Significance
The circadian system drives daily rhythms in physiology and behavior. Mammals in nature can change their daily activity rhythms, but causes and consequences of this behavioral plasticity are unknown. Here we show that nocturnal mice become diurnal when challenged by cold or hunger. Negative energy balance changes hormonal, physiological and behavioral rhythms without modifying the rhythm of the circadian pacemaker in the suprachiasmatic nucleus. This response is adaptive because activity during daytime warmth and resting in a buffered environment during the cold nighttime generally reduces energy expenditure. This mechanism may explain why nighttime activity in humans generally evokes higher energy uptake and subsequent obesity and metabolic syndrome, as seen in late chronotypes and night shift workers.
Keywords: circadian rhythms, behavioral neurobiology, behavioral plasticity, chronotype ecology
Abstract
The mammalian circadian system synchronizes daily timing of activity and rest with the environmental light–dark cycle. Although the underlying molecular oscillatory mechanism is well studied, factors that influence phenotypic plasticity in daily activity patterns (temporal niche switching, chronotype) are presently unknown. Molecular evidence suggests that metabolism may influence the circadian molecular clock, but evidence at the level of the organism is lacking. Here we show that a metabolic challenge by cold and hunger induces diurnality in otherwise nocturnal mice. Lowering ambient temperature changes the phase of circadian light–dark entrainment in mice by increasing daytime and decreasing nighttime activity. This effect is further enhanced by simulated food shortage, which identifies metabolic balance as the underlying common factor influencing circadian organization. Clock gene expression analysis shows that the underlying neuronal mechanism is downstream from or parallel to the main circadian pacemaker (the hypothalamic suprachiasmatic nucleus) and that the behavioral phenotype is accompanied by phase adjustment of peripheral tissues. These findings indicate that nocturnal mammals can display considerable plasticity in circadian organization and may adopt a diurnal phenotype when energetically challenged. Our previously defined circadian thermoenergetics hypothesis proposes that such circadian plasticity, which naturally occurs in nocturnal mammals, reflects adaptive maintenance of energy balance. Quantification of energy expenditure shows that diurnality under natural conditions reduces thermoregulatory costs in small burrowing mammals like mice. Metabolic feedback on circadian organization thus provides functional benefits by reducing energy expenditure. Our findings may help to clarify relationships between sleep–wake patterns and metabolic phenotypes in humans.
Environmental differences between day and night have resulted in daily (circadian) rhythms in physiology and behavior of most organisms. Many mammals are night active (nocturnal), which most likely reduces predation risk (1). Being active at night is however likely to be energetically costly because nocturnal mammals cannot escape the low temperatures at night by sleeping in sheltered places. Nocturnal mammals that are energetically challenged therefore may become day active (diurnal) to reduce energy expenditure (2). Nocturnal and diurnal activity patterns are indeed subject to phenotypic plasticity (temporal niche switching) under natural conditions (2).
The central circadian pacemaker in mammals is located in the suprachiasmatic nucleus (SCN) and coordinates tissue specific circadian rhythms throughout the body (3). In most cells, circadian rhythms originate from molecular feedback loops involving gene transcription (4–6) or a putative metabolic oscillator (7–10). At the molecular and systemic level regulatory loops interact to control circadian and metabolic function (11–13), but a causal relationship between organismal metabolism and circadian phase of entrainment has not been shown. Here we aim to identify effects of metabolism on circadian organization by manipulating energy balance in mice.
Ambient temperature (Ta) is a potent modifier of metabolic rate in mammals (14), but the period of circadian clocks is generally buffered against changes in Ta (temperature compensation; refs. 15–17). In nature, however, evolutionary selection pressures act on phase of entrainment relative to the light–dark (LD) cycle, rather than on circadian period per se. Because Ta fluctuates during the day, daily energy expenditure can be reduced by synchronizing rest-associated thermal insulation strategies with the coldest part of the day [circadian thermoenergetics (CTE) hypothesis; ref. 2]. Negative energy balance can therefore be compensated by shifting activity into the warmer phase (i.e., light phase) of the day, resulting in a reduction of energy expenditure under natural conditions.
According to the CTE hypothesis, both increasing energy expenditure and decreasing energy intake are predicted to lead to a relative increase in activity levels during the light phase (diurnality). Here, this prediction is tested by assessing phase of entrainment at different Ta and combining the effect of Ta on energy expenditure with decreased energy intake [Working for Food (WFF) protocol; ref. 18]. Entrainment to the LD cycle and the internal distribution of clocks in the SCN and peripheral tissues are assessed to confirm the diurnal phenotype in these energetically challenged mice. Finally, the energetic consequences of shifting to diurnality are quantified for mice living in natural conditions to assess the adaptive significance of diurnality in energetically challenged small mammals.
Results
Phase of Entrainment Depends on Energetic Balance.
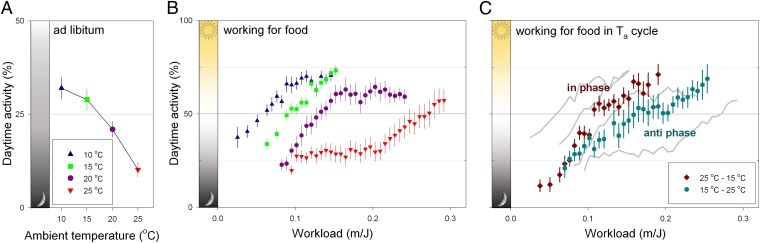
To test the prediction from the CTE hypothesis that negative energy balance will induce diurnal activity patterns, we manipulated constant Ta. Reducing constant Ta from 25 °C to 10 °C caused a threefold increase in relative activity during the light phase (F3,72 = 10.37, P < 0.0001; Fig. 1A). To test whether the effect of Ta on circadian phase of entrainment was a direct effect of Ta on clock output or the result of a negative energy balance, energy balance was also manipulated by reducing energy intake. Letting mice work for a food reward at gradually increasing workload levels (WFF protocol; ref. 18) at various Tas reduced energy intake without restricting food availability to a specific time of day (Fig. S1). Increasing workload also induced a shift from nocturnal toward diurnal activity patterns and lower Ta facilitated this shift (F3,43 = 72.29, P < 0.0001; Fig. 1B). This effect is not due to fragmentation of activity or lengthening of the activity phase because it is also observed when activity onset, offset or center of gravity are used as markers for the phase of entrainment (Fig. S2). These data indicate that negative energy balance can indeed induce a diurnal activity pattern in an otherwise nocturnal mammal.
Fig. 1.
Low Ta and simulated food shortage (working for food, WFF) stimulate daytime activity in mice. (A) In ad libitum fed mice the percentage of total running wheel activity performed during the light phase increases significantly with lowered Ta (actograms in Fig. S5). (B) At lower Tas, the switch point between nocturnality and diurnality occurs significantly earlier in the WFF protocol (actograms in Fig. S6). (C) Mice became diurnal during the WFF protocol when performed in temperature cycles both in phase (25–15 °C) and in antiphase (15–25 °C) with the LD cycle (see example of actograms in Fig. S7). Workload indicates the distance (in m) that mice had to run to obtain 1 Joule food reward. Data are plotted as mean ± SEM.
Diurnal Mice Remain Entrained to the LD Cycle.
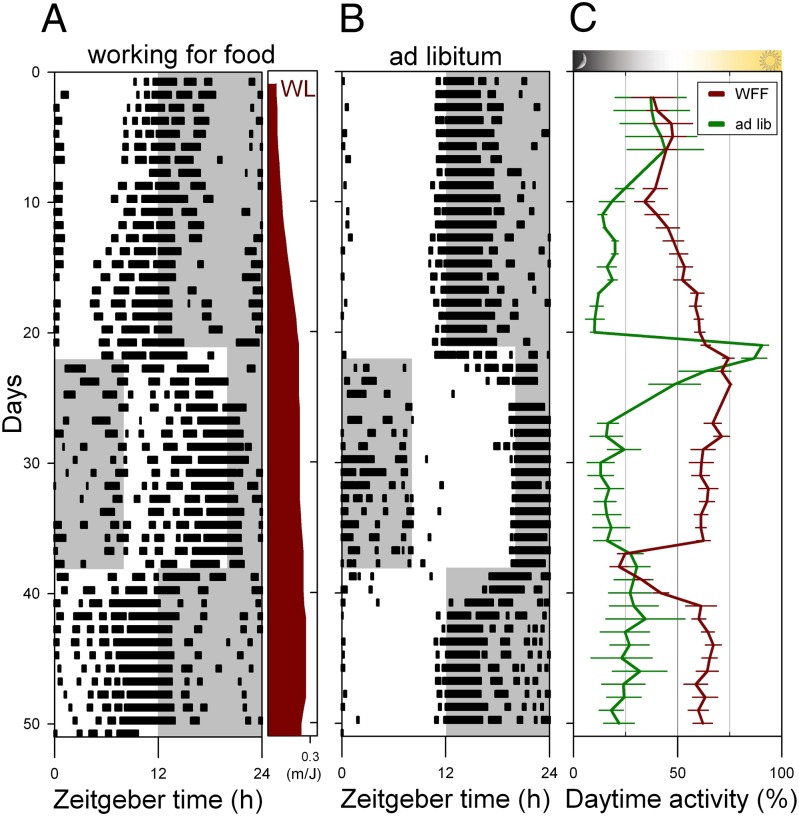
The observed increase in daytime activity can be explained as an advanced termination of sleep induced by hunger or it may reflect an entrained circadian phase optimization of activity to the light phase of the day. To discriminate between these two possibilities, the LD cycle during the WFF protocol was delayed by 8 h, followed by an 8-h advance 16 days later. The observed transient entrainment patterns during the first days following the LD cycle shifts indicate that the underlying mechanism relies on a circadian oscillator. After these transient days, all WFF mice returned to their diurnal phenotype, showing circadian light entrainment (Fig. 2). Under energetically challenging conditions in nature, the LD cycle and Ta rhythm might both be used as entraining cues for the circadian rhythm. The relative strength of both environmental signals (Ta cycle and LD cycle) was tested by comparing a 25–15 °C Ta cycle in phase with the LD cycle to a Ta cycle in antiphase with the LD cycle. Antiphase Ta cycles did not prevent WFF mice from becoming diurnal (Fig. 1C).
Fig. 2.
Entrainment to the LD cycle is preserved under simulated food shortage conditions. Diurnal working for food mice (A) and nocturnal ad libitum fed controls (B) reentrain transiently to shifts of the LD cycle. (C) The percentage of daytime activity of both groups (mean ± SEM) is shown for the full duration of the experiment with a 8-h delay on day 21 and a 8-h advance of the LD cycle on day 38. WL is workload in the WFF protocol.
Diurnality Is Associated with Circadian Reorganization of Internal Clocks.
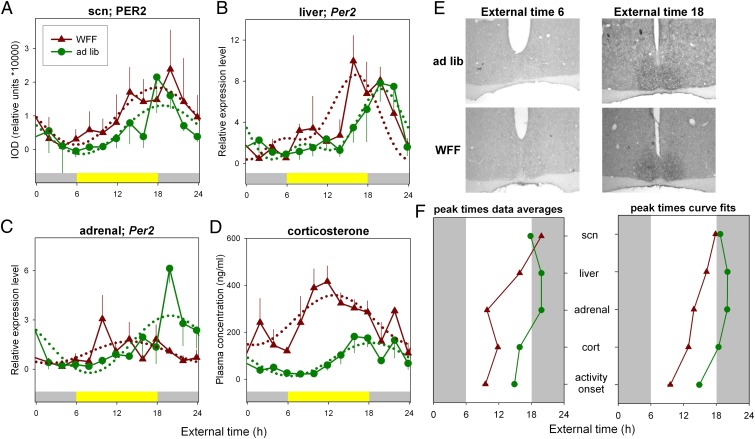
To better understand the mechanisms underlying diurnality in mice, the phase distribution of internal clocks in the SCN and peripheral tissues was assessed (Fig. 3). Patterns of expression of the circadian clock protein period2 (PER2) in the SCN did not change under WFF conditions (F3,63 = 1.23, P = 0.31; Fig. 3A), indicating that the mechanism responsible for a shift to diurnal activity patterns in mice is downstream from the SCN or parallel to it. Gene expression patterns indicate that WFF advanced Per2 mRNA rhythms in liver and adrenal glands by 3.85 h and 6.1 h, respectively (liver: F5,61 = 4.27, P = 0.002; adrenal F3,60 = 5.95, P = 0.001). Elevated plasma corticosterone levels peaked 5.5 h earlier in WFF animals (F3,61 = 23.01, P < 0.0001), which corresponds closely to the earlier activity onset (t21 = 7.43, P < 0.0001).
Fig. 3.
Internal circadian organization changes under simulated food shortage conditions. (A) SCN PER2 protein expression profiles for WFF (red) and ad libitum (green) fed mice do not show a phase shift of the SCN rhythm. Liver (B) and adrenal (C) Per2 mRNA expression is phase advanced in the WFF compared with the ad libitum fed group. (D) The plasma corticosterone concentration profile is phase advanced in mice undergoing the WFF protocol. (E) PER2 protein expression in coronal SCN sections at external time 6 and 18 for WFF and control mice. (F) Timing of peak expression as displayed (A–D) for WFF and ad libitum fed mice. (Left) Peak time using highest average value per day. (Right) Peak time as derived by harmonic regression (CircWave) analysis.
Diurnality Provides Energetic Benefits.
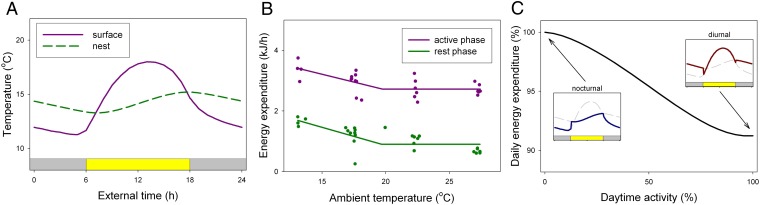
To investigate the possible benefits of the observed shift to diurnality under energetically challenging conditions, the expected energy expenditure for a mouse under natural conditions was quantified. The average daily surface Ta rhythm (September 2001–2010) and associated nest temperature rhythm in our outdoor mouse enclosures (Fig. 4A) were combined with laboratory measurements of energy expenditure during the active and rest phase (Fig. 4B) to calculate daily energy expenditure (Fig. 4C). Because nest temperatures are buffered by thermal capacity of the surrounding material, they typically have lower amplitudes and a delayed phase compared with the surface Ta rhythm (Fig. 4A). Diurnal mammals will therefore encounter higher temperatures than nocturnal mammals (Fig. 4C). When mice are active during the light phase and rest in their nests at night, the average encountered temperature also increases and daily energy expenditure can be reduced by 8.8% (Fig. 4C). This number is likely a minimal estimate because it does not take into account additional energy saving strategies during the rest phase (e.g., thermal insulation, huddling, timing of daily torpor), which are all more efficient during the night than during daytime.
Fig. 4.
Quantification of the energetic benefit of diurnality. (A) Daily variation in average September surface and nest temperatures. The nest temperature is buffered from the surface temperature by nest insulation leading to a later peak phase and reduced amplitude. The expected nest temperature is calculated based on the nest insulation constant measured for mouse nests in our outdoor mouse enclosures. (B) Scholander curves showing the relation between Ta and energy expenditure for the rest and active phase of WFF mice. (C) Calculated daily energy expenditure decreases with increasing daytime activity for mice subjected to natural daily temperature cycles. Daily energy expenditure varies between 60.7 and 55.4 kJ/day. (Insets) The encountered temperatures for a completely nocturnal (Left) and diurnal (Right) mouse. Due to the buffered nest temperature, compared with nocturnal mice, diurnal mice encounter higher temperatures during both their active and rest phase.
Discussion
Together our results show that circadian organization in nocturnal mammals shows considerable phenotypic plasticity in response to energetic challenges. The extent of circadian reorganization depends on the severity of the energetic challenge. The diurnal phenotype in our mice is accompanied by an unchanged SCN phase while peripheral clocks are phase advanced, approaching a situation normally observed in diurnal mammals. In nature, diurnality will generate energetic benefits, which have to be balanced against day/night differences in predation risk and foraging efficiency.
The lack of a phase shift of the clock gene rhythm in the SCN is in line with earlier studies showing that patterns of electrical activity (19, 20), glucose metabolism (21) and Per1 and Per2 clock gene expression (22–25) of the SCN do not differ between nocturnal and diurnal mammal species. The finding that the SCN remains entrained to the LD cycle with a stable phase angle, unaffected by changed phasing of body temperature (18) and glucocorticoid rhythms seems a specific feature of the SCN (26, 27). These results indicate that the mechanism responsible for a shift to diurnal activity patterns in mice is downstream from the SCN or parallel to it. A possible model includes a slave oscillator driving activity and peripheral rhythms (activity oscillator), with its phase relation to the SCN depending on metabolic feedback (Fig. S3).
The phase advance of peripheral oscillators suggest that the diurnal phenotype in mice under WFF conditions indeed approaches the phenotype of a true diurnal mammal where the phase relationship between the LD cycle and the SCN is similar, but the phase relationship between the SCN and peripheral rhythms is reversed (28). The observed phase advances of the liver and adrenal rhythms are comparable to those observed in animals fed during the light phase (e.g., 29), suggesting the feeding rhythm as a possible mechanism behind the phase advance of the peripheral rhythms. The correspondence between the timing of behavior and corticosterone secretion is consistent with a role for the adrenal glands in circadian organization (30).
Induction of diurnality by the energetic challenges described here is consistent with results in mice that become diurnal while earning food on a moderate workload while lactating (31). This finding provides further evidence that a negative energy balance indeed drives the diurnality response. To fully understand how the circadian system organizes behavior and physiology, it is important to understand whether diurnality is directly induced by low Ta or food intake rate, or whether it is induced through a common pathway that signals the energetic state of the animal.
The shift from nocturnality to diurnality seems independent of the actual energetic consequences of the observed phase shift. Under constant Ta, diurnality does not provide energetic benefits, whereas diurnality increases energy expenditure under inverted Ta cycles. The observed induction of diurnality by WFF in constant Ta and inverted Ta cycles therefore indicates that mice in the wild use the highly stable LD cycle as a proxy (proximate factor; ref. 32) for the more variable Ta cycle, to ultimately reach an energetically optimal phase of entrainment (ultimate factor). Our results further indicate that the effect of Ta on phase of entrainment under ad libitum conditions (Fig. 1A) is underestimating the effect of Ta on phase of entrainment under natural conditions, when mice have to search for their food at intermediate workload levels (Fig. 1B). The energetic benefit of diurnality is therefore likely to be one of the important factors involved in shaping the seasonal changes in temporal organization of many species (reviewed in ref. 2). A description of the ultimate consequences of seasonal changes in temporal organization should thus combine the energetic consequences of these changes with seasonal changes in predation risk and foraging opportunities; all being direct or indirect consequences of changing day length.
Under natural conditions, mammals can increase the energetic benefit of diurnality by combining diurnality with different energy saving strategies during the resting phase (e.g., thermal insulation, huddling, daily torpor). Lowering body temperature during the resting phase is one of the main strategies used by energetically challenged mammals to reduced energy expenditure (33). Indeed, mice in the WFF protocol also reduce body temperature during the rest phase (Fig. S4) and may even show daily torpor bouts when challenged by more challenging workloads than used here (18). The CTE hypothesis predicts that further energetic savings can be obtained when the daily torpor bout is synchronized with the coldest part of the day, which can only occur after the activity has shifted to daytime. In line with this prediction, a recent field study found that within a population of golden spiny mice, mice with the lowest food intake not only showed the longest torpor bouts but also concentrated activity during the warmest part of the day (34).
Using the CTE hypothesis we predict that diurnal mammals will not shift when energetically challenged because their activity rhythm is already optimized for minimized energy expenditure. However, a positive energy balance could allow for a more nocturnal temporal organization, which would increase energy expenditure and restore the energy balance. This may even occur in diurnal animals, provided that a change in predation risk does not outweigh the benefits of a restored energy balance. The positive correlation between body mass index and late chronotypes in humans (35) supports the notion that metabolic homeostasis can influence circadian phase of entrainment. The CTE hypothesis also predicts that forced activity during the night in diurnal mammals will increase food intake to compensate for the expected increase in thermoregulatory costs of nocturnality. Forced nighttime activity occurs during night shiftwork in humans, but many nightshift-workers do not encounter the expected lower night temperatures. Additional thermoregulatory costs will therefore be absent and a surplus of energy intake induced by the phase shifted activity rhythm may thus result in obesity and metabolic syndrome. A positive relationship between nighttime activity and obesity and metabolic syndrome has indeed been found in human experiments (36), social jet-lag (35), and night shift workers (37, 38).
Methods
Animals, WFF Procedure, and Activity Analysis.
All procedures were approved by the Animal Experimentation Committee of the University of Groningen (DEC 5454). Three- to 9-mo-old CBA/CaJ mice from our breeding colony were housed in running wheel cages at the experimental temperature in climate controlled rooms in a 12-h:12-h LD cycle (240–420 lux). In the WFF protocol, mice had to earn 45 mg food pellets (612 J per pellet) by running in a wheel while the workload, e.g., the distance (starting workload: 65.3 m/kJ) they had to run to obtain a food pellet, was increased (6.5 m/kJ) daily. The WFF protocol allowed the mice to work and receive food at all times of day.
The WFF protocol was performed at four different constant Tas (10 °C, 15 °C, 20 °C, 25 °C; n = 12 per temperature). Running wheel activity was recorded in 2-min bins and split in daily intervals. The total number of running wheel revolutions during the light phase was divided by the total daily running wheel activity to calculate the daily daytime activity percentage. For ad libitum fed animals (10 °C: n = 20; 15 °C: n = 19; 20 °C: n = 27; 25 °C: n = 10) the individual daytime activity percentage was determined on experimental day 9–18. Changes in activity patterns were analyzed by one-way ANOVA analysis using SAS JMP 7.0.
For the phase shifting experiment (WFF: n = 7; ad libitum: n = 3; Ta = 20 °C), the workload was increased to induce diurnality (day 1 to day 21) and kept constant throughout the rest of the experiment (day 22 to day 51). On day 22, the LD cycle was delayed by 8 h for 18 days after which the LD cycle was shifted to the original phase.
Tissue Sampling, Gene Expression, and Blood Hormone Analysis.
Thirty-six WFF and 36 ad libitum fed male mice housed at 20 °C were killed at 2-h intervals over the 24-h LD cycle (12 time points). Cardiac blood was sampled after which an aortic ligature was placed to enable transcardial brain perfusion with fixative while avoiding perfusion of abdominal organs. Mice were killed by isoflurane overdose (Pharmachemie). Cardiac blood was drawn using heparinized syringes followed by plasma separation (centrifugation at 4 °C and 2,600 × g) and storage at −80 °C. Liver and adrenals were removed, snap frozen in liquid nitrogen and stored at −80 °C. Brains were perfused with 0.01 M PBS (PBS) + 10 units/mL heparin for 2 min followed by 4% (40 g/L) paraform-aldehyde in 0.1 M PBS for 20 min and stored at 4 °C in PBS + 0.1% sodium-azide solution.
Plasma corticosterone was measured by radio immunoassay (RIA, ImmuChem, MP Biomedicals). Relative Per2 mRNA levels in liver and adrenal was measured by standard CYBR green (Applied Biosystems 4385617) real-time quantitative PCR (Applied Biosystems 7500 Fast Real-Time PCR System) using β-actin as a control. Mouse Per2 specific primers (accession NM_011066.3): AGGCACCTCCAACATGCAA (fwd), GGATGCCCCGCTTCTAGAC (rev), and β-actin specific primers (accession NM_007393): CCTCTGAACCCTAAGGCCAA (fwd), AGCCTGGATGGCTACGTACA (rev).
Immunocytochemistry (ICC) analysis for PER2 was performed on 20 μm thin SCN containing brain sections (primary antibody: rabbit anti-mouse PER2 1:2,000, Alpha Diagnostics Per21-A; secondary antibody: biotinylated goat anti-rabbit 1:300, Jackson Immuno Research 111-066-006) using diaminobenzidine (DAB) staining after avidin–biotin amplification (Vectastain Elite, Brunswig Chemie 6-PK-6100). Background corrected integrated optical density analysis in three 60-μm separated medial SCN sections was performed by National Institutes of Health ImageJ software (NIH, http://rsbweb.nih.gov/ij/). Optical density data, gene expression data, and hormone levels were statistically analyzed using forward harmonic regression followed by an F-test on the residuals (CircWave procedure). Differences between WFF and ad libitum groups were tested after fitting a wave function through both groups separately compared with both groups combined.
Surface and Nest Temperatures.
The average daily surface temperature cycle in September (2001–2010) was obtained from the Koninklijk Nederlands Meteorologisch Instituut (KNMI), weather station Eelde, the Netherlands (World Meteorological Organization, station #06280; 53° 07'N, 06° 35'E). The relationship between nest temperature and surface temperature was estimated by calculating the nest insulation constant k in mouse nests in outdoor enclosures at the laboratory in Groningen, the Netherlands (53° 14'N, 06° 32'E). Simultaneously measured nest temperatures and surface temperatures were used to determine k using the Newtonian Cooling Law equation: Tnest = Tnest,previous − k × (Tnest, previous − Tsurface) in 1-min steps. The daily nest temperature profile was calculated using the average September surface temperature cycle and nest insulation constant (k = 0.072 h−1) in steps of 10 min.
Metabolic Measurements and Calculation Energy Expenditure.
Energy expenditure was determined every 4 min by measuring O2 use and CO2 production using a dried air open-circuit indirect calorimetry system (O2: Servomex Xentra 4100; CO2: Servomex 1440; flow: 20 l/h, type 5850 Brooks mass flow controller) at different Tas (Fig. 4B) in high-workload WFF mice. Energy expenditure during the active and rest phase was calculated as the maximal and minimal values respectively of the 2-h running average of measured energy expenditure. Expected energy expenditure was calculated for all temporal niches ranging from nocturnal to diurnal for a mouse with a 12-h activity and 12-h rest phase using the measured energy expenditures during active and rest phase and surface and nest temperature cycles.
Supplementary Material
Acknowledgments
We thank Simon Verhulst and Marcel Visser for their critical comments on the manuscript. This work was funded by the Marie Curie Fellowship 221095 (to V.P.) and an Ubbo Emmius Fellowship (to S.J.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1413135111/-/DCSupplemental.
References
- 1.Gerkema MP, Davies WIL, Foster RG, Menaker M, Hut RA. The nocturnal bottleneck and the evolution of activity patterns in mammals. Proc Biol Sci. 2013;280(1765):20130508. doi: 10.1098/rspb.2013.0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hut RA, Kronfeld-Schor N, van der Vinne V, De la Iglesia H. In search of a temporal niche: Environmental factors. Prog Brain Res. 2012;199:281–304. doi: 10.1016/B978-0-444-59427-3.00017-4. [DOI] [PubMed] [Google Scholar]
- 3.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 4.van der Horst GTJ, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398(6728):627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 5.Shearman LP, et al. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288(5468):1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 6.Preitner N, et al. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110(2):251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 7.Asher G, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134(2):317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 8.Nakahata Y, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134(2):329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Neill JS, Reddy AB. Circadian clocks in human red blood cells. Nature. 2011;469(7331):498–503. doi: 10.1038/nature09702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edgar RS, et al. Peroxiredoxins are conserved markers of circadian rhythms. Nature. 2012;485(7399):459–464. doi: 10.1038/nature11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330(6009):1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalsbeek A, et al. Circadian disruption and SCN control of energy metabolism. FEBS Lett. 2011;585(10):1412–1426. doi: 10.1016/j.febslet.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laposky AD, et al. Altered sleep regulation in leptin-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2006;290(4):R894–R903. doi: 10.1152/ajpregu.00304.2005. [DOI] [PubMed] [Google Scholar]
- 14.Speakman J. Factors influencing the daily energy expenditure of small mammals. Proc Nutr Soc. 1997;56(3):1119–1136. doi: 10.1079/pns19970115. [DOI] [PubMed] [Google Scholar]
- 15.Pittendrigh CS. On temperature independence in the clock system controlling emergence time in Drosophila. Proc Natl Acad Sci USA. 1954;40(10):1018–1029. doi: 10.1073/pnas.40.10.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96(2):271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 17.Sawyer LA, et al. Natural variation in a Drosophila clock gene and temperature compensation. Science. 1997;278(5346):2117–2120. doi: 10.1126/science.278.5346.2117. [DOI] [PubMed] [Google Scholar]
- 18.Hut RA, Pilorz V, Boerema AS, Strijkstra AM, Daan S. Working for food shifts nocturnal mouse activity into the day. PLoS ONE. 2011;6(3):e17527. doi: 10.1371/journal.pone.0017527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inouye ST, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci USA. 1979;76(11):5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato T, Kawamura H. Circadian rhythms in multiple unit activity inside and outside the suprachiasmatic nucleus in the diurnal chipmunk (Eutamias sibiricus) Neurosci Res. 1984;1(1):45–52. doi: 10.1016/0168-0102(84)90029-4. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz WJ, Reppert SM, Eagan SM, Moore-Ede MC. In vivo metabolic activity of the suprachiasmatic nuclei: A comparative study. Brain Res. 1983;274(1):184–187. doi: 10.1016/0006-8993(83)90538-3. [DOI] [PubMed] [Google Scholar]
- 22.Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: Differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998;20(6):1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]
- 23.Lambert CM, Machida KK, Smale L, Nunez AA, Weaver DR. Analysis of the prokineticin 2 system in a diurnal rodent, the unstriped Nile grass rat (Arvicanthis niloticus) J Biol Rhythms. 2005;20(3):206–218. doi: 10.1177/0748730405275135. [DOI] [PubMed] [Google Scholar]
- 24.Lincoln G, Messager S, Andersson H, Hazlerigg D. Temporal expression of seven clock genes in the suprachiasmatic nucleus and the pars tuberalis of the sheep: Evidence for an internal coincidence timer. Proc Natl Acad Sci USA. 2002;99(21):13890–13895. doi: 10.1073/pnas.212517599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vosko AM, Hagenauer MH, Hummer DL, Lee TM. Period gene expression in the diurnal degu (Octodon degus) differs from the nocturnal laboratory rat (Rattus norvegicus) Am J Physiol Regul Integr Comp Physiol. 2009;296(2):R353–R361. doi: 10.1152/ajpregu.90392.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oster H, et al. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4(2):163–173. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330(6002):379–385. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambert CM, Weaver DR. Peripheral gene expression rhythms in a diurnal rodent. J Biol Rhythms. 2006;21(1):77–79. doi: 10.1177/0748730405281843. [DOI] [PubMed] [Google Scholar]
- 29.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291(5503):490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 30.Pezük P, Mohawk JA, Wang LA, Menaker M. Glucocorticoids as entraining signals for peripheral circadian oscillators. Endocrinology. 2012;153(10):4775–4783. doi: 10.1210/en.2012-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perrigo G. Breeding and feeding strategies in deer mice and house mice when females are challenged to work for their food. Anim Behav. 1987;35:1298–1316. [Google Scholar]
- 32.Baker JR. 1938. The evolution of breeding seasons. Evolution: Essays on Aspects of Evolutionary Biology, ed DeBeer J (Clarendon, Oxford), pp 161–177.
- 33.Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol. 2004;66:239–274. doi: 10.1146/annurev.physiol.66.032102.115105. [DOI] [PubMed] [Google Scholar]
- 34.Levy O, Dayan T, Rotics S, Kronfeld-Schor N. Foraging sequence, energy intake and torpor: An individual-based field study of energy balancing in desert golden spiny mice. Ecol Lett. 2012;15(11):1240–1248. doi: 10.1111/j.1461-0248.2012.01845.x. [DOI] [PubMed] [Google Scholar]
- 35.Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22(10):939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 36.Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106(11):4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pietroiusti A, et al. Incidence of metabolic syndrome among night-shift healthcare workers. Occup Environ Med. 2010;67(1):54–57. doi: 10.1136/oem.2009.046797. [DOI] [PubMed] [Google Scholar]
- 38.Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: Two prospective cohort studies in women. PLoS Med. 2011;8(12):e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.