It has been hypothesized that memories are encoded by modification of synaptic strengths through cellular mechanisms such as long-term potentiation (LTP) and long-term depression (LTD)1. However the causal link between these synaptic processes and memory has been difficult to demonstrate2. Here we show that fear conditioning3–8, a type of associative memory, can be inactivated and reactivated by LTD and LTP, respectively. We begin by conditioning an animal to associate a foot-shock with optogenetic stimulation of auditory inputs targeting the amygdala, a brain region known to be essential for fear conditioning3–8. Subsequent optogenetic delivery of LTD conditioning to the auditory input inactivates memory of the shock. And finally, subsequent optogenetic delivery of LTP conditioning to the auditory input reactivates memory of the shock. Thus, we have engineered inactivation and reactivation of a memory using LTD and LTP, supporting a causal link between these synaptic processes and memory.
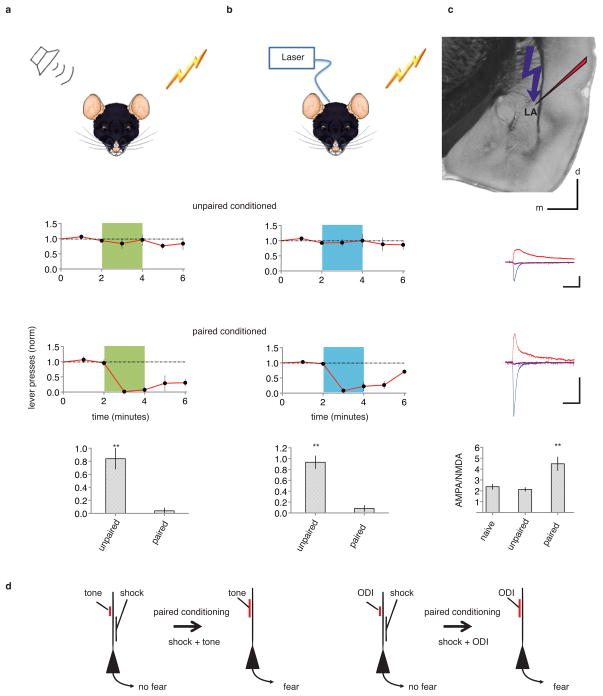
To examine the relation between synaptic plasticity and memory, we used cued-fear conditioning3–8 in rats, wherein a neutral conditioned stimulus (CS), such as a tone, when paired with an aversive unconditioned stimulus (US), results in a tone-driven conditioned response (CR) indicating memory of the aversive stimulus. Temporally (but not non-temporally) pairing a tone with a shock led to a robust CR (reduced lever pressing to a previously learned cued lever-press task9; Extended Data Fig. 1) during subsequent testing with a tone alone (ref. 3–8, Fig. 1a). To investigate the synaptic basis underlying this associative memory we replaced a tone with optogenetic stimulation of neural inputs to the lateral amygdala originating from auditory nuclei. We injected an adeno-associated virus (AAV) expressing a variant of the light-activated channel ChR2, oChIEF, that can follow 50–100 Hz stimuli10, into the medial geniculate nucleus and auditory cortex (Extended Data Fig. 2). After the channel reached axonal terminals in the lateral amygdala (Extended Data Fig. 3), a cannula permitting light delivery was placed targeting the dorsal tip of the lateral amygdala (Extended Data Fig. 4). An optical CS alone (a two minute 10 Hz train of 2 ms pulses, see methods) had no effect on lever pressing (Extended Data Fig. 5). However, temporally (but not non-termporally) pairing the optical CS with a foot shock (see methods) led to a CR (Fig. 1b) that was sensitive to extinction (see below) and blocked by NMDA receptor inhibition during conditioning (Extended Data Fig. 6), indicating the generation of an associative memory.
Figure 1. Fear conditioning with tone or optogenetics.
a, Top, diagram of rat receiving tone and shock during conditioning. Rats exposed to unpaired (N=5, middle) or temporally paired (N=5, bottom) tone and shock were tested one day later by a tone (green). Time plots: normalized number of lever presses (1 minute bins) to a previously learned cued lever-press task. Bar graph: normalized number of lever presses during the first minute of tone. b, Top, diagram of rat receiving optogenetically driven input (ODI) stimulation and shock during conditioning. Rats (N=8) received unpaired (middle) and one day later temporally paired (bottom) ODI and shock. Time graphs as in a, except animals were tested by 10 Hz ODI (blue). Bar graph as in a for 10 Hz ODI. c, top, experimental design; averaged optically-driven synaptic responses obtained at −60 mV (blue), +40 mV (red) and 0 mV holding potential for cells from animals that received unpaired (top) or paired (bottom) conditioning. Traces were scaled to match NMDA-mediated currents. Bar graph plots average AMPA/NMDA (no conditioning, 2.4±0.2, N=11; unpaired conditioning 2.1±0.2, N=10; paired conditioning 4.4±0.6, N=8). Scale bars, 100 pA, 50 ms, 1 mm. d, Synaptic modification model. Temporally pairing of tone (left) or ODI (right) and shock inputs to lateral amygdala neurons leads to potentiation of tone (left) or ODI (right) input, which can contribute in triggering CR. Here and throughout: NS, non significance; *, p<0.05; **, P<0.01; error bars, SEM. See methods for details.
To examine if LTP occurred after pairing optical CS with foot-shock (Fig. 1d, refs3–8), we prepared amygdala brain slices from animals receiving unpaired, paired, or no conditioning, and measured the AMPA receptor component (A) and NMDA receptor component (N) of the optically driven synaptic response (Fig 1c). The A/N ratio increased in animals receiving paired conditioning indicating that LTP had occurred11,12 at optically driven inputs to lateral amygdala neurons.
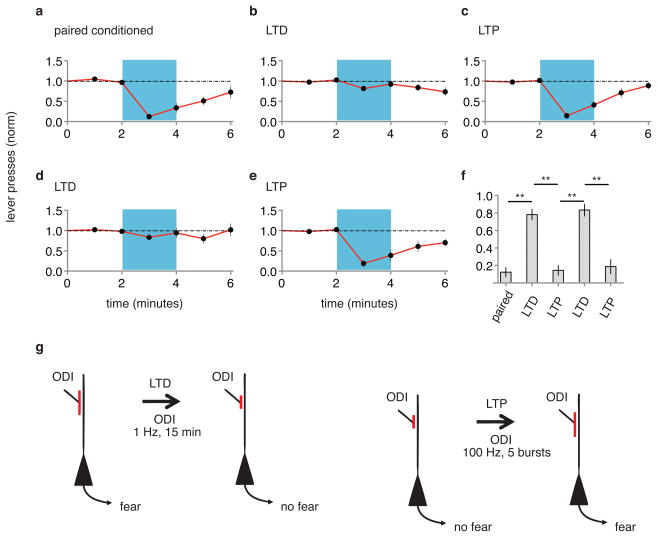
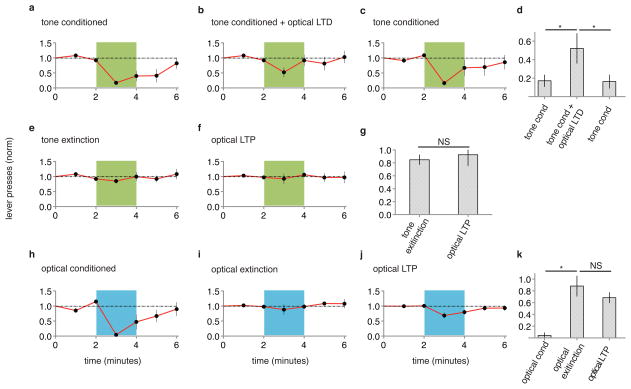
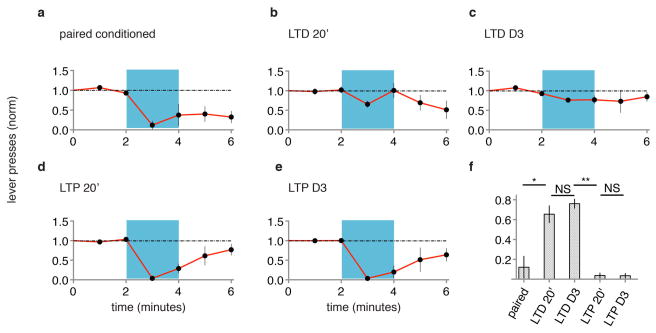
Can memories be inactivated? If LTP occurred at the optically driven synapse onto the lateral amygdala, and this LTP contributes to the formation of a memory, reversing LTP with LTD (refs 13, 14) should inactivate the memory. Animals that displayed CR after paired optical CS-shock conditioning were exposed to an optical LTD protocol (see methods). One day later, animals were tested with optical CS and displayed no CR, indicating inactivation of the memory of the shock by LTD (Fig. 2a, b, f). Can memories be reactivated? To these animals we delivered an optical LTP protocol (see methods). One day later animals displayed a CR (Fig. 2c, f), suggesting reactivation of the memory. Synapses are capable of undergoing multiple rounds of bidirectional plasticity13. We thus delivered a second optical LTD protocol; the next day animals produced no CR (Fig. 2d, f), indicating re-inactivation of the memory. Subsequent optical LTP conditioning recovered the CR (Fig. 2e, f and Extended Data Fig. 7) indicating reactivation of the memory. The behavioral effects of LTD and LTP conditioning were rapid and long lasting (Extended Data Fig. 8). These experiments suggest that a necessary component of the optical CS-triggered memory of the shock can be inactivated by LTD and reactivated by LTP.
Figure 2. LTD inactivates and LTP reactivates memory.
A single group of rats (N = 12) was tested for CR two days following paired conditioning of ODI and shock (a). Graphs as in Fig. 1. After testing, animals were delivered an optical LTD protocol and tested for CR one day later (b). After testing, animals were delivered an optical LTP protocol and tested for CR one day later (c). After testing, animals were delivered another optical LTD protocol and tested for CR one day later (d). After testing, animals were delivered another optical LTP and tested for CR one day later (e). f, Normalized lever presses one minute into optical CS after different protocols (as indicated). g, Cellular models of synaptic modifications occurring in the lateral amygdala that may contribute to behavioral responses following LTD (left) or LTP (right) protocols delivered to ODI.
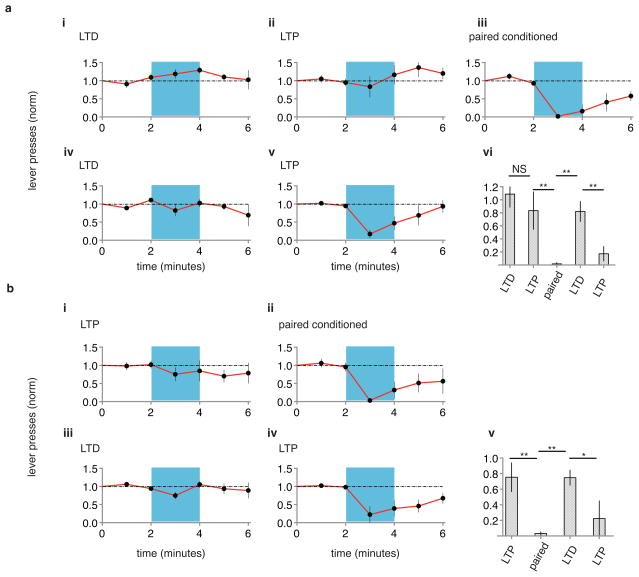
In the experiments described above, is LTP restoring a memory of the shock? Or is the LTP conditioning merely potentiating random inputs that are sufficient to drive lateral amygdala neurons that reduce lever pressing? That is, does the generation of a CR by an LTP protocol require prior optical CS-shock pairing? Indeed, an LTP protocol produced a CR only in animals that had previously received optical CS-shock conditioning (Fig. 3). These results support the view that LTP reactivates the memory that was formed by optical CS-shock pairing and inactivated by LTD.
Figure 3. LTP produces conditioned response only after prior paired conditioning.
A naïve group of animals (a, N=4) was tested for CR one day after LTD protocol (i), one day after subsequent LTP protocol (ii), one day after subsequent paired optical CS-shock conditioning (iii), one day after subsequent LTD protocol (iv) and one day after subsequent LTP protocol (v). vi, Graph of normalized lever presses one minute into optical CS one day following indicated protocols. A separate naïve group of animals (b, N = 5) was tested for CR one day after LTP protocol (i), one day after paired optical CS-shock conditioning (ii), one day after subsequent LTD protocol (iii) and one day after subsequent LTP protocol (iv). v, Graph of normalized lever presses one minute into optical CS one day following indicated protocols. Note that CR is seen following LTP protocol only after prior paired conditioning.
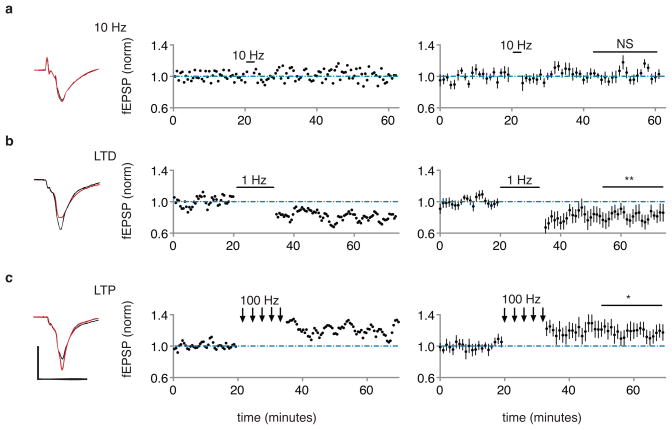
To confirm that the test and conditioning stimuli were producing the expected synaptic effects, we conducted in vivo recordings in the lateral amygdala of anesthetized rats expressing oChIEF in auditory regions (see methods). Brief light pulses at the recording site produced in vivo field responses (that resembled optically-evoked responses in amygdala brain slices; Extended Data Fig. 9), which were not affected by optical CS, depressed by optical LTD conditioning, and potentiated by optical LTP conditioning (Fig. 4; Extended Data Fig. 10). These results confirm that the synaptic stimulation conditioning protocols used to perturb behavior, modify synapses in the expected manner.
Figure 4. In vivo electrophysiological responses to 10Hz, LTD and LTP protocols.
a, b, c, Left, in vivo field response (average of 20 responses) in lateral amygdala to single optical stimulus before (black) and after (red) indicated conditioning protocol. Plot of individual experiment (middle) or average of 10 experiments (right) of field EPSP slope (normalized to baseline period) before and after indicated stimulation. Average baseline normalized value 30–40 minutes following conditioning: 10 Hz, 102.2 ± 5%; 1 Hz, 82 ± 8%; 100 Hz, 118 ± 9%. Scale bars, 1 mV, 10 ms.
To examine further the relationship between these synaptic stimulation conditioning protocols and memory processes, we tested the effects of these protocols on auditory cued-fear conditioning. We first asked if optical LTD could inactivate tone-induced fear conditioning. In two groups of naïve animals we infected unilaterally auditory regions with AAV-oChIEF, and pharmacologically ablated the contralateral amygdala (see methods). One group of animals received tone paired with shock, which led to a tone-evoked CR (Fig. 5a, d). A second group of animals received the same tone paired with shock conditioning immediately followed by an optical LTD protocol. This second group showed significantly reduced tone-evoked CR (Fig. 5b, d); subsequent tone conditioning without an optical LTD protocol produced a tone-evoked CR (Fig. 5c, d). This result is consistent with a memory model in which tone conditioning induces LTP at auditory inputs to the lateral amygdala and that subsequent LTD at these synapses reverses LTP and thereby inactivates the memory.
Figure 5. Optical LTD protocol significantly reduces auditory fear conditioning; optical LTP does not reverse auditory extinction.
Separate groups of animals were exposed to (a) paired tone and shock conditioning (N = 5), or (b) paired tone and shock conditioning followed by optical LTD protocol, and subsequently tested for CR with tone (green). c, Animals shown in (b) were subsequently exposed to paired tone and shock conditioning and tested for CR. d, optical LTD significantly reduces auditory fear conditioning. e, Animals shown in (c) were exposed to auditory extinction protocol and tested for CR; f, animals received optical LTP and tested for CR. g, optical LTP did not reverse auditory extinction. A naïve group of animals (N=5) received paired optical conditioning and tested for CR (h); then received optical extinction protocol (see methods) and tested for CR (i); then received optical LTP protocol and tested for CR (j). k, optical LTP did not reverse optical extinction.
Next we examined extinction, a process whereby repeated exposure to a CS (in the absence of a US) leads to a reduced CR. Can optical LTP reverse extinction of tone conditioning? Animals received tone conditioning and an extinction protocol (see methods), which removed the CR (Fig. 6e). Delivery of an optical LTP protocol did not produce a CR (Fig. 6f, g), consistent with the view that extinction is not a weakening of synapses potentiated during paired conditioning14. Similarly, animals receiving paired optical CS-shock conditioning produced a CR that could be removed by repeated exposure to optical CS (see methods) and optical LTP did not recover the CR (Fig. 6h, i, j, k), again demonstrating that extinction is not LTD.
Prior studies examining the relation between LTP, LTD and memory have employed pharmacological (e.g. ref. 15) or genetic (e.g. refs 16,17) manipulations to perturb and demonstrate parallels between cellular and behavioral processes. Other studies have measured randomly sampled sites in regions required for memory formation to detect changes in biochemistry and synaptic transmission following memory formation18–21. However, selective perturbation of synapses that are employed to form a memory was not possible in these studies. Here, by optogenetically isolating a neural input that can be used to form an associative memory, we can selectively manipulate synapses driven by this input and assess directly the relationship between cellular and behavioral processes.
Formation of an associative memory produced LTP at the lateral amygdala optogenetic input, as indicated by an increased A/N. Such LTP appears to be required as delivery of an LTD conditioning stimulus that can reverse LTP effectively removed the ability of the optogentic input to elicit the memory. Furthermore, subsequent delivery of an LTP conditioning stimulus to the optogenetic neural input restored the CR. Our data support the view that LTP had reactivated the memory of the aversive stimulus, because delivery of an LTP protocol without prior formation of the memory did not evoke a CR. Our findings demonstrate that memories of aversive events formed through activation of selected inputs can be turned off and on by conditioning protocols that produce bidirectional synaptic plasticity at those inputs, strengthening the causal relation between synaptic plasticity and memory formation22.
It is notable that optical LTP in naïve animals did not produce a CR; while in these animals, optical LTP did produce a CR after optical CS-shock pairing and optical LTD. This result suggests that non-specific potentiation of auditory inputs to the lateral amygdala is not sufficient to produce a CR. It may be that specific potentiation onto a subset of inputs, presumably those neurons also activated by the foot shock, is necessary to produce a conditioned response. Furthermore, the pairing of optical CS with shock likely produces additional modifications (not produced by optical LTP alone) that may be required to produce a CR23–26. Thus, LTP at auditory inputs to the amygdala may be necessary but not sufficient to produce an associative memory.
Our studies complement recent studies that have used optogenetics to examine how neuronal assemblies can represent a memory27–29. In those studies synaptic mechanisms were not examined. Our studies suggest that LTP is used to form neuronal assemblies that represent a memory. Furthermore, our findings predict that LTD could be used to disassemble them, and thereby inactivate a memory.
Methods
Subject
Male Sparague-Dawley rats, age 6–8 weeks for virus injection and cannula placement and 10–12 weeks for behavioral and electrophysiological studies, were housed two per cage and kept on a 12/12 hours light-dark cycle (lights on/off at 7 a.m./7 p.m.). The behavioral studies were done during daylight. All procedures involving animals were approved by the Institutional Animal Care and Use Committees of the University of California, San Diego.
Virus
We used a ChR variant, named oChIEF, which is a mammalian codon optimized version of ChIEF30 with the same properties except that it has stronger expression in mammalian cells and has an additional N-terminal amino acid residue. Expression was driven by the neuron-specifc synapsin promoter30.
Surgery
Male Sparague-Dawley rats, age 6–8 weeks, were anesthetized with isoflurane for stereotaxic injection of AAV-oChIEF into the medial geniculate nucleus (AP: −5.1 mm and −5.7 mm; ML: 2.9 mm; DV: −5.5 to −6.7 mm) and the auditory cortex (AP: −5.7 mm; ML: 4.8 mm with a 20° angle; DV: −4.5 to −5.7 mm). 0.4–0.5 μl of virus was injected over a 10–15 minute period. At the end of injection, pipet remained at the site for 5 minutes to allow for diffusion of the virus. An optic fiber cannula (Doric Lenses) was implanted just above the dorsal tip of the lateral amygdala (AP: −3.3 to −3.5 mm; ML: 4.2 mm; DV: −7 mm with a 7° angle) and secured to the skull with screws and dental cement. Rats were injected with 5mg/kg carprofen (NSAID) after surgery.
Excitotoxic Lesion
Rats, age 6–8 weeks, were anesthetized with isoflurane for stereotaxic injection of N-methyl-D-aspartate (NMDA) into one amygdala ((AP: −3 mm; ML: 4.2 mm; DV: −7 to −8 mm with a 7° angle). 0.5 μl of NMDA (20mg/ml) was injected over a 10–15 minute period31. At the end of injection, pipet remained at the site for 5 minutes to allow for diffusion of the solution.
Behavioral assays
Training
Rats were trained to associate lever press for a reward (40 μl of 10% sucrose per lever press). During the training period rats were kept on a restricted water schedule (2 hours daily of water ad libitum). Training context was a modular operant test chamber (12.5×10×13 in.) with a stainless grid floor and open roof located in a sound attenuating cubicle (Med Associates, St. Albans, VT). The test chamber was equipped with a retractable response lever, a liquid dispenser receptacle and a light above the dispenser that signaled when liquid was injected into the dispenser. The consumption of liquid was detected by a head entry detector in the receptacle; each successive liquid reward was subsequently followed with a 15 second delay after head removal from the receptacle. The system was controlled and the data collected through a MED-SYST-16 interface, which was controlled by MED-PCR IV software running on a PC. Rats were initially trained to associate the reward with the light above the dispenser receptacle. In a 45 minute session, rats with at least 60 head entries into the receptacle were selected for lever press training.
Lever-press training was conducted in the same context as above, but this time rats had to press a lever to receive the liquid. The level press turned the light above the receptacle on, which in the previous training session they had associated with liquid in the receptacle. Rats with a minimum of 6 responses/min in the first 10 minute of the training session were selected for conditioning.
Tone conditioning
The conditioning chamber was a box (12×10.5×13 in.) with an electrified grid floor (Coulbourn Instruments, Allentown, PA) within a larger sound-attenuating box. Rats had full access to water 24 hours before conditioning. Conditioning protocol consisted of 10 trials of 20 second tone (tone volume 80 dB), with randomized intervals (average interval duration 3 minutes). In the paired group tones were coterminated with a 0.5 second 0.5 mA footshock (or a single 20 second tone coterminated with 1 second 0.5 mA footshock for mild conditioning, Fig. 5). In unpaired group tones and shocks were separated by at least 1 min. Paired and unpaired groups received equal number of tones (CS) and shocks (US) in the same context; however, only in the paired group did tone and shock coincide. Next day conditioned rats were placed into the test chamber to measure the effect of CS on their lever presses (for details, see the section for Testing).
Optical conditioning
Rats were placed into the conditioning chamber and were attached to an optic fiber patch cord connected to a 473 nm solid-state laser diode (OEM Laser Systems) with 15–20 mW of output from the 200 μm fiber. They were allowed to explore the chamber for 3 min prior to the conditioning. Optical conditioning was 10-trains of blue light (10 pulses of 10 Hz, 2 ms duration) applied at randomized intervals with an average of 3 minutes apart. For paired conditioning, the light stimulus co-terminated with 0.5 s of 0.5 mA footshock; in unpaired conditioning, the light and shock were separated by a minimum of 1 min. Paired and unpaired groups received equal number of light stimuli and shocks in the same context; however, only in the paired group did light and shock coincide. The delivery of shock and light was controlled by a pulse generator (Master-8; AMPI, Jerusalem, Israel). After the conditioning rats remained in the box for additional three minutes before returning to their home cage.
Testing
After the conditioning, rats were water restricted for 24 hours before they were tested for lever press. Testing was done in the same context as training except that the floor was a plastic sheet with white and red strips. Testing was a 7 minute session in which rats had to press a lever to receive the liquid (10% sucrose). Rats were attached to the optic fiber patch cord, placed into the chamber, and allowed to explore the environment for 5 minutes before having access to the lever. The testing session, where rats had free access to the lever, was a 3 minute period of no light, followed by two minutes of light on (10 Hz of pulses with 2 ms duration), and two minutes of no light. At the end of the session rats were returned to their home cage. Only rats that in two consecutive days showed consistent reduction (>30%) in the lever press during the light-on period were used for further behavioral phases. Those which failed the test were examined histologically to locate the position of cannula and viral injection (Extended Data Fig. 4).
Tone-conditioned rats were tested in the same way except that they received 2 minutes of tone instead of light stimulation.
LTD induction
Within one hour following testing, rats were placed in a separate context, a translucent plastic container (22.5×15×12 in.), attached to the optic fiber patch cord and allowed to explore the environment for 3 minutes before LTD induction. Optical LTD was induced with 900 pulses of light, each 2 ms, at 1 Hz. After the induction rats remained in the chamber for 3 additional minutes before returning to their home cage.
LTP induction
Within one hour following testing, rats were placed in a separate context, a cardboard box (20.5×15.5×14.5 in.), attached to the optic fiber patch cord and allowed to explore the environment for 3 minutes before LTP induction. Optical LTP was induced with 5 trains of light (each train 100 pulses, 100 Hz) at 3 minute inter-train intervals. After the induction rats remained in the chamber for 3 additional minutes before returning to their home cage. During all behavioral assays the light intensity remained the same for each animal. At the end of the experiment, animals were perfused and the location of the optic fiber was verified.
Systemic injection of MK80132
Rats were anesthetized with isoflurane for 5 minutes before being given an intraperitoneal injection of MK801 (0.2 mg/kg) in sterile saline. Conditioning protocol was administered 30 minutes following injection.
Perfusion, slicing and imaging
Prior to perfusion, rats were administered a ketamine/dexdomitor (75 and 5 mg/kg respectively) mixture intraperitoneal injection. Rats were then transcardially perfused with ~150 mL of saline followed by ~150 mL of 4% paraformaldehyde in 0.1M phosphate buffer solution (PB, pH 7.4). Brains were then fixed overnight in the same solution and rinsed and stored in 0.1M PB for slicing.
Brains were sliced coronally in 150 μm sections using a vibratome sectioning system and stored in PB. Slices were imaged using an Olympus MVX10 epifluorescent microscope to verify AAV-oChIEF-tdTomato expression in the MGN, auditory cortex, and their projections to the dorsal lateral amygdala. Additionally, appropriate positioning of the optic fiber cannula over the lateral amygdala was verified.
In vitro recording
For extracellular field potential recordings acute slices (as described in33) were prepared from 3–4 month old rats expressing AAV-oChIEF in the medial geniculate nucleus /and or auditory cortex. Extracellular field potentials were recorded with Axopatch-1D amplifiers (Axon Instruments) in dorsal tip of the lateral amygdala with glass electrodes (1–2 MΩ) filled with the perfusion solution. The auditory projection to the lateral amygdala was evoked by optical stimulation above the recording site. To measure AMPA-R field potential 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX) (10 μM) was added at the end of the experiments. Data were acquired and analyzed using custom software written in Igor Pro (Wavemetrics). Perfusion solution contained: 119 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 26 mM NaHCO3, 1 mM NaH2PO4, 11 mM glucose (pH 7.4), and gassed with 5% CO2/ 95% O2.
For whole cell recording, acute slices (as described in34–36) were prepared from 3–4 month old rats expressing AAV-oChIEF in the medial geniculate nucleus and/or auditory cortex. Whole-cell recordings were obtained from individual cells in dorsal tip of the lateral amygdala using glass pipettes (3–4 MΩ) filled with internal solution containing, in mM, cesium methanesulfonate 115, CsCl 20, HEPES 10, MgCl2 2.5, Na2ATP 4, Na3GTP 0.4, sodium phosphocreatine 10, and EGTA 0.6, at pH 7.25. External perfusion consisted of artificial cerebrospinal fluid (ACSF), containing 119 mM NaCl, 2.5 mM KCl, 26 mM NaHCO3, 1 mM NaH2PO4, 11 mM glucose, supplemented with 1 mM MgCl2, and 2 mM CaCl2, 100 μM picrotoxin and 1 mM Sodium L-ascorbate. Synaptic responses were evoked every 10 second by stimulating auditory projections to the lateral amygdala using 2 ms of blue light generated by the epifluorescence microscope and passed through the 60X objective lenses placed immediately above the recorded cell. AMPA:NMDA ratio was calculated as the ratio of peak current at −60mV to the current at +40mV, 50 ms after stimulus; both values subtracted from the current at 0mV.
In vivo recording
Four weeks after injection of AAV-oChIEF-tdTomato into auditory regions (8 animals were injected in both MGN and auditory cortex; 2 animals were injected in only auditory cortex; results were pooled), rats were anesthetized with a set of three injections of 700 μl urethane (330 mg/ml) given at 10 minute intervals 2 hours before the recording37 and then mounted on a custom made stereotaxic frame with an adjustable angle, to hold the head in a fixed position during the recording. The body temperature was regulated by a heating pad. Using aseptic surgical tools the skull was exposed and a hole (~3 mm) was made, centered at −3.3mm AP and 4.2mm ML. The recording electrode was a glass pipet (4–5mΩ) filled with 0.9% NaCl. The recording electrode was connected to a Axopatch-1D amplifier. The signal was amplified (X1000), filtered (2K Hz) and digitized at 10 kHz using an Instrutech A/D interface. Data were acquired and analyzed using custom software written in Igor Pro (Wavemetrics).
For optical stimulation, the optic fiber was glued to the glass pipet so that the tip of the fiber was 500 μm above the tip of the glass pipet to form an optrode. The optic fiber was connected to a 473 nm solid-state laser diode (OEM Laser Systems). The parameters for the optical stimulation were identical to those used during behavior (2 ms duration, 15–20 mW intensity). The optrode was slowly lowered in at a 7° angle following the start of stimulation. After establishing a stable baseline of at least 30 minutes (stimulation frequency 0.033Hz) at the recording site (DV: −7 to −7.5), 2 minutes of 10 Hz stimulation was evoked, which was followed by 40 minutes of 0.033 Hz stimulation. Subsequent LTD and LTP, with the same parameters used in the behavioral assay, were induced 40 minutes apart. Electrode resistance and light intensity were monitored before and immediately after the recordings to ensure that there was no change in the course of recording. All animals were perfused after the recordings and the position of the recording site verified.
Analysis
The number of lever presses were binned for each minute and normalized to the two minute period before light stimulation. Suppression ratio was measured by dividing the number of lever press during the first minute of conditioning stimulus (tone or optical stimulation) by that immediately preceding the stimulus.
To minimize the voltage dependent conductance component the initial slope of field excitatory postsynaptic potentials13 were measured using a custom written MatLab program.
Excitatory postsynaptic current amplitude was measured by averaging a fixed 3-ms window covering the peak amplitude and subtracting from an average current window before stimulation. All values given in the text and figures indicate mean ± SEM. Student’s paired and non-paired t-tests were used with p < 0.05 considered as significant. All behavioral data were also analyzed with Wilcoxon rank-sum test (Matlab statistic toolbox) and yielded the same significance values as the t-test.
Extended Data
Extended Data Figure 1. Freezing correlates well with reduction in lever presses to previously learned task.
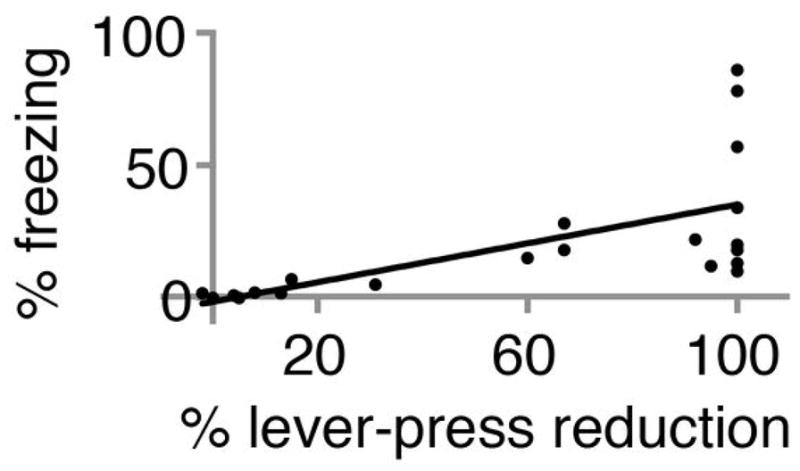
Plot of percent freezing versus percent reduction in lever presses to previously learned task. Best fit line indicates significant positive correlation (R2 = 0.4; p < 0.01; F-test). Data includes results from 3 manipulations (paired optical CS-shock conditioning, optical LTD and optical LTP). The percent change in lever presses to previously learned task (60% ± 9%) was significantly greater than change in percent freezing (20% ± 5%; N = 21; p < 0.001, paired Students t-test)
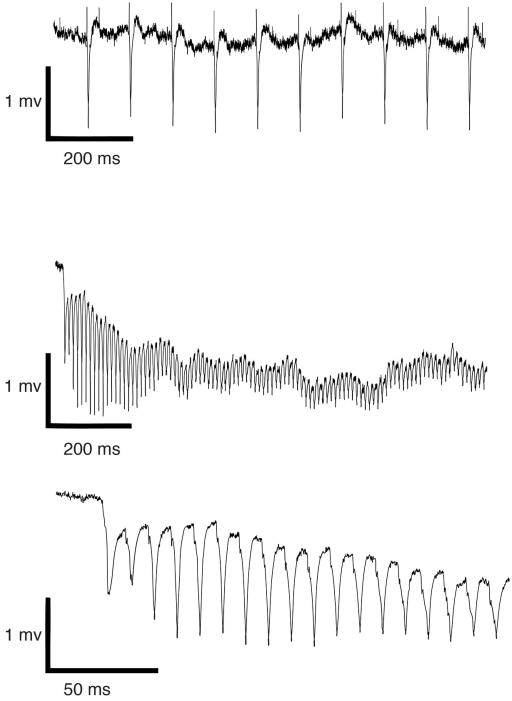
Extended Data Figure 2. In vivo, optically evoked synaptic responses in lateral amygdala.
Field responses to 10 Hz (top) and 100 Hz optical stimulation (middle, bottom), obtained from animal infected with AAV-oChIEF in auditory regions four weeks prior to recording. Note that responses follow stimulation faithfully.
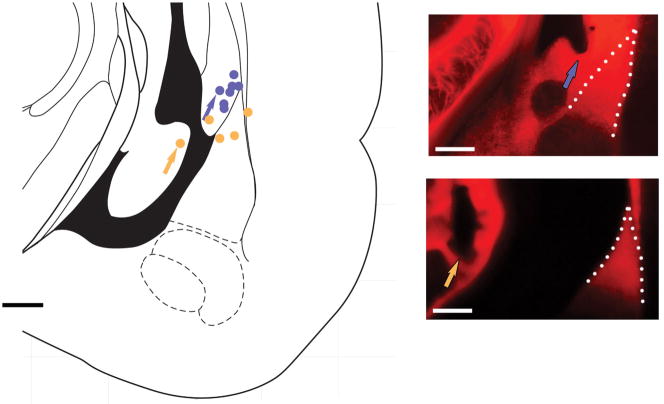
Extended Data Figure 3. Expression of oChIEF in auditory regions reaches lateral amygdala.
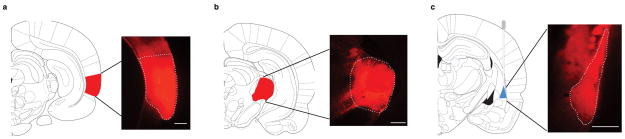
Diagram (left) and epifluorescent image (right) of coronal section of rat brain indicating areas expressing AAV-oChIEF-tdTomato 3–4 weeks after in vivo injection in auditory cortex (a) and medial geniculate nucleus (b). c, axonal expression of AAV-oChIEF-tdTomato in lateral amygdala (dashed white line); approximate placement of cannula and light (blue) indicated. Scale bars, 500 μm.
Extended Data Figure 4. Optic fiber locations in representative group of rats used in the behavioral assays.
Histologically assessed optic fiber tip location for rats which responded (blue circles; upper panel, right, is one example) or did not respond (orange circles; lower panel, right, is one example) to optical conditioning. The arrow on the panels shows the location of the tip of optic fiber. Lateral amygdala is indicated by dashed line. Note that the ventricle opened during tissue sectioning in the lower image. Scale bars, 500 μm.
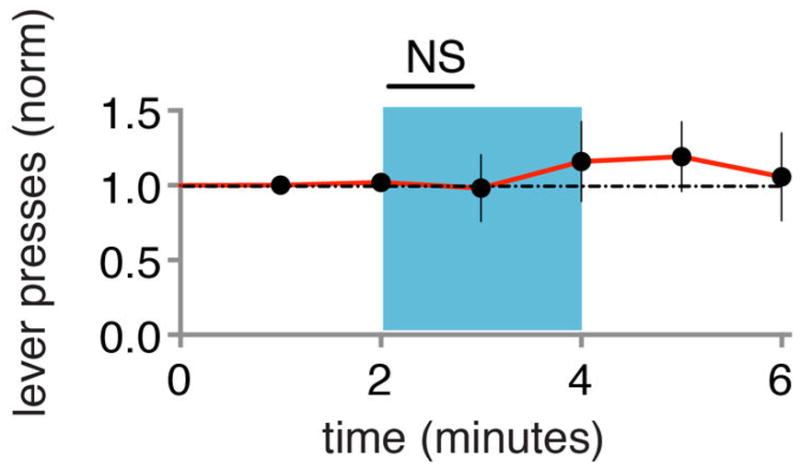
Extended Data Figure 5. 10 Hz test protocol does not produce CR.
Test for CR (blue) in naïve animals (N=8), as measured by changes in lever presses normalized to baseline period. Subsequent delivery of paired optical CS and shock produced CR in these animals (not shown). Each point represents data collected over 1 minute.
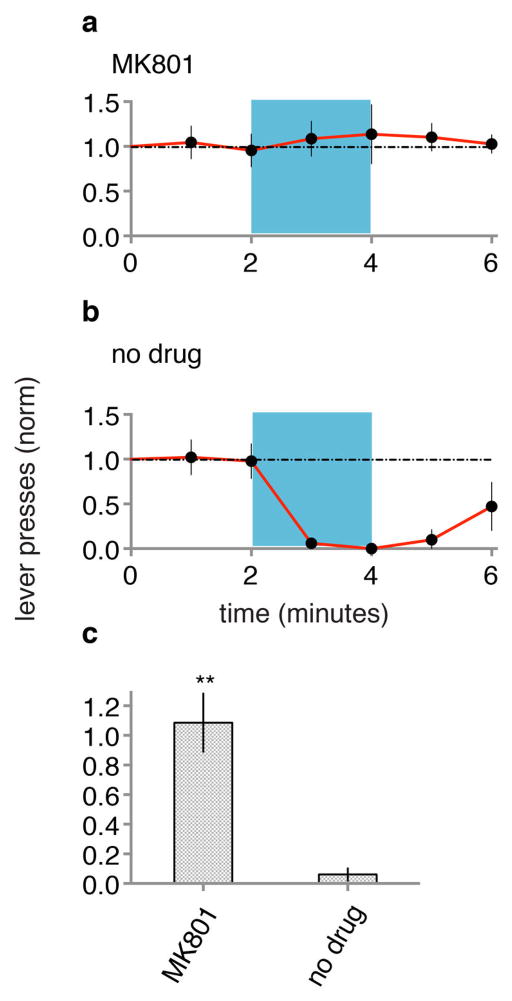
Extended Data Figure 6. Systemic NMDA receptor blockade during conditioning blocks ODI-induced conditioned response.
Animals (N = 5) were injected with MK801 (see methods) and given optical CS paired with foot shock and subsequently tested one day later for CR (a). The same group of animals was then given optical CS paired with foot shock (in the absence of MK801) and subsequently tested one day later for CR (b). c, MK801 signifcantly blocked conditioning.
Extended Data Figure 7. LTD and LTP remove and reactivate memory.
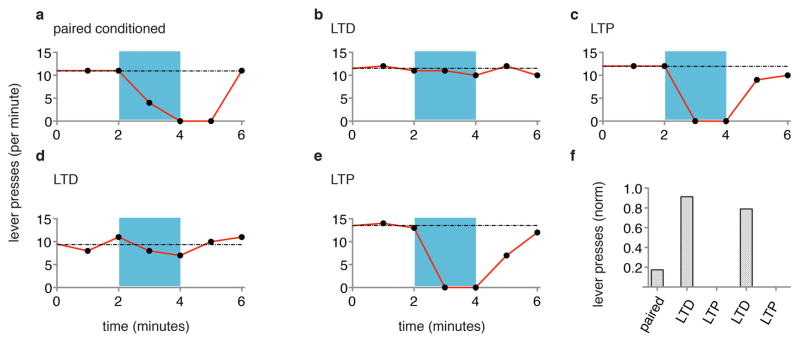
Data from individual rat, measuring lever presses per minute before, during (blue) and after optical CS, one day after paired conditioning of optical CS and shock (a), one day after subsequent optical LTD protocol (b), one day after subsequent optical LTP protocol (c), one day after subsequent second optical LTD protocol (d) and one day after subsequent second optical LTP protocol (e). f, Graph of lever presses during first minute into optical CS one day after delivery of indicated conditioning protocols.
Extended Data Figure 8. The effects of LTD and LTP are rapid and long-lasting.
Animals (n=5) were tested for CR one day following pairing of optical CS with shock (a). Within one hour of testing animals received optical LTD protocol and were tested for CR 20 minutes (b) and three days (c) later. Following day three testing animals received optical LTP protocol and were tested for CR 20 minutes (d) and three days (e) later. f, Graph of normalized lever presses for first minute of optical CS following indicated protocols.
Extended Data Figure 9. Optically evoked in vivo and in vitro stimuli produce similar electrophysiological responses.
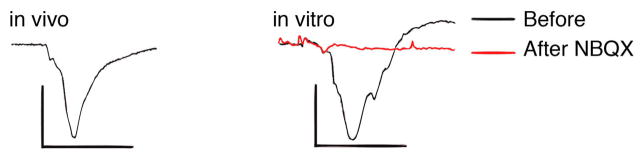
Animals were injected in vivo with AAV-oChIEF-tdTomato in auditory regions 4 weeks prior to recordings. Left, in vivo electrophysiological response obtained from glass electrode placed in lateral amygdala and evoked by light pulse delivered through fiber optic cable placed 500 μm above tip of glass electrode. Right, in vitro brain slice electrophysiological response obtained from glass electrode placed in lateral amygdala and evoked by light pulse delivered through fiber optic cable placed above the brain slice. Black trace is before and red trace after bath application of 10 μM NBQX. Scale bars, 1 mV, 10 ms.
Extended Data Figure 10. LTD reverses LTP and LTP reverses LTD of in vivo optical responses in amygdala.
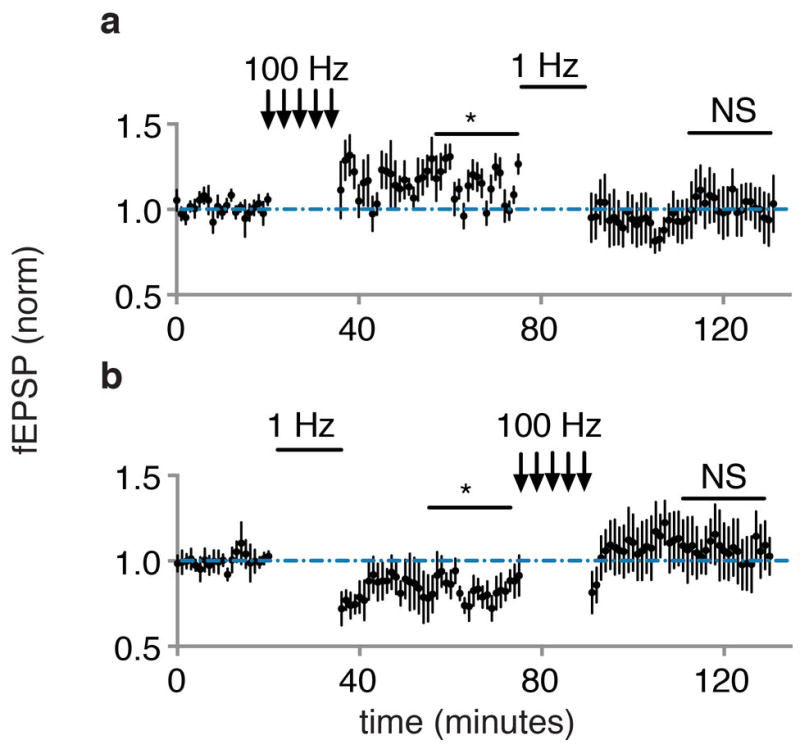
a, plot of baseline normalized fEPSP in vivo optically evoked responses (N = 5) following optical LTP (100 Hz) and optical LTD (1 Hz). b, same as a for a separate group of recordings (N = 5) following optical LTD (1 Hz) and optical LTP (100 Hz). All comparisons to baseline period.
Acknowledgments
We thank Dr. Jeff Isaacson, Larry Squire and members of the Malinow laboratory for helpful suggestions. This study was supported by NIH MH049159 and Cure Alzheimer’s Foundation grants to RM and NIH grant NS27177 to RT; Rt is an Investigator of the HHMI.
Footnotes
Author Contributions: S.N. and R.M. designed the experiments and wrote the manuscript. S.N., R.F. and R.M. analyzed the data. S.N., R.F. and C.D.P performed the experiments. J.Y.L. and R.Y.T. provided oChIEF-tdTomato construct.
References
- 1.Squire LR, Kandel ER. Memory : from mind to molecules. 2. Roberts & Co; 2009. [Google Scholar]
- 2.Stevens CF. A million dollar question: does LTP = memory? Neuron. 1998;20:1–2. doi: 10.1016/s0896-6273(00)80426-2. [DOI] [PubMed] [Google Scholar]
- 3.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 4.Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annual review of psychology. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- 5.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 6.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 7.Sah P, Westbrook RF, Luthi A. Fear conditioning and long-term potentiation in the amygdala: what really is the connection? Ann N Y Acad Sci. 2008;1129:88–95. doi: 10.1196/annals.1417.020. [DOI] [PubMed] [Google Scholar]
- 8.Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiological reviews. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Repa JC, et al. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat Neurosci. 2001;4:724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- 10.Lin JY, Lin MZ, Steinbach P, Tsien RY. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys J. 2009;96:1803–1814. doi: 10.1016/j.bpj.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muller D, Joly M, Lynch G. Contributions of quisqualate and NMDA receptors to the induction and expression of LTP. Science. 1988;242:1694–1697. doi: 10.1126/science.2904701. [DOI] [PubMed] [Google Scholar]
- 12.Kauer JA, Malenka RC, Nicoll RA. A persistent postsynaptic modification mediates long-term potentiation in the hippocampus. Neuron. 1988;1:911–917. doi: 10.1016/0896-6273(88)90148-1. [DOI] [PubMed] [Google Scholar]
- 13.Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- 14.Herry C, et al. Neuronal circuits of fear extinction. European Journal of Neuroscience. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- 15.Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 16.Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- 17.Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- 18.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science (New York, NY) 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 19.Rumpel S, Ledoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science (New York, NY) 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 20.Rogan MT, Stäubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala : Article : Nature. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 21.Shinnick-Gallagher P, McKernan MG. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- 22.Martin SJ, Morris RGM. New life in an old idea: the synaptic plasticity and memory hypothesis revisited. Hippocampus. 2002;12:609–636. doi: 10.1002/hipo.10107. [DOI] [PubMed] [Google Scholar]
- 23.Li H, et al. Experience-dependent modification of a central amygdala fear circuit. Nat Neurosci. 2013;16:332–339. doi: 10.1038/nn.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- 25.Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciocchi S, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garner AR, et al. Generation of a synthetic memory trace. Science. 2012;335:1513–1516. doi: 10.1126/science.1214985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramirez S, et al. Creating a false memory in the hippocampus. Science. 2013;341:387–391. doi: 10.1126/science.1239073. [DOI] [PubMed] [Google Scholar]
- 30.Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nature Publishing Group. 2013 doi: 10.1038/nn.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirby ED, Jensen K, Goosens KA, Kaufer D. Stereotaxic Surgery for Excitotoxic Lesion of Specific Brain Areas in the Adult Rat. JoVE (Journal of Visualized Experiments) :e4079–e4079. doi: 10.3791/4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li XF, Stutzmann GE, LeDoux JE. Convergent but temporally separated inputs to lateral amygdala neurons from the auditory thalamus and auditory cortex use different postsynaptic receptors: in vivo intracellular and extracellular recordings in fear conditioning pathways. Learning & memory (Cold Spring Harbor, NY) 1996;3:229–242. doi: 10.1101/lm.3.2-3.229. [DOI] [PubMed] [Google Scholar]
- 33.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 34.Li B, et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffman DA, Johnston D. Downregulation of transient K+ channels in dendrites of hippocampal CA1 pyramidal neurons by activation of PKA and PKC. J Neurosci. 1998;18:3521–3528. doi: 10.1523/JNEUROSCI.18-10-03521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen BT, et al. Cocaine but Not Natural Reward Self-Administration nor Passive Cocaine Infusion Produces Persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogan MT, LeDoux JE. LTP is accompanied by commensurate enhancement of auditory-evoked responses in a fear conditioning circuit. Neuron. 1995;15:127–136. doi: 10.1016/0896-6273(95)90070-5. [DOI] [PubMed] [Google Scholar]