Significance
Alcohol-dependent subjects frequently develop emotional symptoms that contribute to the persistence of alcohol drinking. These subjects are also characterized by gastrointestinal disturbances. In this study, we showed that alcohol-dependent subjects with altered intestinal permeability had also altered gut-microbiota composition and activity and remained with high scores of depression, anxiety, and alcohol craving after a short-term detoxification program. These results are consistent with the existence of a gut–brain axis in alcohol dependence, in which the gut microbiota could alter the gut-barrier function and influence behavior in alcohol dependence. Therefore, this study opens a previously unidentified field of research for the treatment and the management of alcohol dependence, targeting the gut microbiota.
Keywords: alcohol dependence, gut permeability, gut microbiota, gut–brain axis, behavior
Abstract
Alcohol dependence has traditionally been considered a brain disorder. Alteration in the composition of the gut microbiota has recently been shown to be present in psychiatric disorders, which suggests the possibility of gut-to-brain interactions in the development of alcohol dependence. The aim of the present study was to explore whether changes in gut permeability are linked to gut-microbiota composition and activity in alcohol-dependent subjects. We also investigated whether gut dysfunction is associated with the psychological symptoms of alcohol dependence. Finally, we tested the reversibility of the biological and behavioral parameters after a short-term detoxification program. We found that some, but not all, alcohol-dependent subjects developed gut leakiness, which was associated with higher scores of depression, anxiety, and alcohol craving after 3 wk of abstinence, which may be important psychological factors of relapse. Moreover, subjects with increased gut permeability also had altered composition and activity of the gut microbiota. These results suggest the existence of a gut–brain axis in alcohol dependence, which implicates the gut microbiota as an actor in the gut barrier and in behavioral disorders. Thus, the gut microbiota seems to be a previously unidentified target in the management of alcohol dependence.
Alcohol consumption is the world’s third largest risk factor for disease and disability and accounts for 5.9% of all deaths worldwide (1). Although alcohol exerts large deleterious effects on health, studies to date on the pathophysiology of alcohol dependence have mainly focused on the influence of alcohol consumption on neuronal functions in the brain (2). A limited number of studies have, however, suggested that gut functions might also be altered by chronic alcohol consumption (3, 4). Accordingly, we and others have shown that actively drinking alcohol-dependent (AD) subjects exhibited increased intestinal permeability (IP) and increased plasma levels of gut-derived bacterial products such as lipopolysaccharides and peptidoglycans (5–8). These bacterial products activate specific inflammatory pathways that partially recover after a 3-wk period of alcohol abstinence (5, 6). These recent observations indirectly suggest the possibility that the composition of gut microbiota could be altered in AD subjects and related to behavioral symptoms.
The human gut microbiota consists of a complex community exceeding 100 trillion microorganisms (9) whose collective genome—the microbiome—encodes 100 times more genes than the human genome (10). It is now widely accepted that the gut microbiota should be considered an “exteriorized” organ placed within the body, which provides important physiological functions and is indispensable for human life (10–12). However, the microbial composition or activity of the gut can be modified by diet, antibiotic use, host genetics, and other environmental factors (13). Data suggest that an imbalance of the intestinal microbiota, known as dysbiosis, may contribute to a variety of somatic diseases such as obesity (14), type 2 diabetes (15), inflammatory bowel diseases (16, 17), and allergy (18).
Recent studies suggest that the gut bacteria also influence brain functions and behavior and may therefore play a role in the development of psychiatric disorders (19). Indeed, in experimental studies, researchers observed that germfree mice displayed reduced anxiety-like behavior compared with mice with normal gut microbiota, demonstrating evidence of gut-to-brain interactions (20, 21). Further studies brought forward evidence that the pathways underlying the gut–brain axis are multiple and highly complex, involving brain biochemistry, the vagus nerve, proinflammatory cytokines, and tryptophan metabolism (22). Furthermore, inflammation and tryptophan/kynurenine pathways have been related to the development of depression-like behavior (23–26). In addition, gut bacteria produce neurotransmitters (serotonin, GABA, dopamine, acetylcholine), and bacterial fermentation of dietary fiber induces the release of short-chain fatty acids, which are metabolites with potential neuroactive properties (22). Recent evidence also suggests that Bacteroides fragilis may prevent autism spectrum disorder in a mouse model (27) and that administration of probiotic Bifidobacterium infantis may have antidepressant properties in rats through changes in the tryptophan/kynurenine pathway (26). Although several animal studies support a relation between the gut microbiota and behavior, major questions remain regarding this relation in human health.
Depression and anxiety frequently develop in actively drinking AD subjects and play an important role in the negative reinforcement of drinking tendency (28). These factors are strongly related to the urge to drink, hereafter referred to as alcohol craving (29, 30), an important predictor of relapse after detoxification (31). The possibility that these psychological symptoms of addiction are related to a dysbiosis has so far never been investigated. The aim of the present study was to determine whether gut permeability could be associated to the severity of psychological symptoms (depression, anxiety, and craving) developed by human AD subjects. Then, we assessed the composition and activity of the gut microbiota and tested whether they are related to gut permeability. Finally, we analyzed whether alterations in gut permeability, microbiota composition, and metabolome are reversible after 3 wk of alcohol withdrawal, which is known to induce partial recovery of psychiatric symptoms (32).
Results
IP Is Increased in a Subset of AD Subjects.
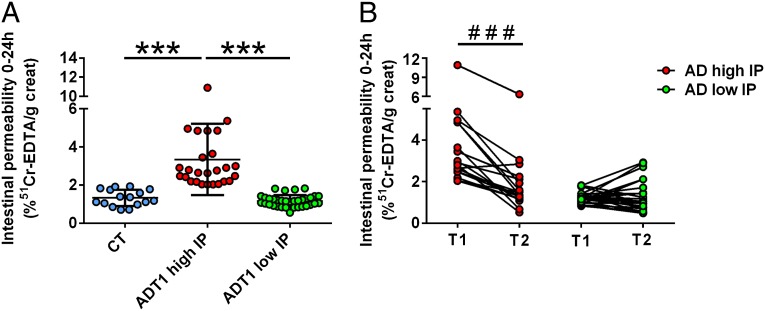
Intestinal permeability was measured by using the 51Cr-EDTA method. Results revealed that, at the second day of alcohol withdrawal (T1), 26 of 60 (43%) patients had elevated gut permeability whereas the remaining 34 (57%) patients had normal gut permeability compared with control subjects (Fig. 1A). Subjects were therefore split into two groups: AD patients with “high” IP and AD patients with “low” IP. This separation of subjects was calculated according to a deviance criterion at a threshold of 1.65 SDs of the mean of the control group. In a normal distribution, this deviance criterion corresponds to the fifth percentile, which is a common threshold to highlight deviance from the mean. The gut permeability level was not related to the amount of alcohol consumed, which was similar in both subgroups of patients (P = 0.72). The demographic characteristics of the subjects included in this preliminary study are shown in Table 1. After 19 d of alcohol abstinence (T2), the gut permeability of AD subjects with high IP decreased significantly, the mean being equivalent to that observed in the control group and in the group of AD subjects with low IP at T1 (Fig. 1B). The detailed results of small-bowel and colon permeabilities are presented in Table S1. We also examined the type of alcoholic beverages consumed by each subject and found that, on average, the consumption of beer was similar in both groups of AD patients (with high and low IP). However, the consumption of wine tended to be lower in AD subjects with high IP, and the consumption of spirits tended to be higher in AD subjects with high IP (Fig. S1).
Fig. 1.
Intestinal permeability was measured by using the 51Cr-EDTA method. (A) Results revealed that, at T1, 26 of 60 patients had elevated gut permeability whereas the remaining 34 patients had normal gut permeability compared with control subjects. Subjects were therefore split into two groups: AD patients with “high” IP and AD patients with “low” IP. (B) A 3-wk alcohol withdrawal induced a total recovery of gut permeability in AD subjects with high IP. Subjects that relapsed during the detoxification program were excluded from analysis, and results were obtained in 43 subjects. AD, alcohol-dependent subjects; CT, control subjects; IP, intestinal permeability. T1 and T2 refer to the beginning and end of alcohol withdrawal, respectively. ***P < 0.001, ###P < 0.001.
Table 1.
Demographic and clinical characteristics of the control and alcohol-dependent groups in the preliminary and main studies
| Study data | CT | AD high IP | AD low IP |
| Preliminary study | |||
| No. of subjects | 15 | 26 | 34 |
| Sex | 8M/7F | 19M/7F | 28M/6F |
| Age, y | 48 ± 11 | 48 ± 11 | 48 ± 10 |
| BMI, kg/m2 | 26.1 ± 3.1 | 23.3 ± 3.9 | 24.8 ± 4.7 |
| Alcohol intake, g/d | 11 ± 7 | 188 ± 84*** | 177 ± 104*** |
| Main study | |||
| No. of subjects | 15 | 6 | 7 |
| Sex | 8M/7F | 3M/3F | 5M/2F |
| Age, y | 48 ± 11 | 52 ± 9 | 52 ± 10 |
| BMI, kg/m2 | 26.1 ± 3.1 | 24.6 ± 4.5 | 26.4 ± 5.3 |
| Alcohol intake, g/d | 11 ± 7 | 126 ± 55 *** | 145 ± 86 *** |
| Albumin, g/dL | nd | 4.40 ± 0.36 | 4.46 ± 0.30 |
| Prealbumin, mg/dL | nd | 30.7 ± 6.8 | 34.4 ± 5.4 |
Data are means ± SD. AD high IP and AD low IP refer to alcohol-dependent subjects with high and low intestinal permeability, respectively. CT, control group; F, female; M, male; nd, not defined. ***P < 0.001 (AD vs. CT).
Gut-Barrier Alteration Is Associated with the Persistence of Psychological Symptoms at the End of Alcohol Withdrawal.
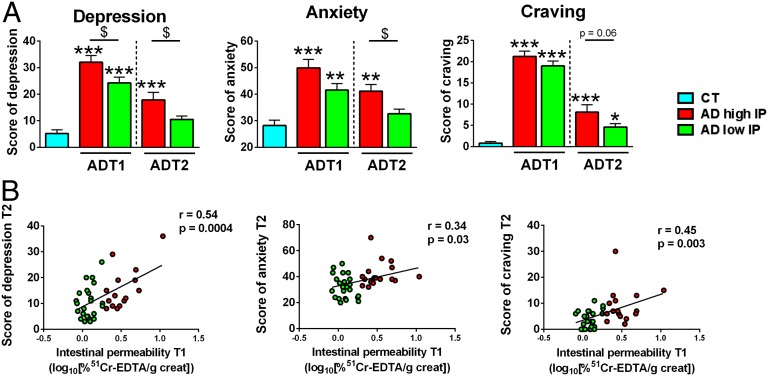
We assessed the psychological status of AD subjects because alcohol dependence is firstly a psychiatric disorder. At the beginning of detoxification, all psychological scores (depression, anxiety, and alcohol craving) were higher in AD subjects than in controls, as described in our previous study (4). Alcohol withdrawal is known to be associated with an improvement in psychological symptoms. Indeed, we found that, at the end of the detoxification, the depression and anxiety scores of AD subjects with low IP recovered completely and returned to the same level as that of controls. However, the AD subjects with high IP were still characterized by higher levels of depression, anxiety, and craving (Fig. 2A). Correlation analysis revealed that IP measured at the beginning of withdrawal was positively associated with all of the psychological symptoms measured at the end of the detoxification program (Fig. 2B). These results suggest that the gut-barrier function could be involved in the persistence of psychological symptoms after detoxification.
Fig. 2.
Increased intestinal permeability of AD subjects was associated with the persistence of psychological symptoms at the end of alcohol withdrawal. (A) Scores of psychological factors in CT and AD subjects at the beginning (T1) and end (T2) of withdrawal showing that AD subjects with high IP had higher score of depression, anxiety, and alcohol craving at T2. *P < 0.05 vs. CT; **P < 0.01 vs. CT; ***P < 0.001 vs. CT; $P < 0.05. (B) Associations of IP measured at T1 with psychological factors assessed at T2. Values are Pearson’s moment correlation coefficients. AD subjects with high IP and low IP are depicted in red and green, respectively. AD, alcohol-dependent; CT, control; IP, intestinal permeability.
The Gut-Microbiota Profile Is Altered in AD Subjects with High IP.
In a subset of 13 AD subjects, we analyzed the gut-microbiota composition and functionality and tested whether they could be related to the gut permeability. The demographic and clinical characteristics of these 13 subjects, which were also split into high and low IP groups, are shown in Table 1. Both groups of AD subjects had elevated concentrations of all inflammatory markers (TNFα, IL-1β, IL-6, IL-8, and IL-10) and of high-sensitivity C-reactive protein (Fig. S2). However, the level of IL-8 was significantly higher in AD subjects with high IP than it was in AD subjects with low IP, and this cytokine was positively correlated with IP (r = 0.79, P = 0.01).
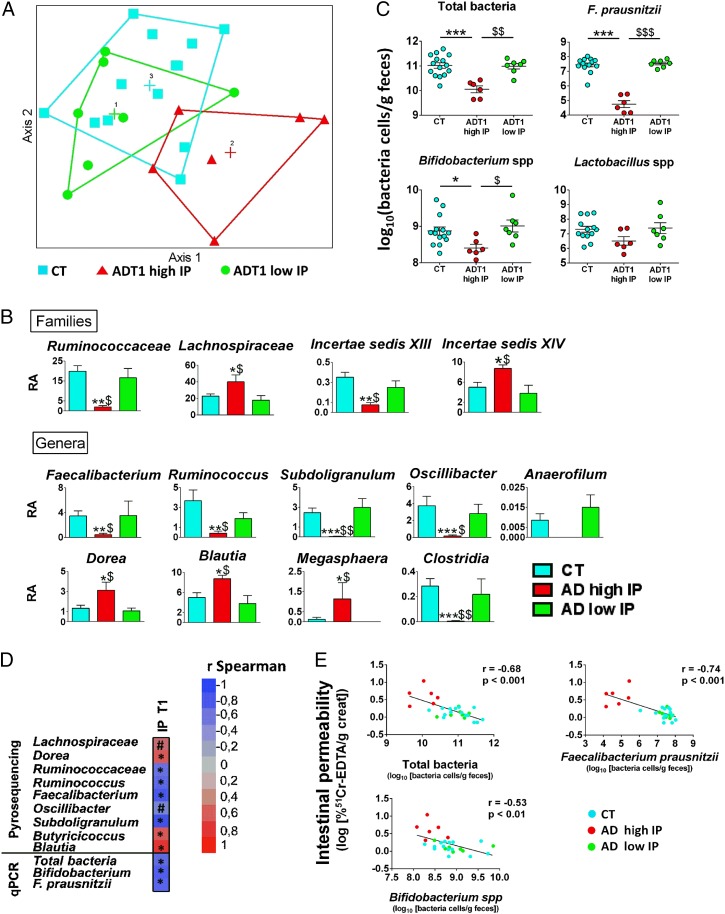
The overall gut-microbiota composition was analyzed by pyrosequencing of the 16S rRNA gene, and profiles of microbial abundance were obtained from each subject. Nonmetric multidimensional scaling revealed that the bacterial profiles of AD subjects with a high IP differed from those of the controls and the AD subjects with low IP (Fig. 3A). We then investigated which bacterial groups were responsible for the changes observed in the profile of AD subjects with high IP. We did not find significant differences between the three groups of subjects at the phylum level of the bacteria. However, at the family level, bacteria from Ruminococcaceae and Incertae Sedis XIII were less abundant whereas those from Lachnospiraceae and Incertae Sedis XIV were more abundant in AD subjects with high IP compared with AD subjects with low IP and controls (Fig. 3B). At the genus level of the bacterial groups, AD subjects with high IP had a drastic decrease in the abundance of Ruminococcus, Faecalibacterium, Subdoligranulum, Oscillibacter, and Anaerofilum. All of these genera belong to the Ruminococcaceae family. The abundance of Dorea, which belongs to the family Lachnospiraceae, was increased in AD subjects with high IP. Additionally, the genera Blautia and Megasphaera were increased whereas Clostridia was decreased in AD subjects with high IP (Fig. 3B). These analyses also revealed that the relative abundance of the taxa mentioned above was similar in AD subjects with low IP and the controls.
Fig. 3.
Gut-microbiota profiles of AD subjects with high and low intestinal permeability at the beginning of alcohol withdrawal. (A) Gut-bacterial profiles were calculated for each subject using the abundance of the bacterial families from 454 pyrosequencing data. Bacterial taxa for which the sum of sequences in all of the samples was less than 0.01% of the total number of sequences were removed from the analysis. Hellinger transformation was applied to the resulting matrix. Subjects were plotted in the map by using nonmetric multidimensional scaling. (B) Relative abundance of bacterial families and genera at the beginning of withdrawal. No significant differences were observed between AD subjects with low IP and controls. Differences observed between AD subjects with high IP and the other two groups are depicted. Results of relative abundance obtained from pyrosequencing are expressed in the percentage of sequences/taxon. *P < 0.05 vs. CT, **P < 0.01 vs. CT, ***P < 0.001 vs. CT, $P < 0.05 vs. ADT1 low IP, $$P < 0.01 vs. ADT1 low IP. (C) Total bacteria, F. prausnitzii, Bifidobacterium spp., and Lactobacillus spp. were quantified by qPCR in CT and AD subjects at the onset (T1) of alcohol withdrawal. *P < 0.05, $P < 0.05, $$P < 0.01, ***P < 0.001, $$$P < 0.001. (D) Chart depicting the correlations between IP and gut bacteria detected by pyrosequencing and qPCR methods at the beginning of detoxification. Asterisk indicates significant correlations (P < 0.05) and # indicates 0.1 < P < 0.05. (E) Correlations between IP at T1 and gut bacteria measured by qPCR. r indicates Pearson’s coefficient. AD subjects with high IP and low IP are depicted in red and green, respectively. AD, alcohol dependent; CT, control; IP, intestinal permeability; RA, relative abundance. T1 refers to the beginning of alcohol withdrawal.
The abundance of common bacterial species was also assessed by using quantitative PCR (qPCR) (Fig. 3C). We found that the total amount of bacteria was significantly lower in AD subjects with high IP compared with the other two groups. We then quantified the level of Faecalibacterium prausnitzii, a bacterial species known for its antiinflammatory properties (33) and found that it was drastically decreased (up to 4 log units) in AD subjects with high IP. This result is consistent with those obtained with the pyrosequencing approach. In addition, F. prausnitzii was negatively correlated with plasma IL-8 levels (r = −0.65, P = 0.003). Finally, we assessed the levels of Bifidobacterium spp. and Lactobacillus spp. and found that the level of Bifidobacterium spp. was significantly lower in AD subjects with high IP compared with controls and AD subjects with low IP. The decrease was not significant for the level of Lactobacillus spp.
An Altered Microbiota Composition Is Associated with Gut-Barrier Dysfunction.
Because only the AD subjects who presented increased IP had an altered gut-microbiota composition compared with control subjects, we hypothesized that some bacteria could be involved in the regulation of the gut-barrier function. We therefore tested the correlations between gut bacteria and IP at T1 (Fig. 3 D and E). Our analysis revealed a negative correlation between IP and the total amount of bacteria, indicating that subjects with a low number of bacteria in the gut had a higher IP. Negative correlations were also found for the bacteria belonging to the Ruminococcaceae family, especially for F. prausnitzii. Bifidobacterium was also negatively correlated with IP. The genera Dorea and Blautia that were increased in AD subjects with high IP were positively correlated with IP. These results support the hypothesis that the microbiota composition may influence gut-barrier function.
Effect of Short-Term Alcohol Withdrawal on Gut-Microbiota Composition.
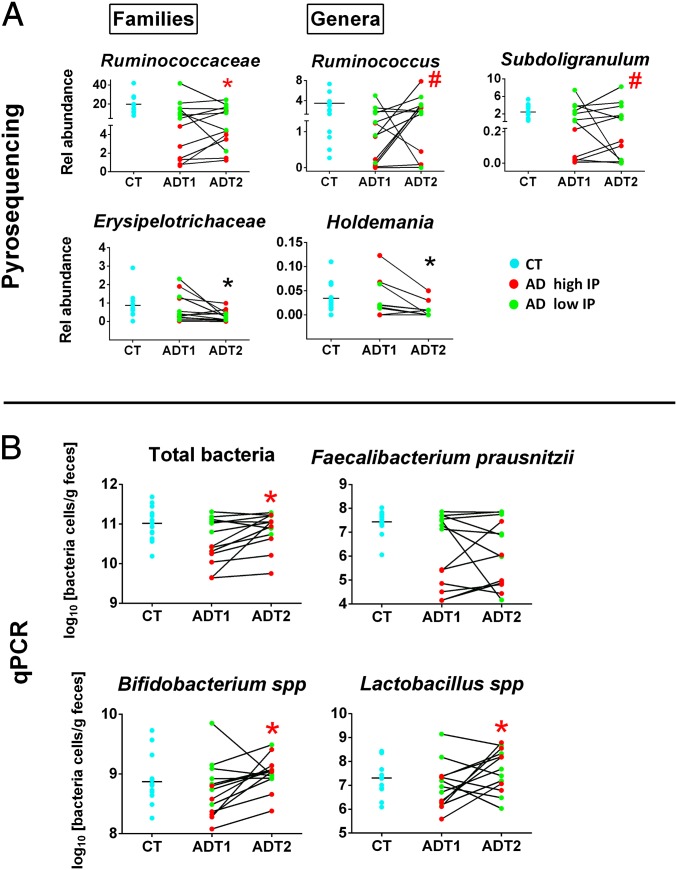
The microbial composition of AD subjects was also assessed at the end of a 3-wk detoxification program (T2). We found that alcohol abstinence induced a significant increase in Ruminococcaceae in subjects with high IP. The genera Ruminococcus and Subdoligranulum also increased at T2 although not significantly. However, the family Erysipelotrichaceae and the genus Holdemania decreased significantly from T1 to T2 in all AD subjects (Fig. 4A). Alcohol withdrawal had no impact on the abundance of the other families or genera that were found to be modified in AD subjects with high IP at T1. However, qPCR analysis revealed that the total amount of bacteria, as well as the levels of Bifidobacterium spp. and Lactobacillus spp., increased significantly during withdrawal in AD subjects with high IP and returned to the levels of controls (Fig. 4B). In contrast, the levels of F. prausnitzii remained unchanged at the end of the detoxification program (Fig. 4B).
Fig. 4.
Effect of alcohol withdrawal on gut-microbiota composition. (A) A significant increase in Ruminococcaceae was observed from T1 to T2 in AD subjects with high IP (*P < 0.05). The genera Ruminococcus and Subdoligranulum also increased during withdrawal in AD subjects with high IP but not significantly (#P = 0.11). The family Erysipelotrichaceae and the genus Holdemania decreased significantly during withdrawal (*P < 0.05) in all subjects. Results of relative abundance obtained from pyrosequencing are expressed in the percentage of sequences/taxon. (B) Abundance of total bacteria, F. prausnitzii, Bifidobacterium spp., and Lactobacillus spp. after 3 wk of alcohol abstinence as measured by qPCR. *P < 0.05 in AD high IP from T1 to T2. AD high IP and low IP are depicted in red and green, respectively. AD, alcohol dependent; CT, control; IP, intestinal permeability. T1 and T2 refer to the beginning and end of alcohol withdrawal, respectively.
The Metabolic Profile Is Altered in AD Subjects with Gut-Barrier Dysfunction.
Metabolomic analyses of fecal samples were also performed in the subset of 13 AD subjects to investigate whether bacterial metabolites could be associated with the altered gut-barrier function. A total of 155 volatile organic compounds (VOCs) were identified. Subject-specific compounds and metabolites present in less than 20% of subjects in both groups (AD and control) were discarded from statistical analysis. Ninety-nine VOCs remained and were considered in the analysis of metabolic profiles. No outlier was detected with principal component analysis. Thirty-eight compounds were common to 80% of the samples (Table S2). Some metabolites, including 2-methyl-1-butanol and methanethiol, were commonly found in controls but were absent in AD subjects (Table S3). On the other hand, metabolites belonging to alcohols, alkanes, and benzenes were found only in AD and not in control subjects (Table S4).
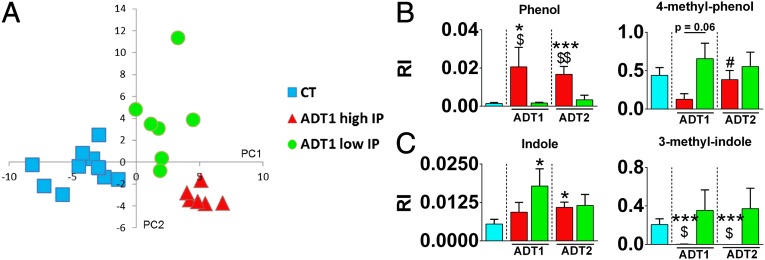
We then performed additional analyses that considered the gut-barrier function of the individuals. Partial least squares-discriminant analysis (PLS-DA) revealed that the metabolic profiles of AD subjects with high IP differed from those with low IP (Fig. 5A). The corresponding loading plot (Fig. S3), showing the metabolites, was used to identify discriminating metabolites, whose relative indices are shown in Fig. S4. Among them, phenolic and indolic compounds, which arise from the metabolism of aromatic amino acids, were found to be associated with the gut-barrier status. Phenol was present in high amount in patients with high IP whereas it was almost absent in subjects with low IP (Fig. 5B). However, the level of 4-methyl phenol was higher in AD subjects with low IP compared with AD subjects with high IP (Fig. 5B). Indole and 3-methyl indole were present in high amounts in AD subjects with low IP but were lower or almost totally absent in AD subjects with high IP (Fig. 5C).
Fig. 5.
Metabolomic profiling of AD subjects with high and low IP. (A) Score plots showing clustering of the metabolite profiles analyzed with partial least squares-discriminant analysis. (B and C) Volatile organic compounds belonging to the chemical classes (B) phenols and (C) indoles. *P < 0.05 compared with CT, ***P < 0.001 compared with CT, $P < 0.05 AD high IP vs. AD low IP at the same study time, $$P < 0.01 AD high IP vs. AD low IP at the same study time, #P < 0.05 compared with ADT1 high IP. AD subjects with high IP and low IP are depicted in red and green, respectively. CT subjects are depicted in blue. AD, alcohol-dependent subjects; CT, control subjects; IP, intestinal permeability; RI, relative indices. T1 and T2 refer to the beginning and end of alcohol withdrawal, respectively.
Discussion
Gut Permeability is Associated with the Severity of Behavioral Markers of Alcohol Dependence.
A limited number of human studies (7, 34–37) have analyzed gut permeability in AD subjects, who are often also diagnosed with alcoholic liver disease. Most of these studies reported an increase in small-bowel permeability whereas a few pointed to increased colon permeability. We have observed two different clusters of AD subjects with distinct permeability features. The high IP group of subjects had a large increase in small-bowel and colon permeabilities that recovered after a detoxification program. In the low IP group, the gut-barrier function remained normal throughout the process.
AD subjects also presented with psychological symptoms, including depression, anxiety, and alcohol craving, which contribute to the negative reinforcement process, a major mechanism involved in the persistence of alcohol dependence (38) that is related to a higher probability of relapse after detoxification. In our previous studies (5, 6), we observed that alcohol withdrawal induced only a partial recovery of these behavioral markers. The present study shows that the recovery of these markers is not evenly distributed among AD subjects. AD subjects with low IP recovered completely at T2 for depression and anxiety. This population seems to present with a less severe form of dependence where affective symptoms recover after detoxification. Conversely, in AD subjects with high IP, the scores of depression, anxiety, and craving remained largely increased, even when they had stopped drinking for more than 2 wk. These observations suggest that gut permeability is related to psychological status at the end of alcohol withdrawal.
If gut permeability does play a role in behavioral changes, one must pay attention to the potential mechanisms by which alterations of the gut-barrier function occur. The possibility of a toxic effect of ethanol on the small-bowel epithelium has been described in healthy subjects (34, 39) and in in vitro studies (40–43). However, patients from both groups consumed the same amount of alcohol, which lessens the implication that ethanol itself is involved in permeability disturbance. The difference in IP in this context might be ascribed to changes in microbial composition and activity. This hypothesis is supported by a rodent study in which antibiotic treatment abolished the ethanol-induced increase in colonic paracellular permeability (44).
Increased Gut Permeability Is Associated with Dysbiosis in AD Subjects.
Consistent with the hypothesis that dysbiosis is linked to gut-barrier alteration, only the subgroup of AD subjects with high IP had altered gut-microbiota composition, which consisted of a large decrease in the overall bacterial load, a drastic decrease in abundance in the Ruminococcaceae family (Ruminococcus, Faecalibacterium, Subdoligranulum, Oscillibacter, and Anaerofilum), and an increase in abundance in the Lachnospiraceae family (Dorea) and the genus Blautia.
Preclinical studies have shown that chronic ethanol administration induces in rats a dysbiosis (45), and in mice a decrease in the level of Ruminococcaceae (46), or a decrease in the level of Firmicutes and an increase in Bacteroidetes (47). In humans, few studies have evaluated the gut microbiome of AD subjects and never in relation to gut permeability. Kirpich et al. (48) observed a decrease in Bifidobacterium and Lactobacillus in the stool cultures of AD subjects compared with those of healthy controls. In 2012, Mutlu et al. showed alterations of the mucosal-associated colonic microbiome in only a subset (31%) of AD subjects (49), indicating that not all alcoholics were dysbiotic, which is in line with our observation that only part of the AD patients had an altered gut-microbiota profile. Furthermore, in the same study, the dysbiotic group included actively drinking and sober alcoholics (>1 mo), suggesting long-lasting dysbiosis, which is consistent with the incomplete recovery of the gut microbiota that we observed after 3 wk of abstinence. Interestingly, we observed that Lactobacillus spp. and Bifidobacterium spp., as well as bacteria from the family Ruminococcaceae, increased during alcohol abstinence, suggesting that these bacteria, known to have a beneficial impact on gut-barrier function (50), could contribute to the recovery of IP at T2. This suggestion is supported by the strong negative correlations that we observed between IP and Bifidobacterium as well as Ruminococcaceae bacteria, particularly F. prausnitzii. This species is also depleted in Crohn’s disease (33) and ulcerative colitis (51) and has been shown to have antiinflammatory properties both in vitro and in vivo (33) and therefore seems to be crucial for gut homeostasis. Indeed, supernatants from F. prausnitzii cultures inhibited IL-8 secretion and NF-κB activation in Caco-2 cells stimulated with IL-1β (33). In our study, AD patients who presented with low levels of F. prausnitzii had higher plasma IL-8 levels, and these variables were significantly and negatively correlated. Taken together, our results show that alterations in microbial composition are associated with increased IP and increased plasma levels of proinflammatory cytokines.
We also hypothesize that metabolites produced by gut bacteria might be at the origin of gut-barrier dysfunction and inflammation. The metabolic profiles calculated by PLS-DA were clearly distinct between control, low IP, and high IP AD subjects. Overall, this observation supports the existence of a relation between metabolites and IP. Basically, two types of microbial fermentation occur in the colon: saccharolytic fermentation and proteolytic fermentation (52). The former is generally considered to be beneficial to the host, and the latter is presumed to be detrimental and might be involved in the etiology of colon cancer and ulcerative colitis (53). The main products of carbohydrate fermentation (i.e., SCFA), which present with beneficial functions (54), were not different between AD and control subjects and unrelated to IP. Metabolite differences among groups therefore resulted mainly from the protein fermentation that leads to the formation of branched-chain fatty acids, indolic compounds, and potentially toxic metabolites such as phenolic and sulfur-containing compounds. The production of phenolic compounds in the gut depends on microbial composition (55) or microbial metabolic activities (56). Phenol that derives from tyrosine breakdown was largely increased in high IP AD subjects compared with the other two groups. The toxic effect of phenol on intestinal epithelial cells has been demonstrated in two independent in vitro studies (57, 58), suggesting that phenol is a potential driver of gut-barrier alterations. Another phenolic compound, 4-methyl phenol (also called p-cresol), was decreased in AD subjects with high IP compared with the other two groups and increased upon alcohol withdrawal; the latter effect could be associated with the increase in Lactobacillus spp., Bifidobacterium spp., and Ruminococcaceae from T1 to T2 observed in AD subjects with high IP because these genera and this family have been shown to be related to the production of p-cresol (59–62). Tryptophan bacterial metabolism results in a large variety of indolic compounds (62–65). In in vitro studies, indolic compounds were shown to improve intestinal cell barrier function and to decrease proinflammatory IL-8 expression (66, 67). Interestingly, the level of 3-methylindole was completely blunted in high IP AD subjects who also presented with a higher IL-8 plasma level. Moreover, a study showed that the beneficial impact of probiotics on gut-barrier function was induced by a protein factor (50). Taken together, these observations are consistent with a protective role of gut microbes that produce indolic compounds and p-cresol on the gut barrier and inflammation and with a detrimental role of bacteria producing phenol.
Study Limitation.
The existence of distinct populations of AD subjects with differences in intestinal permeability that are related to differences in psychological symptoms could be established on a large group. There is, however, one limitation of this study that is the size of the sample for which we could obtain large details on the composition and function of the gut microbiota and on inflammation. The small size of the sample is due to the fact that deep analysis of gut microbiota is expensive and time consuming and that we used a test–retest design that doubles the number of samples.
In conclusion, to our knowledge, this is the first study to investigate gut permeability and gut-microbiota composition and activity and their relationship with the behavioral markers of addiction severity in AD subjects (Fig. 6). The observation that some, but not all, AD subjects develop gut leakiness indicates that chronic alcohol dependence is necessary but not sufficient to cause gut dysfunction. Thus, other cofactors besides direct toxicity of alcohol may be involved. Here, we showed that AD subjects presenting with increased IP also had altered gut-microbial composition and activity. These results strongly suggest that the bacteria present in the gut and/or the metabolites produced by the bacteria may be involved in the regulation of the gut-barrier function and could therefore contribute to the indirect toxicity of alcohol consumption. Moreover, we showed that AD subjects with gut dysfunction had higher scores for depression, anxiety, and alcohol craving at the end of the detoxification program, which is expected to influence the probability of relapse through a negative reinforcement mechanism. These results suggest the existence of a gut–brain axis in alcohol dependence, in which the gut microbiota could alter the gut barrier and influence the severity of alcohol-dependence behaviors. However, the mechanisms underlying the communication between the gut and the brain have not been analyzed in this study but deserve further investigation, in particular the involvement of the vagus nerve as well as the tryptohan/kynurenine pathway in alcohol-dependent subjects.
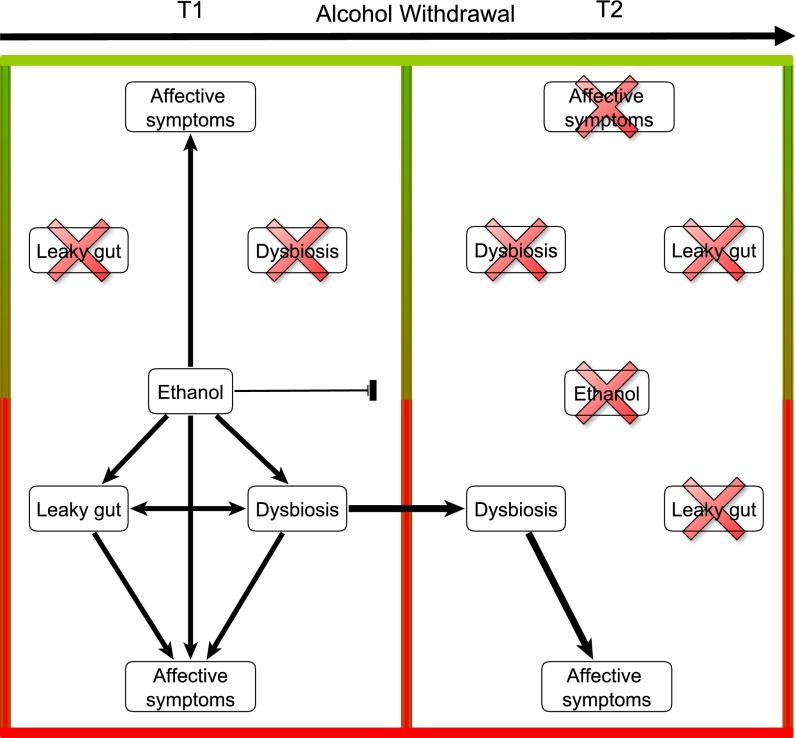
Fig. 6.
Model representing the relationship among alcohol consumption, gut dysfunction, and affective symptoms in the two subsets of alcohol-dependent subjects at both times of alcohol withdrawal. At the beginning of withdrawal (T1), a subset of AD subjects developed affective symptoms that were likely induced by ethanol and that were not associated with gut disorders. In the other subset of AD subjects, alcohol consumption was associated with gut leakiness, gut-microbiota alterations, and affective symptoms. After 3 wk of abstinence (T2), affective symptoms recovered completely in the subset of AD subjects that did not present with gut dysfunction at T1. In the other subset of AD subjects, the gut barrier was restored upon abstinence, but gut dysbiosis was still present at T2 and might be responsible for the persistence of affective symptoms. From these observations, we hypothesize that gut-microbiota alterations could be associated with a more severe form of alcohol dependence and a higher probability of relapse through negative reinforcement mechanisms linked to higher levels of depression, anxiety, and alcohol craving.
Overall, the gut microbiota seems to be an innovative target in the management of patients who are being treated for alcohol dependence. In view of the bacteria that were modulated upon alcohol consumption, which characterize increased gut permeability, we propose that more attention be paid to the nutritional follow-up of abstinent patients. Probiotics and prebiotics are known to improve the composition of the gut microbiota in favor of bacteria that decrease gut-barrier alterations (50, 68) and inflammation (13, 69), and they may improve mood and behavior in several pathological contexts (27, 70–73). Therefore, the use of these nutritional tools to improve gut function and mental health in patients diagnosed with alcohol-use disorders deserves interest.
Methods
Subjects.
A group of AD subjects presenting with a diagnosis of alcohol dependence according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (74) who were clinically evaluated by a psychiatrist (P.d.T.) were incorporated into the study when admitted to the gastroenterology ward for a 3-wk detoxification and rehabilitation program. Subjects had kept drinking until the day of admission to the detoxification ward, or the day before, and were tested twice, on the day after admission (T1) and on day 19 (T2) corresponding to the last day of detoxification. Patients who had metabolic disorders such as obesity [body mass index (BMI) > 30 kg/m2] and diabetes, inflammatory bowel disease, other chronic inflammatory diseases (such as rheumatoid arthritis), or cancer, as well as those who took antibiotics, probiotics, glucocorticoids, or nonsteroidal antiinflammatory drugs during the 2 mo preceding enrollment were excluded from the study. Transient liver elastography (Fibroscan) was performed in all patients on the day of admission to quantify liver stiffness, which correlates with fibrosis grades according to the Metavir classification system of fibrosis (75). From this evaluation, only patients without significant fibrosis (F0 and F1 scores) were selected. Patients with overt cirrhosis, on the basis of laboratory and imaging tests, were excluded (SI Methods). A complete medication and medical history was taken at admission, as well as basic demographic and clinical data related to nutritional status. For a preliminary study, we could select a group of 60 AD subjects that were tested at T1 for intestinal permeability and psychological symptoms. Among them, 44 subjects who had remained abstinent until the end of the detox period were also tested at T2. This sample of subjects was split into high and low IP groups. From this preliminary group, a subset of 13 subjects (8 men and 5 women) was tested for additional gut-microbiota composition and functionality measurements both at T1 and T2. AD subjects were compared for intestinal permeability, psychological dimensions, and gut-microbiota composition and functionality with 15 age-, sex-, and BMI-matched controls who socially consumed less than 20 g of alcohol per day.
Assessment of Alcohol Consumption.
The amount of alcohol consumed the week before hospitalization was evaluated with the time line follow-back approach (76), as detailed in de Timary et al. (77).
Measurement of IP.
IP was assessed by using the radioactive probe 51Cr-EDTA as described previously (5). Briefly, after an overnight fast and emptying of the bladder, patients drank a Nutridrink (200 mL, 150 kcal/100 mL) (Nutricia) containing 50 µCi (1.85 MBq) 51Cr-EDTA. Urine was collected for 24 h. Radioactivity was measured in urine collections with a gamma counter (Cobra5003; Canberra Packard). Urinary excretion was expressed as the percentage of the ingested dose (ID) normalized to creatinine concentration (% ID/g creatinine).
Gut-Microbiota Analysis.
Gut microbiota was analyzed by using two culture-independent methods: pyrosequencing and qPCR of 16S rDNA. These methods are complementary because pyrosequencing allows the creation of a qualitative bacterial profile that considers most of the bacteria present in the gut whereas qPCR is used to quantify specific bacterial targets of particular interest. Moreover, qPCR is more sensitive and can quantify some bacteria that are not detected with pyrosequencing, such as Bifidobacterium spp.
Fecal samples were collected in a sterile container and immediately stored at −80 °C until further processing. Bacterial DNA extraction was performed by using the repeated bead beating procedure with a modified protocol for the QIAamp Stool DNA Mini Kit (Qiagen).
A detailed description of both methods is provided in SI Methods, and primer sequences are mentioned in Table S5.
Analysis of VOCs in Fecal Samples.
Stool samples were transferred to a headspace vial, and VOCs were analyzed on a gas chromatography-mass spectrometry quadrupole (Trace GC, Thermoquest; DSQ II, Thermo Electron), which was coupled online to a purge-and-trap system. Before analysis, 125-mg fecal aliquots were suspended in 5 mL of water. Diethyl acetic acid (1.5 mg/L) was added as an internal standard. A magnetic stirrer, sulfuric acid, and a pinch of sodium sulfate were added to the sample to acidify and salt out the solution. The chromatograms thus obtained were processed by using AMDIS (Automatic Mass Spectral Deconvolution and Identification Software, version 2.71) provided by the US National Institute of Standards and Technology (NIST). Identification of the metabolites in the samples was achieved by manual visual inspection of the mass spectra of unknown peaks with the NIST library. All compounds were relatively quantified compared with diethyl acetic acid. A detailed description of the method is provided in SI Methods.
Assessment of Mood and Psychological Symptoms.
At the beginning and end of detoxification, all patients were tested for depression, anxiety, and alcohol craving with the French versions of self-reported questionnaires: the Beck Depression Inventory, the State-Trait Anxiety Inventory (form YA), and the Obsessive-Compulsive Drinking Scale. These tests have been described in detail previously (5).
Statistical Analysis.
Statistical analyses were performed by using SPSS, version 20.0 (IBM Corp). Assumptions of normality and equality of variances were checked with the Kolmogorov–Smirnov and Levene tests, respectively. If the assumptions were not met, Kruskal–Wallis tests were used to compare controls, AD subjects with high IP, and AD subjects with low IP. If the assumptions were met, parametric ANOVAs were performed. Significant ANOVA results were followed by Bonferroni’s post hoc test for pairwise comparisons. The effect of alcohol withdrawal was assessed by using Wilcoxon or paired t tests to compare data at T1 and T2. Bivariate correlations were performed with Spearman’s rho or Pearson’s product-moment correlation coefficient, depending on data assumptions.
The absolute number of sequences for identified and unclassified taxa obtained by pyrosequencing in each sample was transformed by using the Hellinger method after removing taxa representing less than 0.01% of total abundance. The resulting matrix was used to construct nonmetric multidimensional scaling by using Bray–Curtis dissimilarity with PCOrd, version 6.08 (MjM Software). The Bray–Curtis coefficient is a statistic used to quantify the compositional dissimilarity between two different bacterial profiles.
Relative abundances of taxa were analyzed by using Kruskal–Wallis tests, and qPCR data were analyzed with ANOVA after log-transformation.
Unscrambler X, version 10.2 (CAMO A/S) was used to perform cluster analyses of metabolite profiles. Subject-specific compounds (those detected in only one person) and metabolites present in less than 20% of subjects in both groups (AD and control) were discarded from statistical analysis. Ten control samples were available for this analysis. Principal-component analysis was applied to detect outliers. Clustering of similar metabolite patterns of the samples according to control, AD with high IP, and AD with low IP groups at T1 was then performed by using PLS-DA and was presented as a score plot. The corresponding loading plot, showing the metabolites, was used to identify discriminating metabolites. Kruskal–Wallis tests were applied to compare the relative indices of metabolites between controls, AD subjects with high IP, and AD subjects with low IP.
Study Approval.
The study protocol was approved by the local ethical committee, and all subjects signed an informed consent form before the investigation (B40320096274; Commission d’Éthique Biomédicale Hospitalo-Facultaire de l’UCL).
Supplementary Material
Acknowledgments
We thank all patients and controls who participated in this study, as well as the nurses of the Unité Intégrée d’Hépatologie of Saint-luc Hospital for their technical help. We also warmly thank Greet Vandermeulen, who provided important help for metabolomic analyses, and all the members of the Laboratory for Experimental Psychopathology (Université Catholique de Louvain) for their comments and suggestions. We thank Dr. C. Fillée for help in the storage of samples. This work was supported by Fonds pour la Formation à la Recherche dans l’Industrie et dans l’Agriculture, Belgium Grant 5.1.049.11.F (to S.L.) and Fonds de Recherche Scientifique Médicale Grant 3.4614.12 (to P.d.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1415174111/-/DCSupplemental.
References
- 1. World Health Organization (2014) Global Status Report on Alcohol And Health 2014. Available at: www.who.int/substance_abuse/publications/global_alcohol_report/en/. Accessed May 16, 2014.
- 2.Gilpin NW, Koob GF. Neurobiology of alcohol dependence: Focus on motivational mechanisms. Alcohol Res Health. 2008;31(3):185–195. [PMC free article] [PubMed] [Google Scholar]
- 3.Bode C, Bode JC. Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol. 2003;17(4):575–592. doi: 10.1016/s1521-6918(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 4.Leggio L, et al. Ghrelin system in alcohol-dependent subjects: Role of plasma ghrelin levels in alcohol drinking and craving. Addict Biol. 2012;17(2):452–464. doi: 10.1111/j.1369-1600.2010.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leclercq S, et al. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav Immun. 2012;26(6):911–918. doi: 10.1016/j.bbi.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Leclercq S, De Saeger C, Delzenne N, de Timary P, Stärkel P. Role of inflammatory pathways, blood mononuclear cells, and gut-derived bacterial products in alcohol dependence. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32(5):742–747. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 8.Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: Reevaluation with an improved chromogenic assay. J Hepatol. 1991;12(2):162–169. doi: 10.1016/0168-8278(91)90933-3. [DOI] [PubMed] [Google Scholar]
- 9.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 10.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 11.Guarner F, Malagelada J-R. Gut flora in health and disease. Lancet. 2003;361(9356):512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 12.Kurokawa K, et al. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res. 2007;14(4):169–181. doi: 10.1093/dnares/dsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delzenne NM, Neyrinck AM, Bäckhed F, Cani PD. Targeting gut microbiota in obesity: Effects of prebiotics and probiotics. Nat Rev Endocrinol. 2011;7(11):639–646. doi: 10.1038/nrendo.2011.126. [DOI] [PubMed] [Google Scholar]
- 14.Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson FH, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 16.Manichanh C, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55(2):205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seksik P, et al. Review article: The role of bacteria in onset and perpetuation of inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24(Suppl 3):11–18. doi: 10.1111/j.1365-2036.2006.03053.x. [DOI] [PubMed] [Google Scholar]
- 18.Noverr MC, Huffnagle GB. Does the microbiota regulate immune responses outside the gut? Trends Microbiol. 2004;12(12):562–568. doi: 10.1016/j.tim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Finegold SM, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16(4):444–453. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2011;23(3):255–264, e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 21.Diaz Heijtz R, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108(7):3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cryan JF, Dinan TG. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 23.Capuron L, et al. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7(5):468–473. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- 24.O’Connor JC, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14(5):511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Connor JC, et al. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci. 2009;29(13):4200–4209. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Hsiao EY, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8(11):1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- 29.Andersohn F, Kiefer F. Depressive mood and craving during alcohol withdrawal: Association and interaction. Ger J Psychiatry. 2004;7:6–11. [Google Scholar]
- 30.Cordovil De Sousa Uva M, et al. Distinct effects of protracted withdrawal on affect, craving, selective attention and executive functions among alcohol-dependent patients. Alcohol Alcohol. 2010;45(3):241–246. doi: 10.1093/alcalc/agq012. [DOI] [PubMed] [Google Scholar]
- 31.Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49(11):876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- 32.de Timary P, et al. The associations between self-consciousness, depressive state and craving to drink among alcohol dependent patients undergoing protracted withdrawal. PLoS ONE. 2013;8(8):e71560. doi: 10.1371/journal.pone.0071560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sokol H, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105(43):16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson GM, Orrego H, Israel Y, Devenyi P, Kapur BM. Low-molecular-weight polyethylene glycol as a probe of gastrointestinal permeability after alcohol ingestion. Dig Dis Sci. 1981;26(11):971–977. doi: 10.1007/BF01314757. [DOI] [PubMed] [Google Scholar]
- 35.Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: Possible route of entry for toxic compounds. Lancet. 1984;1(8370):179–182. doi: 10.1016/s0140-6736(84)92109-3. [DOI] [PubMed] [Google Scholar]
- 36.Keshavarzian A, et al. Leaky gut in alcoholic cirrhosis: A possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94(1):200–207. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 37.Elamin EE, Masclee AA, Dekker J, Jonkers DM. Ethanol metabolism and its effects on the intestinal epithelial barrier. Nutr Rev. 2013;71(7):483–499. doi: 10.1111/nure.12027. [DOI] [PubMed] [Google Scholar]
- 38.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59(1):29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 39.Keshavarzian A, Fields JZ, Vaeth J, Holmes EW. The differing effects of acute and chronic alcohol on gastric and intestinal permeability. Am J Gastroenterol. 1994;89(12):2205–2211. [PubMed] [Google Scholar]
- 40.Banan A, Choudhary S, Zhang Y, Fields JZ, Keshavarzian A. Ethanol-induced barrier dysfunction and its prevention by growth factors in human intestinal monolayers: Evidence for oxidative and cytoskeletal mechanisms. J Pharmacol Exp Ther. 1999;291(3):1075–1085. [PubMed] [Google Scholar]
- 41.Ma TY, Nguyen D, Bui V, Nguyen H, Hoa N. Ethanol modulation of intestinal epithelial tight junction barrier. Am J Physiol. 1999;276(4 Pt 1):G965–G974. doi: 10.1152/ajpgi.1999.276.4.G965. [DOI] [PubMed] [Google Scholar]
- 42.Banan A, et al. NF-kappaB activation as a key mechanism in ethanol-induced disruption of the F-actin cytoskeleton and monolayer barrier integrity in intestinal epithelium. Alcohol. 2007;41(6):447–460. doi: 10.1016/j.alcohol.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Elamin E, et al. Effects of ethanol and acetaldehyde on tight junction integrity: In vitro study in a three dimensional intestinal epithelial cell culture model. PLoS ONE. 2012;7(4):e35008. doi: 10.1371/journal.pone.0035008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferrier L, et al. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am J Pathol. 2006;168(4):1148–1154. doi: 10.2353/ajpath.2006.050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mutlu E, et al. Intestinal dysbiosis: A possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009;33(10):1836–1846. doi: 10.1111/j.1530-0277.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bull-Otterson L, et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS ONE. 2013;8(1):e53028. doi: 10.1371/journal.pone.0053028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan AW, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53(1):96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kirpich IA, et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: A pilot study. Alcohol. 2008;42(8):675–682. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mutlu EA, et al. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302(9):G966–G978. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madsen K, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121(3):580–591. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 51.Machiels K, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63(8):1275–1283. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 52.Hamer HM, De Preter V, Windey K, Verbeke K. Functional analysis of colonic bacterial metabolism: Relevant to health? Am J Physiol Gastrointest Liver Physiol. 2012;302(1):G1–G9. doi: 10.1152/ajpgi.00048.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Windey K, De Preter V, Verbeke K. Relevance of protein fermentation to gut health. Mol Nutr Food Res. 2012;56(1):184–196. doi: 10.1002/mnfr.201100542. [DOI] [PubMed] [Google Scholar]
- 54.Nicholson JK, et al. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 55.Bures J, et al. [Excretion of phenol and p-cresol in the urine in fasting obese individuals and in persons treated with total enteral nutrition] Cas Lek Cesk. 1990;129(37):1166–1171. [PubMed] [Google Scholar]
- 56.Lord RS, Bralley JA. Clinical applications of urinary organic acids. Part 2. Dysbiosis markers. Altern Med Rev. 2008;13(4):292–306. [PubMed] [Google Scholar]
- 57.Hughes R, Kurth MJ, McGilligan V, McGlynn H, Rowland I. Effect of colonic bacterial metabolites on Caco-2 cell paracellular permeability in vitro. Nutr Cancer. 2008;60(2):259–266. doi: 10.1080/01635580701649644. [DOI] [PubMed] [Google Scholar]
- 58.McCall IC, et al. Effects of phenol on barrier function of a human intestinal epithelial cell line correlate with altered tight junction protein localization. Toxicol Appl Pharmacol. 2009;241(1):61–70. doi: 10.1016/j.taap.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ward LA, Johnson KA, Robinson IM, Yokoyama MT. Isolation from swine feces of a bacterium which decarboxylates p-hydroxyphenylacetic acid to 4-methylphenol (p-cresol) Appl Environ Microbiol. 1987;53(1):189–192. doi: 10.1128/aem.53.1.189-192.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith EA, Macfarlane GT. Enumeration of human colonic bacteria producing phenolic and indolic compounds: Effects of pH, carbohydrate availability and retention time on dissimilatory aromatic amino acid metabolism. J Appl Bacteriol. 1996;81(3):288–302. doi: 10.1111/j.1365-2672.1996.tb04331.x. [DOI] [PubMed] [Google Scholar]
- 61.Russell WR, et al. Major phenylpropanoid-derived metabolites in the human gut can arise from microbial fermentation of protein. Mol Nutr Food Res. 2013;57(3):523–535. doi: 10.1002/mnfr.201200594. [DOI] [PubMed] [Google Scholar]
- 62.Yokoyama MT, Carlson JR. Microbial metabolites of tryptophan in the intestinal tract with special reference to skatole. Am J Clin Nutr. 1979;32(1):173–178. doi: 10.1093/ajcn/32.1.173. [DOI] [PubMed] [Google Scholar]
- 63.Botsford JL, Demoss RD. Escherichia coli tryptophanase in the enteric environment. J Bacteriol. 1972;109(1):74–80. doi: 10.1128/jb.109.1.74-80.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeMoss RD, Moser K. Tryptophanase in diverse bacterial species. J Bacteriol. 1969;98(1):167–171. doi: 10.1128/jb.98.1.167-171.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng X, et al. The footprints of gut microbial-mammalian co-metabolism. J Proteome Res. 2011;10(12):5512–5522. doi: 10.1021/pr2007945. [DOI] [PubMed] [Google Scholar]
- 66.Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci USA. 2010;107(1):228–233. doi: 10.1073/pnas.0906112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Venkatesh M, et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity. 2014;41(2):296–310. doi: 10.1016/j.immuni.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ait-Belgnaoui A, et al. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37(11):1885–1895. doi: 10.1016/j.psyneuen.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 69.Forsythe P, Bienenstock J. Immunomodulation by commensal and probiotic bacteria. Immunol Invest. 2010;39(4-5):429–448. doi: 10.3109/08820131003667978. [DOI] [PubMed] [Google Scholar]
- 70.Bravo JA, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Messaoudi M, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105(5):755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 72.Rao AV, et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009;1(1):6. doi: 10.1186/1757-4749-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tillisch K, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144(7):1394–1401. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th Ed American Psychiatric Association; Washington: 1994. [Google Scholar]
- 75.Bedossa P, Poynard T. The METAVIR Cooperative Study Group An algorithm for the grading of activity in chronic hepatitis C. Hepatology. 1996;24(2):289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 76.Maisto SA, Sobell LC, Cooper AM, Sobell MB. Comparison of two techniques to obtain retrospective reports of drinking behavior from alcohol abusers. Addict Behav. 1982;7(1):33–38. doi: 10.1016/0306-4603(82)90022-3. [DOI] [PubMed] [Google Scholar]
- 77.de Timary P, et al. The loss of metabolic control on alcohol drinking in heavy drinking alcohol-dependent subjects. PLoS ONE. 2012;7(7):e38682. doi: 10.1371/journal.pone.0038682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.