occidentalis) as defences against bat predation. J Insect Behav. 2006;9(4):533–544. [Google Scholar]
- 50.Lewis JB. Mammals of Amelia County, Virginia. J Mammal. 1940;21(4):422–428. [Google Scholar]
- 51.Arnett EB, Hein CD, Schirmacher MR, Huso MM, Szewczak JM. Evaluating the effectiveness of an ultrasonic acoustic deterrent for reducing bat fatalities at wind turbines. PLoS ONE. 2013;8(6):e65794. doi: 10.1371/journal.pone.0065794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bennett VJ, Hale AM. Red aviation lights on wind turbines do not increase bat-turbine collisions. Anim Conserv. 2014;17(4):354–358. [Google Scholar]
- 53.Hayward B, Davis R. Flight speeds of western bats. J Mammal. 1964;45(2):236–242. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


