Abstract
Oxytocin (OT) and a polymorphism (rs53576) in the oxytocin receptor gene (OXTR) have been independently associated with stress reactivity, whereas oxytocin’s sister peptide, arginine vasopressin (AVP), and polymorphisms in the vasopressin receptor gene (AVPR1A) have been independently associated with aggressive behavior. In this study, 68 men and 98 women were genotyped for the OXTR rs53576 polymorphism and the AVPR1A RS1 polymorphism. Baseline and post-stressor levels of plasma OT, plasma AVP, positive affect, and anger were assessed. Women, but not men, with high levels of post-stressor OT and the GG genotype of rs53576 felt the most positive affect after the stressor. Men, but not women, with high levels of post-stressor AVP and with the 320allele of the RS1 polymorphism reported more post-stressor anger than non-carriers. These data constitute the first evidence that oxytocin and vasopressin receptor genes interact with levels of OT and AVP to predict sex-specific emotional stress responses.
Keywords: OXTR rs53576, oxytocin, AVPR1A RS1, vasopressin, positive emotion, anger
People can greatly vary in their emotional reactions to environmental stressors, and these differential reactions may have important psychological and physiological implications (Moons, Eisenberger, & Taylor, 2010). Understanding the underlying processes that shape these differential emotional responses can identify populations who are likely to respond to stressors with differing emotions and why they do so. Individual differences in functioning of the oxytocin (OT) system might be one such contributor to emotional responses to stress. OT is synthesized in the human hypothalamus (Swaab, Pool, & Nijveldt, 1975; Vandesande & Dierickx, 1975), released from the posterior pituitary (Brownstein, Russell, & Gainer, 1980), and higher levels of endogenous plasma OT have been associated with enhanced maternal bonding (Feldman et al., 2007), more affectionate behavior between partners (Grewen, Girdler, Amico, & Light, 2005; Light, Grewen, & Amico, 2005), increased trust (Taylor, Gonzaga, Klein, Hu, Greendale, & Seeman 2006; Zak, Kurzban, & Matzner, 2005), and muted cortisol responses to psychosocial stressors (Altemus, Deuster, Galliven, Carter, & Gold, 1995; Heinrichs, Meinlschmidt, Neumann, Wagner, Kirschbaum, Ehlert, et al., 2001). Intranasal administration of synthetic OT has also provided experimental evidence that OT can cause more favorable emotional responses to stressors (de Oliviera, Zuardi, Graeff, Queiroz, & Crippa, 2012; Heinrichs, Baumgartner, Kirschbaum, & Ehlert, 2003). Consequently, OT is strongly linked to socioemotional functioning, attenuated stress reactions, and thus may explain variability in how positively people feel in response to stressors.
The rise of molecular genetic techniques has provided the opportunity to study the other side of the oxytocin synapse, namely the oxytocin receptor. Most attention has focused on a single nucleotide polymorphism (rs53576) in intron 3 of the oxytocin receptor gene (OXTR). Relative to carriers of the A-allele, homozygous GG carriers show enhanced psychological resources (Saphire-Bernstein, Way, Kim, Sherman, & Taylor, 2011), more sensitive parenting (Bakermans-Kranenburg & van Ijzendoorn, 2008), greater empathy, and reduced stress reactivity (Rodrigues, Saslow, Garcia, John, & Keltner, 2009). In particular, variations in OXTR have been associated with positive affect (Lucht, Barnow, Sonnenfeld, Rosenberger, Grabe, Schroeder, et al., 2009) and differential emotion regulation strategies (Kim, Sherman, Sasaki, Xu, Chu, Ryu, et al., 2010; Kim, Sherman, Mojaverian, Sasaki, Park, Suh, et al., 2011; Mueller, Thomas, Burbach, Peterson, & Shimizu, 2007). Therefore, much like OT, OXTR polymorphisms may also play a prominent role in reducing the impact of stressors and increasing positive affect.
The literature linking either plasma oxytocin or variation in the OXTR to psychosocial outcomes have been growing rapidly, yet have been progressing independently. This is surprising because, theoretically, one would expect an emotional response to be the result of an interaction between a neurotransmitter and its receptor. Stated differently, because oxytocin mediated neurotransmission is a function of both the concentration of oxytocin in the extracellular space as well as the signaling properties of the receptor a more comprehensive understanding of oxytocinergic function is likely to be obtained by examining both the receptor as well as the neurotransmitter. Therefore, in this study, we examine the behavioral correlates of the interaction between these two variables, as measured by plasma oxytocin and rs53576.
Arginine vasopressin (AVP) is similar to OT in molecular structure; however, in contrast to OT, AVP has been associated with more intense biological stress responses (Ebstein, Israel, Lerer, Uzefovsky, Shalev, Gritsenko, et al., 2009; Shalev, Israel, Uzefovsky, Gritsenko, Kaitz, & Evstein, 2011), more negative responses to emotionally ambiguous stimuli (Thompson, Gupta, Miller, Mills, & Orr, 2004), and increased aggression and hostility in both animal models (Albers & Bamshad, 1998; Ferris, Stolberg, & Delville, 1999; Ferris, 2000; Ferris, Lu, Messenger, Guillon, Heindel, Miller, et al., 2006) and human samples (Coccaro, 1998). AVP has also been linked to anxiety or fear in animal models; however, the preponderance of evidence suggests that aggression may be the predominant consequence of AVP (Thompson et al., 2006). For human samples, whether AVP elicits specific aggression-linked emotions like anger, or threat-linked emotions like fear, or possibly both, has yet to be determined.
In the upstream regulatory region of the AVPR1A gene, there are several microsattelites that affect expression of AVPR1A. The RS1 polymorphism has been associated with behavioral and mental health outcomes such as autism spectrum disorders (Kim, Young, Gonen, Veenstra-VanderWeele, Courchesne, Courchesne, et al., 2002; Tansey, Brookes, Hill, Cochrane, Gill, Skuse, et al., 2010; Yirmiya, Rosenberg, Levi, Salomon, Shulman, Nemanov, et al., 2006), prepulse inhibition (Levin, Heresco-Levy, Bachner-Melman, Israel, Shalev, & Ebstein, 2009), and creativity (Bachner-Melman, Dina, Zohar, Constantini, Lerer, Hoch, et al., 2005). Although the AVP V1a receptor has been shown to have a robust relationship with aggressive behavior in rodents (Ferris et al., 1997), no direct connection between AVPR1A and aggression or hostility in humans has been demonstrated (Pavlov, Chistiakov, & Chekhonin, 2012). However, in one study, people carrying the 320 allele1 were significantly higher in novelty seeking and lower in harm avoidance (Meyer-Lindenberg, Kolachana, Gold, Olsh, Nicodemus, Mattay, 2009); this profile of behavioral approach toward even potentially harmful stimuli is consistent with the greater aggression and approach–oriented behaviors associated with anger (Averill, 1983; Harmon-Jones & Allen, 1998). Therefore, the current study examines how the vasopressin system as a whole may regulate anger in humans, as opposed to an avoidance emotion like fear (Carver & Harmon-Jones, 2009).
Both OT and OXTR are related to stress buffering and positive affect, whereas both AVP and AVPR1A have been tied to aggressive behavior and, potentially, to associated anger responses. Despite the clear overlap between neuropeptide and receptor gene effects, the joint contributions of OT with OXTR or AVP with AVPR1A have not been documented in humans. We hypothesized additive effects such that higher levels of OT in response to a stressor would predict more positive emotional reactions particularly for GG carriers compared to A carriers. In contrast, we hypothesized that higher levels of AVP would predict more anger, but not fear, in those carrying the 320 allele compared to non-carriers.
The literature has repeatedly shown sex differences in the oxytocin and vasopressin systems (e.g., Carter, 2007; Carter, Boone, Pournajafi-Nazaloo, & Bales, 2009; de Vries, 2008; Goodson & Bass, 2001; Grewen, Girdler, Amico, & Light, 2005; Miller et al, 2013; Thompson, George, Walton, Orr, & Benson, 2006; Yamasue, Kuwabara, Kawakubo, & Kasai, 2009). Although prior work has demonstrated that variations in OXTR are indeed consequential for males (Chen et al., 2011; Lucht et al., 2009; Tost et al., 2010), the preponderance of animal and human research and theory suggests that the oxytocin system is particularly influential in females, whereas the vasopressin system may be more influential in males (Gabor, Phan, Clipperton-Allen, Kavaliers, & Choleris, 2012; Taylor et al., 2010). Thus, we examined whether the genetic and neuropeptide effects hypothesized above would be moderated by participant sex such that the OT and OXTR effects would be strongest in women and the AVP and AVPR1A RS1 effects would be strongest in men. Specifically, the benefits of higher OT observed in prior literature were expected to converge with the benefits of homozygous G alleles in women to cause a particularly strong buffering from stress, whereas the more aggressive and hostile aspects of elevated AVP and variations in AVPR1A RS1 would impact feelings of anger in men.
Methods and Materials
Participants
Participants were affiliated with the University of California Los Angeles as students or staff. They completed the experimental protocol in exchange for payment. Procedures were approved by the UCLA Institutional Review Board. All potential participants were screened and were excluded if they were currently in counseling, suffered from mental health conditions (e.g., depression), or used any medications (e.g., Prozac) that could impact the validity of assays. Pregnant or lactating women were also excluded. The final sample consisted of 172 participants (40% male, 60% female) who ranged in age from 18 to 35 (M=21). The participant sample was 37% Asian-American, 2% African-American, 23% European-American, 16% Latino/a, 7% of mixed ethnicity, and 15% self-identified as “other”.2
Trier Social Stress Test
Participants reported to the UCLA Clinical Research Center and were screened for acceptable blood pressure (under 140.00mm HG), pulse (60–100), lung auscultation, and cardiac function. Participants used a salivette to provide one saliva sample 10 minutes after arrival and another sample 30 minutes after that. Saliva samples were immediately placed on ice then transferred to a freezer within minutes. The levels of cortisol in these two samples were average to assess baseline cortisol. A nurse then inserted an indwelling catheter and performed a blood draw from which baseline levels of OT and AVP were assayed. Participants then reported the extent to which they felt specific emotions on 5-point scales (1 = very slightly, 5 = extremely) that were averaged into reliable subscales of positive affect (interested, excited, strong, enthusiastic, proud, alert, inspired, attentive, determined, active; α=.85), anger (irritated with others, hostile, irritable; α=.77), and fear (scared, nervous, jittery, afraid; α=.74).3
Participants then completed the Trier Social Stress Test (TSST), a commonly used laboratory stress challenge known to elicit physiological and emotional stress responses (Kirschbaum, Pirke, & Hellhammer, 1993). Participants were video-recorded performing this task in front of a neutral experimenter and either no audience or an audience of three confederates who were unknown to participants.4 Participants were given 5 min to prepare a speech on why they would make a good administrative assistant, a popular position for students and campus staff. After delivering the 5 min speech, participants completed a mental arithmetic task of counting backwards by 13s from the number 2935 out loud, while the experimenter pressured them to go faster. In order for endocrine changes to be observable, post-stressor samples were taken 25 minutes after starting the TSST; at that time, a third saliva sample was provided and a second blood draw was performed to assess post-stressor levels of OT and AVP. Participants reported their post-stressor level of positive affect (α=.74), anger (α=.74), and fear (α=.75). Then participants provided a fourth saliva sample. Levels of cortisol from the third and fourth samples were averaged to assess post-stressor cortisol reactivity. At the end of the experimental session, participants were debriefed, thanked, and dismissed.
Genotyping
DNA was collected from saliva with Oragene kits (DNA Genotek) and extracted according to manufacturer’s recommendations. The OXTR r53576 SNP was identified using a 5’ nuclease assay to discriminate between the two alleles (Taqman SNP Genotyping Assay C_3290335_10; Applied Biosystems, Inc.). Polymerase chain reactions were performed using the manufacturer’s recommended protocol with 5 µl reaction volumes in 384-well plates with 5 ng of DNA. End point reads of fluorescence levels were obtained with an ABI 7900HT Sequence Detection System. The genotyping success rate was 91%.
AVPR1A RS1 was genotyped using the primers and protocol published in (Meyer-Lindenberg, Kolachana, Gold, Olsh, Nicodemus, Mattay, et al. 2008). The PCR products were electrophoresed on an ABI 3730 DNA analyzer (Applied Biosystems) with a Mapmaker size standard (Bioventures, Murfreesboro, Tennessee). Data collection and analysis used GeneScan and Genotyper software (Applied Biosystems). The genotyping success rate was 95%.
Assay Procedures
Saliva samples were shipped on dry ice overnight to the Behavioral Endocrinology Laboratory at Pennsylvania State University. Cortisol levels were assessed from a 25µl sample, which was assayed in duplicate by radioimmunoassay using the HS-cortisol High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit (Salimetrics LLC, State College, PA). This test allowed for robust results because all saliva samples were within the desired 3.5–9.0 pH range.
Blood samples were drawn into serum tubes containing aprotonin (500 kallikrein-inhibiting units per ml of blood). Samples were centrifuged at 1,600 x g for 15 min at 4 °C. Plasma was then stored in plastic tubes at −80 °C. Frozen samples were batched and shipped overnight on dry ice to Salimetrics, LLC (State College, PA) for analysis. On the day of assay, the samples were thawed completely at room temperature, centrifuged at 1,500 x g for 10 min then pipetted into test wells.
OT was assayed with the commercially available immunoassay (enzyme-linked immunosorbent assay kit; Assay Designs, Inc., Ann Arbor, Michigan, Catalogue No. 900-153). The sample was diluted 5x and 100 µl was used for the assay, which had a lower limit of sensitivity of 11.7 pg/ml and an upper limit of 1,000 pg/ml. The intra-assay coefficient of variation (CV) was 12.7%. The inter-assay CVs, estimated across 15 separate runs for high control (550 pg/ml) and low control (71 pg/ml), were 15.0% and 26.3%, respectively. Baseline and post-stressor OT values were log-transformed to normalize the distribution.
AVP was assayed using a commercially available immunoassay (Assay Designs, Inc., Catalogue No. 900-017). The sample was diluted 2x and 100 µl was used for the assay. The assay had a lower limit of sensitivity of 3.39 pg/ml and an upper-limit of 1,000 pg/ml. The intra-assay CV was 7.4%. The inter-assay CVs, estimated across 10 separate runs for high control (507 pg/ml) and low control (26 pg/ml), were 21.8% and 25.5%, respectively. All plasma samples were tested in duplicate; values that varied by more than 5% were tested again. Baseline and post-stressor AVP values were log-transformed to normalize the distribution.
Statistical Analyses
Deviations from Hardy-Weinberg equilibrium for the OXTR polymorphism and the AVPR1A RS1 polymorphism were calculated with exact tests using Arlequin (Excoffier & Lischer, 2010; see Table 1 for AVPR1A RS1 allele frequencies).
Table 1.
Allele Frequencies of AVPR1A RS1 Polymorphism
| Allele | Count | Percent |
|---|---|---|
| 308 | 5 | 1.5 |
| 312 | 42 | 12.5 |
| 316 | 116 | 34.5 |
| 320 | 89 | 26.5 |
| 324 | 33 | 9.8 |
| 328 | 22 | 6.5 |
| 332 | 27 | 8.0 |
| 336 | 1 | 0.3 |
| 340 | 1 | 0.3 |
Regression analysis was used to test hypotheses. Following prior research (Saphire-Bernstein, Way, Kim, Sherman, & Taylor, 2011; Bakermans-Kranenburg & van Ijzendoorn, 2008; Rodrigues, Saslow, Garcia, John, & Keltner, 2009; Kim, Sherman, Sasaki, Xu, Chu, Ryu, et al., 2010; Riem, Pieper, Out, Bakermans-Kranenburg, & van Ijzendoorn, 2011), comparisons were made between homozygous GG participants and those carrying an A-allele (AA or AG). In separate analyses, post-stressor positive affect, anger, and fear were regressed on the OXTR polymorphism (coded GG = −1, A carrier = 1), participant sex (Men = −1, Women = 1), centered post-stressor OT, and all interactions. These analyses controlled for ethnic group, centered baseline OT, and centered baseline levels of the target emotion (i.e., positive affect, anger, or fear) in order to isolate responses to the laboratory stressor specifically. In separate analyses, post-stressor positive affect, anger, and fear were regressed on AVPR1A RS1 polymorphism (320 non-carriers = −1, 320 carriers = 1), centered post-stressor AVP, sex, and all interactions. The comparison of carriers of the 320 allele with all other alleles was based on prior research showing allele 320 carriers had lower amygdala activity (Meyer-Lindenberg et al., 2008). This model controlled for ethnic group, baseline AVP and baseline emotion. All tests were two-tailed with α set to p < .05.
Importantly, the analysis controlled for any differences in baseline levels of OT, AVP, positive affect, anger, or fear in our participants. In short, these analyses specifically examined reactivity to a psychological stressor regardless of baseline differences across genetic or sex groups. Additionally, although the levels of neuropeptides may be partly determined by receptor polymorphisms, the inclusion of genetic polymorphism and neuropeptide level in the same analysis means that the results reflect the independent power of each factor to predict emotional responses to stressors.
Results
Stress Manipulation Check
We examined cortisol reactivity to verify that the TSST effectively induced a stress response. As expected, on average participants showed an overall increase in salivary cortisol from baseline levels (M = .150 pg/ml, SE = .009) to post-stressor levels (M = .247 pg/ml, SE = .016), F(1, 169) = 49.69, p<.001, ηp2 = .23. OT, OXTR, AVP, and AVPR1A RS1 were not related to post-stressor cortisol, all ps>.05.
Gene Distribution
Genotypes for the AVPR1A RS1 and OXTR polymorphisms did not deviate from Hardy-Weinberg equilibrium for the sample as a whole (p = .33, and .52, respectively) or each ethnic category (European-American, Asian-American, other; ps > .16). Polymorphism distributions were examined across sex groups and three ethnic categories (European-American, Asian-American, other). The distribution of AVPR1A RS1 genotypes did not significantly differ across sex groups, p > .06, or racial groups, p > .12. OXTR genotypes did not differ across sex groups, p > .17; however, OXTR polymorphisms did differ by ethnicity group, χ2 = 16.418, p = .003 (European-American = 48% GG, 46% AG, 6% AA; Asian-American = 20% GG, 47% AG, 33% AA; Other = 41% GG, 45% AG, 14% AA). Consequently, all analyses were conducted controlling for ethnicity group with two effects coded variables (European-American = 1, Asian American = 0, other = −1; European-American = 0, Asian-American = 1, other = −1).5
Preliminary Analyses
Table 2 presents means and mean differences between the OXTR and AVPR1A RS1 polymorphisms. Overall, GG participants had significantly less oxytocin than A carriers at both baseline and post-stressor. Importantly, the subsequent analyses control for these default differences by including baseline levels of neuropeptides as covariates, thus isolating the results to reactivity in biological and psychological systems. On average, participants carrying the 320 allele reported higher levels of anger after the stressor than participants without the 320 allele.
Table 2.
Means (SE) in OT, AVP, positive affect, anger, and fear by OXTR and AVPR1A RS1 polymorphism.
| OXTR rs53576 | AVPR1A RS1 | |||
|---|---|---|---|---|
| Variable | GG | A Carrier | 320 Carrier | Non-Carrier |
| (M=19, F=36) | (M=43, F=61) | (M=31, F=59) | (M=38, F=40) | |
| 1. Baseline OT | 5.257 (.070) | 5.434 (.053) | 5.360 (.061) | 5.406 (.058) |
| 2. Baseline AVP | 3.997 (.080) | 4.157 (.060) | 4.134 (.066) | 4.054 (.063) |
| 3. Baseline Pos. Affect | 3.270 (.091) | 3.310 (.068) | 3.362 (.073) | 3.271 (.068) |
| 4. Baseline Anger | 1.636 (.098) | 1.863 (.073) | 1.811 (.083) | 1.773 (.078) |
| 5. Baseline Fear | 1.560 (.085) | 1.525 (.063) | 1.483 (.069) | 1.573 (.065) |
| 6. Post-stressor OT | 5.174 (.069) | 5.377 (.052) | 5.314 (.058) | 5.318 (.058) |
| 7. Post-stressor AVP | 4.009 (.079) | 4.139 (.059) | 4.130 (.065) | 4.047 (.062) |
| 8. Post-stressor Pos. Affect | 3.473 (.104) | 3.281 (.078) | 3.308 (.086) | 3.434 (.081) |
| 9. Post-stressor Anger | 1.362 (.097) | 1.543 (.074) | 1.609 (.079) | 1.361 (.075) |
| 10. Post-stressor Fear | 1.529 (.088) | 1.560 (.067) | 1.529 (.072) | 1.554 (.068) |
Note: Bold values denote significant differences (at p < .05) on that variable between the two polymorphisms of OXTR, or between the two polymorphisms of AVPR1A RS1. Post-stressor OT differs across rs53576 polymorphisms at p=.017. Post-stressor anger differs across AVPR1A polymorphisms at p=.032. OT and AVP values are provided in pg/ml. Comparisons control for effect of ethnicity group. M= number of men and F= number of women per genetic group.
Table 3 provides the correlations among the continuous measures of OT, AVP, and emotions at baseline and post-stressor. As would be expected, baseline variables consistently correlated with corresponding post-stressor levels of that variable.
Table 3.
Correlations for OT, AVP, and emotion report variables.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Baseline OT | _ | .149 | −.005 | −.018 | −.059 | .865*** | .170* | .029 | .017 | −.064 |
| 2. Baseline AVP | _ | −.040 | −.011 | −.123 | .110 | .955*** | .115 | .111 | −.100 | |
| 3. Baseline Pos. Affect | _ | −.129 | −.032 | .006 | −.027 | .613*** | −.155* | −.101 | ||
| 4. Baseline Anger | _ | .263** | −.021 | −.005 | −.154* | .513*** | .269*** | |||
| 5. Baseline Fear | _ | −.020 | −.077 | −.117 | .266*** | .539*** | ||||
| 6. Post-stressor OT | _ | .130 | .028 | −.006 | −.026 | |||||
| 7. Post-stressor AVP | _ | .111 | .100 | −.073 | ||||||
| 8. Post-stressor Pos. Affect | _ | −.157* | −.070 | |||||||
| 9. Post-stressor Anger | _ | .423*** | ||||||||
| 10. Post-stressor Fear | _ |
Note: Correlations control for effect of ethnicity group.
p < .05,
p < .01,
p < .001
Oxytocin Analyses
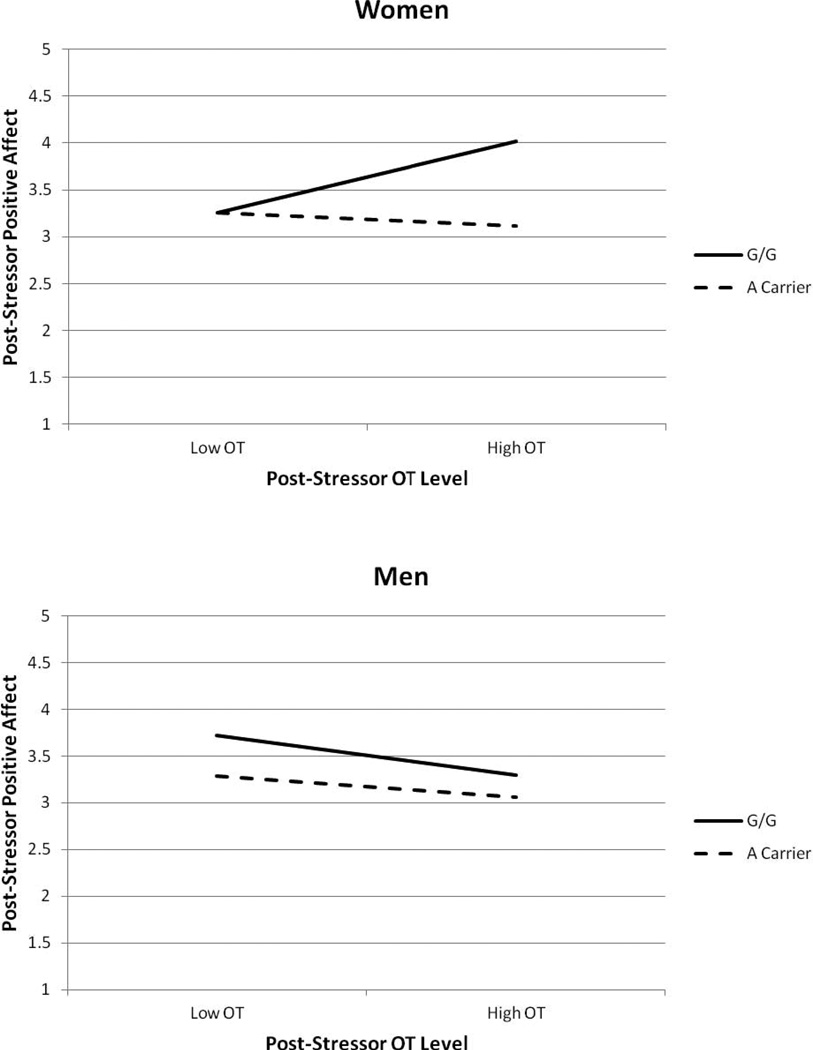
Regression analysis revealed that, overall, A carriers felt less post-stressor positive affect than GGs, b = −0.171, p = .002. Neither the sex main effect, p = .211, nor the post-stressor OT main effect, p = .960, reached significance. The OXTR by sex interaction was significant, ΔR2 = .018, p = .027, as was the post-stressor OT by sex interaction, ΔR2 = .032, p = .004. However, both of these were qualified by a significant three-way interaction of OXTR polymorphism, sex, and post-stressor OT, ΔR2 = .022, p = .014 (Figure 1).
Figure 1.
Positive affect reported after the stressor as a function of participant sex, OXTR polymorphism, and post-stressor OT levels. Women (top panel) showed a significant OXTR polymorphism by post-sressor OT interaction, but men (bottom panel) did not. Analysis controlled for ethnicity group, baseline positive affect, and baseline OT.
Follow-up analysis revealed that the OXTR by post-stressor OT interaction was not significant for men, ΔR2 = .001, p = .559, but was significant for women, ΔR2 = .039, p = .001. Specifically, among women with high levels of post-stressor OT (+1 SD), GG women reacted with more positive affect than women carrying the A-allele, b = −0.948, p = .001. In contrast, for women with low levels of post-stressor OT (−1 SD), GG women reacted similarly to women carrying the A-allele, b = −0.120, p = .482. Additionally, for GG women, greater post-stressor OT was associated with more positive affect in response to the stressor, b = 0.720, p = .002. In contrast, post-stressor OT was not related to positive affect for women who carried an A-allele, b = −0.138, p = .542.
A similar analysis of post-stressor anger failed to reveal the same three-way interaction, ΔR2 = .001, p = .741.6 Only a significant main effect of sex emerged such that women reported feeling less anger in response to the stressor than men, b = −0.182, p = .001. Additionally, no significant main effects or interactions emerged when examining post-stressor fear (all ps > .22). This suggests that the effects of oxytocin signaling were confined to positive emotion.
Vasopressin Analyses
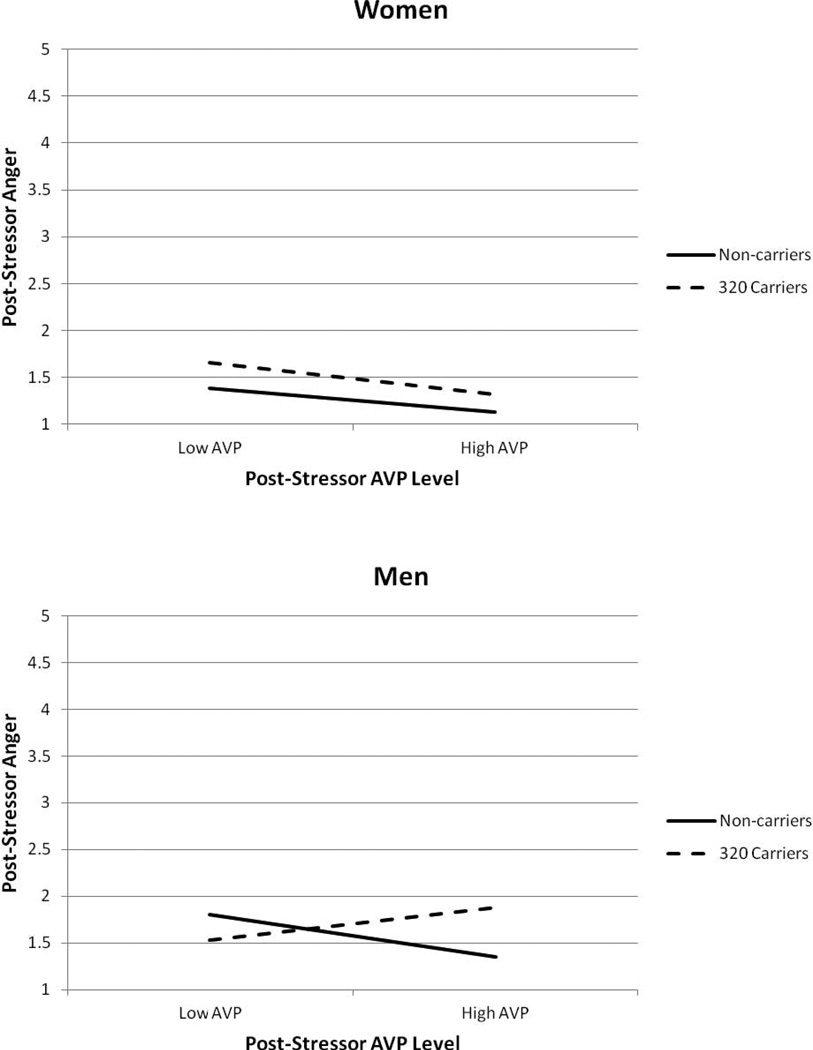
Regression analysis confirmed that, overall, women felt less anger in response to the stressor than men, b = −0.135, p = .005. No other main effects or two-way interactions reached statistical significance. However, a significant three-way interaction for AVPR1A RS1 polymorphism, sex, and post-stressor AVP emerged, ΔR2 = .021, p = .026 (Figure 2).
Figure 2.
Anger reported after the stressor as a function of participant sex, AVPR1A RS1 polymorphism, and post-stressor AVP levels. Men (bottom panel) showed a significant RS1 polymorphism by post-sressor AVP interaction, but women (top panel) did not. Analysis controlled for ethnicity group, baseline anger, and baseline AVP.
Follow-up analysis revealed that the AVPR1A RS1 by post-stressor AVP interaction was not significant for women, ΔR2 = .001, p = .677, but was significant for men, ΔR2 = .025, p = .015. Specifically, for men with high levels of post-stressor AVP (+1 SD), 320 carriers felt angrier in response to the stressor than non-carriers, b = 0.522, p = .012. In contrast, for men with low post-stressor AVP (−1 SD), 320 carriers and non-carriers felt similar levels of anger, b = −0.269, p = .236. Neither 320 carriers (b = .293, p =.391) nor non-carriers (b = −.382, p =.222) showed a significant association between post-stressor AVP and anger in response to the TSST.
A similar analysis of positive affect failed to reveal the same three-way interaction, ΔR2 = .002, p = .499. Only a main effect of AVPR1A RS1 polymorphism emerged such that 320 carriers felt less post-stressor positive affect than non-carriers, b = −.096, p = .049. Additionally, no significant main effects or interactions emerged when examining post-stressor fear (all ps > .36). Therefore, AVP and AVPR1A RS1polymorphisms may not be associated with general negative affect, but instead be associated specifically with anger.
Discussion
The current research establishes that circulating neuropeptides and polymorphisms in their receptor genes interact to predict emotional reactions to acute stressors. In response to a stressor, women carrying the rs53576 GG genotype of the OXTR with high levels of OT output seem particularly insulated and show relatively high levels of positive affect after an acute stressor. In contrast, men carrying the 320 allele of AVPR1A RS1 with high AVP output are more likely than non-carrier men to feel angry and hostile after an acute stressor; however, they were no more likely to show fear, thus indicating a specific emotional response rather than a general negative affective reaction. Together, these results suggest sex-specific effects of the oxytocin and vasopressin systems in regulating emotional reactions to stress.
A primary contribution of this research is in identifying a potential interaction between these neuropeptides and genetic variation in their respective receptors. Although not previously shown, such a finding is to be expected, because the overall effect of signaling in a neurochemical system is a function of both the receptor and the neurochemical. Examining the interaction between neuropeptide levels and genetic variants may help to explain inconsistencies in prior research as well as individual differences seen in the literature.
The interaction between these neuropeptides and variation in their receptor genes is analogous to rs53576 moderating the effects of intranasal oxytocin administration on face preference ratings (Marsh et al., 2012). Consistent with our results, Marsh et al. (2012) found that the GG individuals were more responsive to oxytocin administration. Studying the interaction between a genetic variant and a neurochemical, whether endogenous as in our data or exogenous as in Marsh et al., (2012), is likely to provide a clearer understanding of the relationship between emotional response and the oxytocin or vasopressin systems. Such studies will also inform the use of OT as a psychopharmacological treatment of psychiatric conditions such as autism (Bartz & Hollander, 2008; Andari, Duhamel, Zalla, Herbrecht, Leboyer, & Sirigu, 2010). Treatment effectiveness may depend on patient’s genetic profile as has been found in the treatment of schizophrenia, where genetic variants have been found to moderate the clinical effect of a drug (Zhang et al., 2010) or a drug’s side-effect profile (Sicard et al., 2010).
The oxytocin system was distinctively linked to positive affective responses and not to anger or fear responses. This finding contributes to evidence identifying the oxytocin system as an enhancer of positive outcomes and as a buffer to threat with an especially influential role in women (Light, Grewen, Amico, 2005; Rodrigues, Saslow, Garcia, John, & Keltner, 2009; Tabak, McCullough, Szeto, Mendez, & McCabe, 2011; DeVries, Devries, Taymans, & Carter, 1996; Carter & Altemus). For example, compared to A-allele carriers, those carrying the GG polymorphism of the OXTR rs53576 gene had better psychological resources including enhanced mastery, greater self-esteem, and more optimism (Saphire-Bernstein, Way, Kim, Sherman, & Taylor, 2011), which then presumably minimized depressive symptomatology. Thus, the oxytocin system may be particularly likely to enhance positive outcomes, such as more positive emotion in response to stress, that subsequently mitigate downstream negative outcomes.
Our results are consistent with prior research examining either the OT system or OXTR polymorphisms separately. Nursing mothers, whose OT levels are naturally elevated, showed reduced stress reactivity (Mezzacappa & Katkin, 2002; Mezzacappa, 2004). Examination of OXTR rs53576 has revealed that GG carriers are most likely to employ a culturally normative and effective form of emotion regulation (Kim, Sherman, Sasaki, Xu, Chu, Ryu, et al., 2011; Kim, Sherman, Mojaverian, Sasaki, Park, Suh, et al., 2011), thereby suggesting a self-regulatory mechanism by which positive affect can be increased in stressful situations. Further, beyond the pleasant subjective experience, positive affect may also provide a significant buffer against subsequent stress (Feder, Nestler, & Charney, 2009; Nikolova, Bogdan, & Brigidi, & Hariri, 2012).
In contrast to the oxytocin system, the vasopressin system was linked to anger reactivity. Importantly, this anger response was specific, as neither AVP nor RS1 polymorphisms were related to fear reactions. Indeed, both anger and fear are plausible responses to the TSST; however, the vasopressin system was clearly predictive of only anger responses. Thus, the vasopressin system does not merely amplify stress or reduce the overall ability to cope with stress, but instead seems to be specifically linked to anger and hostility. This distinctive relationship between the vasopressin system and anger is consistent with prior research in both the human (Coccaro, 1998) and the animal (Ferris, Stolberg, & Delville, 1999; Ferris, Lu, Messenger, Guillon, Heindel, Miller, et al., 2006).
A striking component of the current results concerns the differences between men and women (Choleris, Gustafsson, Korach, Muglia, Pfaff, & Ogawa, 2003). Consistent with prior evidence (Chen et al., 2011; Lucht et al., 2009; Tost et al., 2009), we do not contend that the oxytocin and vasopressin systems uniquely impact women and men, respectively. However, the sex-linked differences observed in the effects of the oxytocin and vasopressin systems are consistent with differences in the regulation of these systems by sex hormones (Gimpl & Fahrenholz, 2001). Indeed, both animal and human research has indicated that OT is more important to women’s behavior than men’s, and that the reverse is true or AVP (DeVries, DeVries, Taymans, & Carter, 1996; Taylor, Saphire-Bernstein, & Seeman, 2010). The current results underscore the importance of not only examining interactive effects of receptor polymorphisms and neuropeptide levels, but also of doing so for each sex separately. Importantly, such sex differences in the roles of OT, AVP, and their respective receptor polymorphisms suggest that men and women may chronically deal with environmental stressors differently. Over a lifetime, these different strategies and their differential efficacy may play a role in men and women’s disparate susceptibility to chronic stress, mood disorders, and associated health outcomes. Further elucidating how oxytocin and vasopressin genetic profiles and circulating neuropeptides contribute to sex differences in stress management may inform mental and physical health sex disparities.
These sex differences in the effects of OT and AVP are of theoretical importance for affective science. As researchers continue to explore the biological basis of affective phenomena, there is no reason to assume that sex differences in the underlying biology are limited to quantitative differences in the levels of particular neurochemicals or receptors. Rather, as the data presented here indicate, it may be that the neurochemical systems critical for affect exhibit qualitative sex differences in organization. In other words, a particular neurochemical may have a fundamentally different role in each sex. There is compelling evidence that the neurochemical systems regulating and responding to the affective experience of pain are organized fundamentally differently in males and females (Mogil & Baily, 2010). For example, a similar three-way interaction between sex, stress, and a polymorphism in the AVPR1A promoter (rs10877969) was found to influence response to physical pain in humans and was validated in a rodent model (Mogil et al., 2011). As this same pattern of response was seen here with respect to anger, it suggests that the relationship between neuropeptide systems and affect is organized differently in each sex.
Limitations
In the current study, OT and AVP were assessed from peripheral blood draws, which raises questions as to the relationship between central and peripheral measures of these neuropeptides. One view has been that central and peripheral release of oxytocin and vasopressin are differentially regulated. In response to some stressors (e.g. physical shaking; Nishioka, Anselmo-Franci, Li, Callahan, & Morris, 1998), activation of oxytocin and vasopressin neurons in the hypothalamic paraventricular and supraoptic nuclei triggers release of these peptides into the circulation, which is correlated with release of these peptides from the soma and dendrites into the hypothalamus. It has been postulated that this somatodendritic release is the source of oxytocin and vasopressin that diffuses to forebrain sites regulating behavior (Ludwig, Callahan, & Morris, 1995; Leng & Ludwig, 2008; Ludwig & Leng, 2006). In contrast, other stressors, including psychological stressors, can trigger somatodendritic release of either oxytocin (Engelmann, Ebner, Landgraf, Holsboer, & Wotjak, 1999) or vasopressin (Ebner, Wotjak, Landgraf, & Engelmann, 2005) into the hypothalamus and surrounding parenchyma without axonal release into the peripheral circulation. However, recent anatomical work in rodents has identified magnocellular oxytocin neurons in the hypothalamus that project to forebrain areas (Ross, Cole, Smith, Neumann, Landgraf, Murphy, et al., 2009) and release oxytocin (Knobloch, Charlet, Hoffmann, Eliava, Khrulev, Cetin, et al., 2012). This suggests that there could be a coupling between oxytocin released into the peripheral circulation and oxytocin released into forebrain areas regulating behavior. This would be consistent with the many studies in which peripheral levels of OT and AVP predict psychological, emotional, and behavioral outcomes (Taylor, Saphire-Bernstein, & Seeman, 2010; Uvnäs-Moberg, Widström, Nissen, & Björvell, 1990; Uvnäs-Moberg, Johansson, Lupoli, & Svennersten-Sjuanja, 2001; Uvnäs-Moberg, Arn, & Magnusson, 2005). Peripheral OT and AVP likely reflect proportional increases or decreases in brain levels of these neuropeptides. Although the current sample may be limited by a relatively small sample, future research elucidating and elaborating on these findings can do so with larger samples.
Due to the challenges of studying neuorochemical processes within the brain, it is difficult to disentangle the precise mechanisms underlying the interaction between neuropeptide and receptor reported here. The oxytocin receptor and vasopressin 1A receptor are not just located post-synaptically on cells receiving oxytocin and vasopressin signals. These receptors are also located on the cell bodies and dendrites of the oxytocin and vasopressin neurons themselves (Brown, Bains, Ludwig, & Stern, 2013). These autoreceptors regulate the activity of oxytocin and vasopressin neurons and can thereby impact the extracellular concentrations of oxytocin and vasopressin (Neumann & Landgraf, 2012). Critical for determining whether the reported results are mediated by autoreceptors or post-synaptic receptors is understanding whether or not the studied polymorphisms have differential effects on the expression or function of the receptor when it is expressed in the oxytocin or vasopressin neuron or a post-synaptic neuron. Although it is not clear how rs53576 could impact the OT receptor function or density, there is evidence that the AVPR1A RS1 polymorphism influences gene expression in a neural cell line (Tansey et al., 2011). However, it is not yet clear if these effects are differentially regulated in different cell types.
Beyond the potential effects of the rs53576 and AVPR1A RS1 polymorphisms on levels of OT and AVP, the causes of the relatively low or high levels of OT and AVP in the plasma are not entirely clear. The perceived intensity of the stressor is likely determined by early cognitive appraisals (Lazarus & Folkman, 1984; Tomaka, Blascovich, Kibler, & Ernst, 1997; Smith & Ellsworth, 1985), which likely contribute to variation in OT and AVP production or output. At the genetic level, variations in the genes responsible for OT and AVP production may play a role in the variable levels of stress-linked neuropeptides (Ebstein, Israel, Lerer, Uzefovsky, Shalev, Gritsenko, et al., 2009). In short, the potential reasons why people respond to a stressor with lower or higher levels of OT and AVP are not yet fully understood.
Although the links between OT and positive affect as well as between AVP and anger have been shown here, the neural mechanisms by which neuropeptides translate into differential emotional responses are not clear. If OT and AVP do cause positive affect and anger, respectively, then changes in emotion-related neural regions would be expected. Some evidence has shown that OT does impact the amygdala, a crucial neural region for emotional processing. Greater OT reduces amygdala activity in response to negative stimuli but increases it in response to positive stimuli (Gamer, Zurowski, & Büchel, 2010). This pattern can be interpreted as reduced threat and enhanced attention to positive stimuli suggesting that there is indeed a neural basis for the relationship between OT and positive affect. Similarly, variations in AVPR1A RS1, including the 320 allele, have also been linked to activity in the amygdala (Meyer-Lindberg, Kolachana, Gold, Olsh, Nicodemus, Mattay, et al., 2008). Although these neural pathways are not fully mapped, there is good indication that the oxytocin and vasopressin systems can and do impact emotions via differential activation of emotion-linked neural regions.
Conclusion
The current investigation demonstrated that polymorphisms in the OXTR rs53576 and AVPR1A RS1 receptor genes in combination with circulating levels of OT and AVP, respectively, predicted men and women’s emotional responses to an acute stressor. This constitutes the first documented examination of how biological sex, receptor polymorphisms, and endogenous neuropeptide levels jointly predict emotional outcomes in humans. Future behavioral and pharmacological researchers may wish to consider more closely how receptor polymorphisms can modulate the effects of OT and AVP on men and women.
Acknowledgments
This research was supported by grants from the National Institute on Aging (AG030309), the National Science Foundation (BCS-1124552), and the National Center for Advancing Translational Sciences (8KL2TR000112-05).
Footnotes
Because the primers used to genotype RS1differ across studies, allele lengths reported in the literature differ. For example, the 320 allele examined here and in Meyer-Lindenberg et al., 2009 corresponds to allele 314 of Bachner-Mehlman et al., 2004.
rs53576 could not be genotyped for 13 participants (six female) and four women could not be genotyped for RS1, thus these subjects were not included in OXTR and APR1A analyses, respectively.
Before reporting their baseline emotions participants also completed questions regarding their general health, the UCLA loneliness scale (Russell, Peplau, & Cutrona, 1980), the type and quality of their relationships, life satisfaction, recent changes in level of contact in their relationships, the current composition of their immediate family, and the Brief COPE (Carver, 1997).
Confederates acted in negative or positive ways but this factor had no impact on results and did not interact with other predictors and is therefore only included as a covariate in all analyses.
The observed interactions persisted if Latino/a was coded as a separate group.
Overall there were no outliers with the exception of a single participant’s post-stressor anger score (Z=3.63). However, exclusion of this participant from analyses had no impact on the reported findings.
Contributor Information
Wesley G. Moons, Email: wgmoons@ucdavis.edu.
Baldwin M. Way, Email: way.37@osu.edu.
Shelley E. Taylor, Email: taylors@ucla.edu.
References
- Albers HE, Bamshad M. Role of vasopressin and oxytocin in the control of social behavior in Syrian hamsters (Mesocricetus auratus) Advances in Brain Vasopressin. 1998;119:395–408. doi: 10.1016/s0079-6123(08)61583-6. [DOI] [PubMed] [Google Scholar]
- Altemus M, Deuster PA, Galliven E, Carter CS, Gold PW. Suppression of hypothalmic-pituitary-adrenal axis responses to stress in lactating women. Journal of Clinical Endocrinology & Metabolism. 1995;80:2954–2959. doi: 10.1210/jcem.80.10.7559880. [DOI] [PubMed] [Google Scholar]
- Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proceedings of the National Academy of Sciences. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averill JR. Studies on anger and aggression: Implications for theories of emotion. American Psychologist. 1983;38:1145. doi: 10.1037//0003-066x.38.11.1145. [DOI] [PubMed] [Google Scholar]
- Bachner-Melman R, Dina C, Zohar AH, Constantini N, Lerer E, Hoch S, et al. AVPR1A and SLC6A4 gene polymorphisms are associated with creative dance performance. PLoS Genetics. 2005;1:e42. doi: 10.1371/journal.pgen.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Social Cognitive and Affective Neuroscience. 2008;3:128–134. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Hollander E. Oxytocin and experimental therapeutics in autism spectrum disorders. Progress in Brain Research. 2008;170:451–462. doi: 10.1016/S0079-6123(08)00435-4. [DOI] [PubMed] [Google Scholar]
- Brown CH, Bains JS, Ludwig M, Stern JE. Physiological regulation of magnocellular neurosecretory cell activity: Integration of intrinsic, local and afferent mechanisms. Journal of Neuroendocrinology. 2013;25:678–710. doi: 10.1111/jne.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein MJ, Russell JT, Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980;20:373–378. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- Carter CS. Sex differences in oxytocin and vasopressin: Implications for autism spectrum disorders? Behavioural Brain Research. 2007;176:170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Carter CS, Altemus M. Integrative functions of lactational hormones in social behavior and stress management. Annals of the New York Academy of Science. 1997;807:164–174. doi: 10.1111/j.1749-6632.1997.tb51918.x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Boone EM, Pournajafi-Nazarloo H, Bales KL. Consequences of early experiences and exposure to oxytocin and vasopressin are sexually dimorphic. Developmental Neuroscience. 2009;31:332–341. doi: 10.1159/000216544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS. You want to measure coping but your protocol’s too long: Consider the Brief COPE. International Journal of Behavioral Medicine. 1997;4:92–100. doi: 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- Carver CS, Harmon-Jones E. Anger is an approach-related affect: Evidence and implications. Psychological Bulletin. 2009;135:183–204. doi: 10.1037/a0013965. [DOI] [PubMed] [Google Scholar]
- Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and-beta knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF. Impulsive aggression: A behavior in search of clinical definition. Harvard Review of Psychiatry. 1998;5:336–339. doi: 10.3109/10673229809003583. [DOI] [PubMed] [Google Scholar]
- de Oliveira DCG, Zuardi AW, Graeff FG, Queiroz RH, Crippa JA. Anxiolytic-like effect of oxytocin in the simulated public speaking test. Journal of Psychopharmacology. 2012;26:497–504. doi: 10.1177/0269881111400642. [DOI] [PubMed] [Google Scholar]
- de Vries GJ. Sex differences in vasopressin and oxytocing inneveration of the brain. Progressive Brain Research. 2008;170:17–27. doi: 10.1016/S0079-6123(08)00402-0. [DOI] [PubMed] [Google Scholar]
- de Vries AC, de Vries MB, Taymans SE, Carter CS. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proceedings of the National Academy of Science. 1996;93:11980–11984. doi: 10.1073/pnas.93.21.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner K, Wotjak CT, Landgraf R, Engelmann M. Neuroendocrine and behavioral response to social confrontation: residents versus intruders, active versus passive coping styles. Hormones & Behavior. 2005;47:14–21. doi: 10.1016/j.yhbeh.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Israel S, Lerer E, Uzefovsky F, Shalev I, Gritsenko I, et al. Arginine vasopressin and oxytocin modulate human social behavior. In: Atran S, et al., editors. Values, Empathy, and Fairness across Social Barriers. 2009. pp. 87–102. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Ebner K, Landgraf R, Holsboer F, Wotjak CT. Emotional stress triggers intrahypothalamic but not peripheral release of oxytocin in male rats. Journal of Neuroendocrinology. 1999;11:867–872. doi: 10.1046/j.1365-2826.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- Excoffier L, Lischer HEL. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources. 2010;10:564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nature Reviews Neuroscience. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation - Plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychological Science. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Jr, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. The Journal of Neuroscience. 1997;17:4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Stolberg T, Delville Y. Serotonin regulation of aggressive behavior in male golden hamsters (Mesocricetus auratus) Behavioral Neuroscience. 1999;113:804–815. doi: 10.1037//0735-7044.113.4.804. [DOI] [PubMed] [Google Scholar]
- Ferris CF. Adolescent stress and neural plasticity in hamsters: a vasopressin-serotonin model of inappropriate aggressive behaviour. Experimental Physiology. 2000;85:85s–90s. doi: 10.1111/j.1469-445x.2000.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Lu SF, Messenger T, Guillon CD, Heindel N, Miller M, et al. Orally active vasopressin V1a receptor antagonist, SRX251, selectively blocks aggressive behavior. Pharmacology, Biochemistry and Behavior. 2006;83:169–174. doi: 10.1016/j.pbb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Gabor CS, Phan A, Clipperton-Allen AE, Kavaliers M, Choleris E. Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behavioral Neuroscience. 2012;126:97–109. doi: 10.1037/a0026464. [DOI] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, Büchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proceedings of the National Academy of Sciences. 2010;107:9400–9405. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The Oxytocin Receptor System: Structure, function, and regulation. Physiological Reviews. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Research Reviews. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosomatic Medicine. 2005;67:531–538. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJB. Anger and frontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. Journal of Personality and Social Psychology. 1998;74:1310–1316. doi: 10.1037//0022-3514.74.5.1310. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Meinlschmidt G, Neumann I, Wagner S, Kirschbaum C, Ehlert U, et al. Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. Journal of Clinical Endocrinology & Metabolism. 2001;86:4798–4804. doi: 10.1210/jcem.86.10.7919. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Domes G. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans, in Advances in Vasopressin and Oxytocin. In: Neumann ID, Landgraf R, editors. Genes to Behaviour to Disease. 2008. pp. 337–350. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Young LJ, Gonen D, Veenstra-VanderWeele J, Courchesne R, Courchesne E, et al. Transmission disequilibrium testing of arginine vasopressin receptor 1A (AVPR1A) polymorphisms in autism. Molecular Psychiatry. 2002;7:503–507. doi: 10.1038/sj.mp.4001125. [DOI] [PubMed] [Google Scholar]
- Kim HS, Sherman DK, Sasaki JY, Xu J, Chu TQ, Ryu C, et al. Culture, distress, and oxytocin receptor polymorphism (OXTR) interact to influence emotional support seeking. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15717–15721. doi: 10.1073/pnas.1010830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Sherman DK, Mojaverian T, Sasaki JY, Park J, Suh EM, et al. Gene-Culture Interaction: Oxytocin Receptor Polymorphism (OXTR) and Emotion Regulation. Social Psychological and Personality Science. 2011;2:665–672. [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The “Trier Social Stress Test”: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal, and coping. Springer Publishing Company; 1984. [Google Scholar]
- Leng G, Ludwig M. Neurotransmitters and peptides: whispered secrets and public announcements. Journal of Physiology-London. 2008;586:5625–5632. doi: 10.1113/jphysiol.2008.159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R, Heresco-Levy U, Bachner-Melman R, Israel S, Shalev I, Ebstein RP. Association between arginine vasopressin 1a receptor (AVPR1A) promoter region polymorphisms and prepulse inhibition. Psychoneuroendocrinology. 2009;34:901–908. doi: 10.1016/j.psyneuen.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Light KC, Grewen KM, Amico JA. More frequent partner hugs and higher oxytocin levels are linked to lower blood pressure and heart rate in premenopausal women. Biological Psychology. 2005;69:5–21. doi: 10.1016/j.biopsycho.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Lucht MJ, Barnow S, Sonnenfeld C, Rosenberger A, Grabe HJ, Schroeder W, et al. Associations between the oxytocin receptor gene (OXTR) and affect, loneliness and intelligence in normal subjects. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2009;33:860–866. doi: 10.1016/j.pnpbp.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Callahan MF, Morris M. Effects of tetrodotoxin on osmotically stimulated central and peripheral vasopressin and oxytocin release. Neuroendocrinology. 1995;62:619–627. doi: 10.1159/000127058. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide dependent behaviours. Nature Reviews Neuroscience. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Yu HH, Pine DS, Gorodetsky EK, Goldman D, Blair RJR. The influence of oxytocin administration on responses to infant faces and potential moderation by OXTR genotype. Psychopharmacology. 2012;224:469–476. doi: 10.1007/s00213-012-2775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindberg A, Kolachana B, Gold B, Olsh A, Nicodemus KK, Mattay V, et al. Genetic variants in AVPR1A linked to autism predict amygdala activation and personality traits in healthy humans. Molecular Psychiatry. 2008;14:968–975. doi: 10.1038/mp.2008.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews Neuroscience. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Mezzacappa ES, Katkin ES. Breast-feeding is associated with reduced perceived stress and negative mood in mothers. Health Psychology. 2002;21:187. [PubMed] [Google Scholar]
- Mezzacappa ES. Breastfeeding and maternal stress response and health. Nutrition Reviews. 2004;62:261–268. doi: 10.1111/j.1753-4887.2004.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Miller M, Bales KL, Taylor SL, Yoon J, Hostetler CM, Carter CS, Solomon M. Oxytocin and vasopressin in children and adolescents with autism spectrum disorders: Sex differences and associations with symptoms. Autism Research. 2013;6:91–102. doi: 10.1002/aur.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Bailey AL. Sex and gender differences in pain and analgesia. Progress in Brain Research. 2010;186:141–157. doi: 10.1016/B978-0-444-53630-3.00009-9. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Sorge RE, LaCroix-Fralish ML, Smith SB, Fortin A, Sotocinal SG, Ritchie J, et al. Pain sensitivity and vasopressin analgesia are mediated by a gene-sex-environment interaction. Nature Neuroscience. 2011;14(12):1569–1573. doi: 10.1038/nn.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moons WG, Eisenberger NI, Taylor SE. Anger and fear responses to stress have different biological profiles. Brain, Behavior, and Immunity. 2010;24:215–219. doi: 10.1016/j.bbi.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Mueller KL, Thomas MS, Burbach BJ, Peterson EJ, Shimizu Y. Adhesion and degranulation-promoting adapter protein (ADAP) positively regulates T cell sensitivity to antigen and T cell survival. Journal of Immunology. 2007;179:3559–3569. doi: 10.4049/jimmunol.179.6.3559. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends in Neurosciences. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Nikolova YS, Bogdan E, Brigidi BD, Hariri AR. Ventral striatum reacitvity to reward and recent life stress interact to predict positive affect. Biological Psychiatry. 2012;72:157–163. doi: 10.1016/j.biopsych.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Nishioka T, Anselmo-Franci JA, Li P, Callahan MF, Morris M. Stress increases oxytocin release within the hypothalamic paraventricular nucleus. Brain Research. 1998;781:57–61. doi: 10.1016/s0006-8993(97)01159-1. [DOI] [PubMed] [Google Scholar]
- Pavlov KA, Chistiakov DA, Chekhonin VP. Genetic determinants of aggression and impulsivity in humans. Journal of Applied Genetics. 2012;53:61–82. doi: 10.1007/s13353-011-0069-6. [DOI] [PubMed] [Google Scholar]
- Riem MME, Pieper S, Out D, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Oxytocin receptor gene and depressive symptoms associated with physiological reactivity to infant crying. Social Cognitive and Affective Neuroscience. 2011;6:294–300. doi: 10.1093/scan/nsq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21437–21441. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, et al. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D, Peplau LA, Cutrona CE. The revised UCLA Loneliness Scale: Concurrent and discriminant validity evidence. Journal of Personality and Social Psychology. 1980;39:472–480. doi: 10.1037//0022-3514.39.3.472. [DOI] [PubMed] [Google Scholar]
- Saphire-Bernstein S, Way BM, Kim HS, Sherman DK, Taylor SE. Oxytocin receptor gene (OXTR) is related to psychological resources. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15118–15122. doi: 10.1073/pnas.1113137108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Israel S, Uzefovsky F, Gritsenko I, Kaitz M, Ebstein RP. Vasopressin needs an audience: Neuropeptide elicited stress responses are contingent upon perceived social evaluative threats. Hormones and Behavior. 2011;60:121–127. doi: 10.1016/j.yhbeh.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Sicard MN, Zai CC, Tiwari AK, Souza RP, Meltzer HY, Lieberman JA, Kennedy JL, Muller DJ. Polymorphisms of the HTR2C gene and antipsychotic-induced weight gain: An update and meta-analysis. Pharmacogenomics. 2010;11:1561–1571. doi: 10.2217/pgs.10.123. [DOI] [PubMed] [Google Scholar]
- Smith CA, Ellsworth PC. Patterns of cognitive appraisal in emotion. Journal of Personality and Social Psychology. 1985;48:813–838. [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Maier W, Hurlemann R. Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Frontiers in Neuroendocrinology. 2011;32:426–450. doi: 10.1016/j.yfrne.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Pool CW, Nijveldt F. Immunofluorescence of vasopressin and oxytocin in the rat hypothalamo-neurohypophyseal system. Journal of Neural Transmission. 1975;36:195–215. doi: 10.1007/BF01253126. [DOI] [PubMed] [Google Scholar]
- Tabak BA, McCullough ME, Szeto A, Mendez AJ, McCabe PM. Oxytocin indexes relational distress following interpersonal harms in women. Psychoneuroendocrinology. 2011;36:115–122. doi: 10.1016/j.psyneuen.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey KE, Brookes KJ, Hill MJ, Cochrane LE, Gill M, Skuse D, et al. Oxytocin receptor (OXTR) does not play a major role in the aetiology of autism: Genetic and molecular studies. Neuroscience Letters. 2010;474:163–167. doi: 10.1016/j.neulet.2010.03.035. [DOI] [PubMed] [Google Scholar]
- Tansey KE, Hill MJ, Cochrane LE, Gill M, Anney RJ, Gallagher L. Functionality of promoter microsatellites of arginine vasopressin receptor 1A (AVPR1A): Implications for autism. Molecular Autism. 2011;2 doi: 10.1186/2040-2392-2-3. 3-2392-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Gonzaga GC, Klein LC, Hu P, Greendale GA, Seeman TE. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocorticaI axis activity in older women. Psychosomatic Medicine. 2006;68:238–245. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Saphire-Bernstein S, Seeman TE. Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychological Science. 2010;21:3–7. doi: 10.1177/0956797609356507. [DOI] [PubMed] [Google Scholar]
- Thompson RR, Gupta S, Miller K, Mills S, Orr S. The effects of vasopressin on human facial responses related to social communication. Psychoneuroendocrinology. 2004;29:35–80. doi: 10.1016/s0306-4530(02)00133-6. [DOI] [PubMed] [Google Scholar]
- Thompson RR, George K, Walton JC, Orr SP, Benson J. Sex-specific influences of vasopressin on human social communication. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7889–7894. doi: 10.1073/pnas.0600406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaka J, Blascovich J, Kibler J, Ernst JM. Cognitive and psychological antecedents of threat and challenge appraisal. Journal of Personality and Social Psychology. 1997;73:63–72. doi: 10.1037//0022-3514.73.1.63. [DOI] [PubMed] [Google Scholar]
- Uvnäs-Moberg K, Widström AM, Nissen E, Björvell H. Personality traits in women 4 days postpartum and their correlation with plasma levels of oxytocin and prolactin. Journal of Psychosomatic Obstetrics & Gynecology. 1990;11:261–273. [Google Scholar]
- Uvnäs-Moberg K, Johansson B, Lupoli B, Svennersten-Sjaunja K. Oxytocin facilitates behavioural, metabolic and physiological adaptations during lactation. Applied Animal Behaviour Science. 2001;72:225–234. doi: 10.1016/s0168-1591(01)00112-5. [DOI] [PubMed] [Google Scholar]
- Uvnäs-Moberg K, Arn I, Magnusson D. The psychobiology of emotion: The role of the oxytocinergic system. International Journal of Behavioral Medicine. 2005;12:59–65. doi: 10.1207/s15327558ijbm1202_3. [DOI] [PubMed] [Google Scholar]
- Vandesande F, Dierickx K. Identification of the vasopressin producing and of the oxytocin producing neurons in the hypothalamic magnocellular neurosecretory system of the rat. Cell and Tissue Research. 1975;164:153–162. doi: 10.1007/BF00218970. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Kuwabara J, Kawakubo Y, Kasai K. Oxytocin, sexually dimorphic features of the social brain, and autism. Psychiatry and Clinical Neuroscience. 2009;63:129–140. doi: 10.1111/j.1440-1819.2009.01944.x. [DOI] [PubMed] [Google Scholar]
- Yirmiya N, Rosenberg C, Levi S, Salomon S, Shulman C, Nemanov L, et al. Association between the arginine vasopressin 1a receptor (AVPR1A) gene and autism in a family-based study: mediation by socialization skills. Molecular Psychiatry. 2006;11:488–494. doi: 10.1038/sj.mp.4001812. [DOI] [PubMed] [Google Scholar]
- Zak PJ, Kurzban R, Matzner WT. Oxytocin is associated with human trustworthiness. Hormones and Behavior. 2005;48:522–527. doi: 10.1016/j.yhbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Zhang JP, Lencz T, Malhotra AK. D2 receptor genetic variation and clinical response to antipsychotic drug treatment: A meta-analysis. The American Journal of Psychiatry. 2010;167:763–772. doi: 10.1176/appi.ajp.2009.09040598. [DOI] [PMC free article] [PubMed] [Google Scholar]