Summary
Background
Isolated fibrous tumor of the pleura (SFTP – Solitary fibrous tumour of the pleura/localized fibrous tumour of the pleura) is a rare primary tumour of the pleura of mesenchymal origin. In most cases, it is a benign lesion. It is composed of spindle cells similar to fibroblasts and derives probably from submesothelial mesenchyme.
The aim of the study was to analyze clinical symptoms, incidence, possibility of suggesting the diagnosis on the basis of imaging tests, and confirmation of the diagnosis in pathological tests with regard to studies of histochemistry examination.
Material/Methods
Clinical and morphological material obtained from 14 patients from Department of Thoracic Surgery of Subcarpathian Chest Disease Center treated between year 2004 and 2010 was analysed. In the first stage, selected cases of patients with isolated fibrous tumour of the pleura were chosen from the archives and the analysis of their medical history was carried out. Basic information about age, gender, medical history, smoking habit, physical examination and results of imaging, endoscopic and morphological examinations were noted. The second parallel component of the study was pathomorphological examinations of the surgical material obtained from the patients, including the assessment of morphology and immunohistochemistry.
Results
Of the 14 examined patients, fibrous tumour occurred in 8 men and 6 women. The age range of the patients was 37–73 years, with a peak attributable to the 6th decade of life. In 8 patients the tumour was detected incidentally during routine examinations. In 7 patients there were no clinical signs of respiratory disease, and if present, then the most common complaint was shortness of breath. Regarding symptoms not connected with the respiratory system, anemia occurred most frequently. Fibrous tumour of the pleura was more often associated with the visceral pleura than with the parietal pleura. The largest lesion was approximately 20 cm in size.
Conclusions
Fibrous tumour of the pleura is a pleura-based neoplasm which is usually detected incidentally, and is often asymptomatic or poorly symptomatic. Computed tomography imaging allows to suggest a correct diagnosis. Histopathological diagnosis is based on immunohistochemical examinations.
MeSH Keywords: Immunohistochemistry, Solitary Fibrous Tumor, Pleural –blood, Tomography, Spiral Computed
Background
Solitary fibrous tumour of the pleura (SFTP) or localized fibrous tumour of the pleura is a rare, slow-growing primary pleura-based tumour of mesenchymal origin. In most cases it is a benign lesion. However, approximately 20–30% of the removed SFTP had a malignant component [1]. It usually derives from the visceral pleura (60–80%), more rarely from the parietal pleura [1–5]. Tumours located in the orbit, thyroid, nasopharynx, nasal cavity and meninges occur as well [3]. The incidence is ~2.8 per 100 000 people [3] and accounts for 5% of pleural tumors. It is second most common primary pleural tumour, after mesothelioma [3,6,7]. The first information on fibrous tumour is dated 1870 (E. Wagner), but the first case report on this disease entity was created in 1931 (Klemperer and Rabin) [2,3,8]. Introduction of immunohistochemical methods and use of antibodies to mesenchymal elements and electron microscope allowed for recognition of SFTP origin [3–9]. Radiographic features (chest X-ray) include a solitary round or oval opacification of a various size, often with continuity with the chest wall or located in the interlobar fissure [1–8,10,11]. Computed tomography is a more accurate examination, which allows for assessment of its size, morphology, detailed location and anatomical relations. Other imaging examinations: MRI, USG, DSA, PET are performed less often, but can show infiltration of adjacent tissues and tumour vasculature, control aspiration biopsy and determine the benign or malignant nature of the lesion [2–6,8].
Aims of the study
To analyze clinical symptoms, incidence, possibility of suggesting the diagnosis on the basis of imaging examinations, confirmation of the diagnosis in pathological tests with regard to histochemical examinations.
Material and Methods
Clinical and morphological material obtained from 14 patients (8 men and 6 women) from Department of Thoracic Surgery of Subcarpathian Chest Disease Center in Rzeszow treated between year 2004 and 2010 was analysed. The age range of the patients was 37–73 years, with a peak in the 6th decade of life (7 patients). In the first stage, selected cases of the patients with isolated fibrous tumour of the pleura were chosen from the archives and the analysis of their medical history was carried out. Basic information about age, gender, medical history, smoking habit, physical examination, imaging test results (performed in various centres), endoscopic and morphological examinations were noted. The second parallel component of the study were pathomorphological examinations of the surgical material obtained from the patients, including the assessment of morphology and immunohistochemistry.
Results
In 8 out of 14 patients the tumour was discovered incidentally on a routine chest radiograph. In one patient it was detected during POLYTRAUMA examination. One patient had previously undergone surgery due to left renal hemangiopericytoma and had a chest CT scan performed due to health status deterioration.
In 7 patients there were no clinical signs of respiratory disease, in 5 patients the only complaint was shortness of breath; chest pain – in two, shortness of breath and chest pain – in one, dyspnea, cough, hemoptysis and elevated temperature – in one.
Regarding the symptoms not associated with the respiratory system, anemia occurred in four patients, ischemic heart disease – in three, arterial hypertension – in two, diabetes mellitus – in one, weight loss – in one, elevated level of leukocytes – in one, history of ischemic stroke - in one, history of oncological disorder – in one.
In the analyzed material the lesion was located in the right lung in 9 patients and in the left lung in 5 patients. In three cases the tumour was pedunculated. In 4 cases it was associated with the parietal pleura and in 10 cases – with the visceral pleura. In 5 cases the tumour was surrounded with lung parenchyma. In 2 cases a fluid in the pleural cavity was also present.
In chest X-ray tumours were described as round or oval opacifications of various size (Figures 1A, 2A, 3A, 3B).
Figure 1.
A 46-year-old female patient, with no clinical history. (A) A routine chest X-ray – a round 12-mm opacification (asterisk) in the right upper lung field. (B) Chest CT, axial plane of the right upper lobe, a 10×12×10 mm nodule adjacent (asterisk) to an oblique fissure, no obvious enhancement after administration of contrast medium. Histopathological diagnosis of intrapulmonary solitary fibrous tumour.
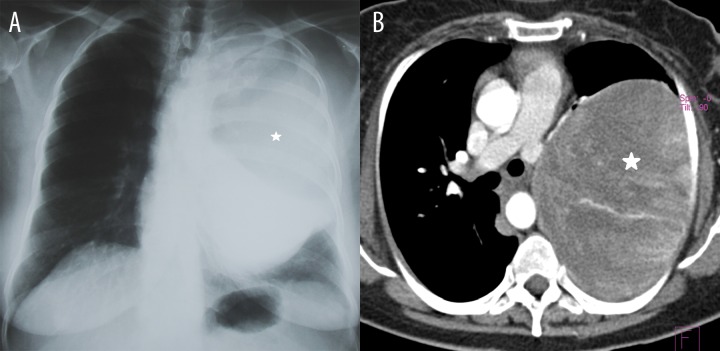
Figure 2.
A 49-year-old female patient. Shortness of breath, weight loss, anemia. (A) Chest X-ray – an opacification (asterisk) in the left lung. (B) Chest CT, axial plane. A giant tumour (asterisk) of the left lung (upper lobe and superior segment of the lower lobe) with heterogeneous enhancement after administration of contrast medium.
Figure 3.
A 37-year-old male patient. (A, B) Routine chest X-ray. Tumour (asterisk) in the left lower lung field. (C, D) Chest CT, axial plane. In the left lower lobe there is a 8×7×5 cm tumour (asterisk) adjacent to the diaphragmatic pleura, with heterogeneous enhancement after administration of contrast medium.
In CT scans tumours were well-circumscribed solid lesions, homogeneous (small lesions) or heterogenous (large lesions). The attenuation was between 30 and 40 HU in the native phase (some lesions had hyperdense areas caused by bleeding). After administration of contrast medium the lesions enhanced by approximately 40 HU, homo- or heterogenously (due to the presence of cystic, necrotic or hemorrhagic components). Single small calcifications were also observed within tumours. Smaller lesions formed an obtuse angle with the chest wall, and in case of larger tumours the angle was acute (Figures 1B, 2B, 3C, 3D, 4A, 4B).
Figure 4.
A 59-year-old male patient, with no respiratory tract symptoms. Chest CT, axial plane. (A) In the lateral segment of the right middle lobe there is a 41×20×29 mm oval tumour (asterisk) adjacent to the chest wall, (B) with heterogenous enhancement after administration of contrast medium and no rib destruction. It forms an obtuse angle with the chest wall.
In the histopathological examinations, macroscopic assessment was performed, with the description of size, shape and consistency of the tumour, surface and cross-sectional area appearance, presence of calcifications, necrosis, hemorrhages and cystic areas. The appearance of lung parenchyma outside the tumour was also assessed. In order to differentiate the observed changes, immunohistochemical examinations were performed, with the use of immunological peroxidase-antiperoxidase method. Antibodies to mesenchymal (vimentin, CD34), smooth muscle (desmin, actin), epithelial (cytokeratin, EMA), neural (S100) and mesothelial (calretinin) components were used. A level of Bcl2 protein and proliferative activity of Ki67 were also determined.
In the examined material the size of the tumour varied from 1 to 20 cm, in 8 cases the shape was round, in 4 – oval, in 2 cases the obtained material was incomplete or fragmented. Three tumours had a peduncle, 11 were completely encapsulated, one was partially encapsulated (with infiltration of the pleura), 7 were hard in consistency and 6 – soft. A total of 7 lesions had a smooth, lobular external surface, 4 – completely smooth, one – with infiltration of the pleura. The colour of the cross-sectional area was grey-yellow in 6 cases, grey – in 6, brown – in one; in one case the colour was impossible to assess due to fragmentation. Ten tumours had a solid structure, two of them – solid-cystic, one of them – necrotic lesions, and one of them was unevaluable.
In the microscopic assessment 5 tumours were surrounded by lung parenchyma, 10 of them were associated with the visceral pleura, and 4 of them – with the parietal pleura. Some of the tumours were mixed cellularity variants, other had homogenous cellularity. Necrosis was observed in 5 cases and hemorrhages – in 3 cases. Atypia and cell polymorphism were present in three tumours, proliferative activity higher than 4/10 per hpf – in three as well (Table 1).
Table 1.
Microscopic evaluation of the operational preparations.
| Benign fibrous variant/prevailing collagen-rich parenchyma | 9 | 69% |
| Benign hypercellular variant/hemangiopericytoma-like | 5 | 38% |
| Hypercellular variant | 3 | 23% |
| Necrosis | 5 | 38% |
| Atypia and cell polymorphism | 3 | 23% |
| Proliferative activity higher than 4/10 hpf | 3 | 23% |
| Hemorrhages | 3 | 23% |
In immunohistochemical examinations the following results were obtained: positive results for CD34 in 9 cases, for Bcl2 in 2 cases, for Ki67 in none, for vimentin in 9 cases, for calretinin in none, for S100 in one case, for SMA in none, for EMA in none, for desmin in none (Table 2).
Table 2.
Immunohistochemical tests – markers used for diagnosis.
| Benign | Malignant | |
|---|---|---|
| Clinical presentation | ||
| Symptomatic6 | + | +++ |
| Incidental discovery | ++ | +/− |
| Pain | +/− | +++ |
| Shortness of breath | +/− | +++ |
| Macroscopic features | ||
| Atypical location | Rarely | Often |
| Size <10 cm | Rarely | Often |
| Encapsulation | Often | Rarely |
| Peduncule presence | Often | Rarely |
| Necrosis | Rarely | Often |
| Areas of hemorrhage | Rarely | Often |
| Calcifications | Often | Rarely |
| Microscopic features | ||
| Number of cells | Low | High |
| Proliferative activity | Low | High |
| Cell polymorphism | Low | High |
| Necrosis | Rarely | Often |
| Infiltration of adjacent structures | None | Often |
Discussion
Solitary fibrous tumour of the pleura (SFTP) or localized fibrous tumour of the pleura is a rare, slow-growing primary pleura-based tumour of mesenchymal origin. It is composed of spindle cells similar to fibroblasts and derives probably from submesothelial mesenchyme. It usually derives from the visceral pleura (in the examined material 71%, in the data from literature 60–80%), more rarely from the parietal pleura [1–5]. The incidence is ~2.8 per 100 000 people [3] and accounts for 5% of pleural tumors. This is second most common primary pleural tumour, after mesothelioma [3,6,7].
Difficulties in the exact determination of the tumour’s origin resulted in using many names, e.g.: localized mesothelioma, localized fibrous mesothelioma, fibrous mesothelioma, benign mesothelioma, submesothelial fibroma [1,8]. Only introduction of immunohistochemical methods, use of antibodies to mesenchymal (vimentin, CD34), smooth muscle (desmin, actin), epithelial (cytokeratin, EMA), neural (S100) and mesothelial (calretinin) components, determination of bcl2 protein (anti-apoptotic protein) and Ki67 (protein of proliferative activity), and use of electron microscope allowed to determine SFTP origin [3–9].
Pathogenetic mechanisms that lead to SFTP development are not known. There was no association observed between developing the tumour and exposure to asbestos and smoking. Some of the authors point to chromosomal abnormalities regarding chromosomes 8, 12 and 21 [3].
Fibrous tumour of the pleura can occur at any age, but usually presents in the 6th to 7th decade (in the examined material there were seven patients aged 53–59). It is slightly more prevalent in women – 1.2:1. The diameter of the tumour varies from 1 to 39 cm and it can weight even up to 5.2 kg [2,8,10].
Most of the lesions are incidentally discovered, e.g. on routine chest radiographs or during examinations performed due to trauma, which is confirmed by the literature data [7].
Presence of the clinical symptoms is usually associated with large tumours which exert a mass effect or cause para-neoplastic syndroms. The most common complaints are: shortness of breath, chest pain and cough. The less common ones include hemoptysis, weight loss, night sweats, shivers, pneumonia, hypoglycemia associated with the secretion of the insulin-like growth factor II, hypertrophic pulmonary osteoartropathy (HPO) presenting with nail clubbing, large joint pain and increase in the size of hands and feet [1–8,10,11]. Pleural effusion is a rare symptom.
Radiographic features (chest X-ray) include a solitary round or oval opacification of a various size, often with continuity with the chest wall or located in the interlobar fissure [1–8,10,11]. As these tumours are often pedunculated (around 60% of tumours from the visceral pleura and around 30% from the parietal pleura), radiographs performed in various patient’s positions show different locations due to movement of the lesion within the chest [1].
In CT scans, small SFTP present as homogenous, well-defined nodules with a smooth external surface, forming an obtuse angle with the pleura. Larger lesions are usually heterogenous, often with areas of necrosis and calcifications (punctate or linear), forming an acute angle with the pleura. Calcifications can be present both in benign and malignant lesions. After administration of contrast medium the enhancement is homogenous in smaller lesions and usually heterogenous in larger ones (as a result of necrosis, sclerosis and hemorrhages) [2–6,8].
Magnetic resonance imaging is performed occasionally. It can show fibrous and collagenous nature of the lesion and determine the degree of diaphragm infiltration. In T1- and T2-weighted images tumours present as heterogenous masses of low or intermediate signal (also in contrast-enhanced T1-weighted images). High signal in T2-weighted images depends on the presence of necrotic and cystic components, and areas of myxomatous degeneration [2,3,6,8].
Arteriography is an important diagnostic method, which can show feeding blood vessels, especially in extrapleural and pedunculated tumours. Additionally, it allows to perform embolization before surgery, which minimizes the risk of hemorrhage [3,8].
Ultrasonography is particularly useful in performing biopsies of peripheral lesions [3,8].
The use of PET in the work-up of fibrous tumours is not common, but a high level of 18-FDG metabolism can indicate a malignant lesion [3,8].
Transthoracic biopsy is not always possible due to unfavourable location of the tumour (e.g. behind the scapula), its elasticity or mobility (especially in pedunculated tumours) [7].
Radiographic features that even before surgery can suggest the diagnosis of SFTP include: well-defined tumour, smooth surface, calcifications, continuity with the chest wall and clinical symptoms.
Macroscopically the tumours present as encapsulated or well-circumscribed round or oval lesions, soft or hard, with a smooth external surface, sometimes with an adjacent piece of fibrous tissue corresponding to the peduncle. They are solid or solid-cystic, of homogenous or lobulated structure and whitish, grey, grey-pink or grey-yellow. They may contain hemorrhagic and necrotic components [1,4,7,8,10].
Microscopic features can be various, even within a single tumour; there are areas of spindle cells with herringbone pattern, storiform pattern and hemangiopericytoma-like pattern, or hypocellular areas with prevailing fibrous stroma. Extensive sclerosis or myxomatous lesions can often be present [8,10]. Usually there is no cell atypia or nuclear polymorphism present, and proliferative activity is not higher than 3 figures per 10 hpf [6].
Tumour size above 10 cm, accompanying pleural effusion and marked continuity with parietal pleura can suggest malignant nature of the tumour. The presence of necrosis, hemorrhages, infiltration of adjacent tissues, hypercellularity with oval cells, cell atypia, nuclear polymorphism and proliferative activity higher than 4 figures per 10 hpf also suggest a malignant form of solitary fibrous tumour [3,8] (Table 3).
Table 3.
Prognosis of SFTP nature.
| Marker | Number of positive results / number of examined cases | Number of negative results / number of examined cases |
|---|---|---|
| CD 34 | 9/10 | 1/10 |
| Ki 67 | 2/3 | 1/3 |
| Bcl 2 | 0/0 | 0/0 |
| Vimentin | 9/9 | 0/9 |
| Calretinin | 0/2 | 2/2 |
| S100 | 1/8 | 7/8 |
| SMA | 0/5 | 5/5 |
| EMA | 0/2 | 2/2 |
| CK | 0/5 | 5/5 |
| Desmin | 0/5 | 5/5 |
Differential diagnosis includes a broad spectrum of spindle-cell tumours, both showing continuity with the pleura, lung, mediastinum and chest wall, and metastases from other organs. Immunohistochemical examinations play a significant role in differential diagnostics. The most important ones are: positive in 95–100% of tumours reaction to CD34, vimentin and, in slightly lower proportion of tumour groups, to bcl2, and negative reaction to S100 protein, desmin and actin in smooth muscles, cytokeratin and calretinin (according to the literature data). They allow to rule out a peripheral sarcomatoid carcinoma of the lung, desmoplastic or sarcomatoid form of malignant mesothelioma, synovial sarcoma, schwannoma, inflammatory pseudotumour, extraintestinal gastrointestinal stromal tumour and other less common lesions [1,3,6,8].
Irrespective of the nature of the tumour (benign or malignant), the treatment of choice is surgical removal. Due to the possibility of recurrence, clinical and radiological follow-up are recommended. In case of a benign nonpedunculated SFTP, the risk of recurrence is <2%, in benign pedunculated <14%, in malignant SFTP – 14% and 63%, respectively [3].
Conclusions
SFTP is a pleural tumour occurring at any age, with incidence peak in the 6th decade of life, more often associated with the visceral pleura – which was confirmed by the analysis of the collected cases.
In most cases, SFTP is discovered incidentally, e.g. on a routine chest radiograph or during examination performed due to trauma (in the examined material in 9 out of 14 patients).
In a half of the cases no clinical symptoms were present. Most commonly reported complaints included shortness of breath and chest pain.
CT examination is an important imaging method, which shows a distinctive pattern of changes, and, combined with clinical data, allows the radiologist to suggest the SFTP diagnosis.
The diagnosis is based on histopathological examinations, and immunohistochemical tests provide differential diagnosis.
References
- 1.Masaya O, Hiroyasu Y, Sung-Soo Ch, et al. Solitary fibrous tumor of the pleura presenting dry cough induced by postural posittion. Ann Thorac Cardiovasc Surg. 2009;15(6):401–3. [PubMed] [Google Scholar]
- 2.Robinson LA. Solitary fibrous tumor of the pleura. Cancel Control, Journal of the Moffiti Cancer Center. 2006;13(4):264–69. doi: 10.1177/107327480601300403. [DOI] [PubMed] [Google Scholar]
- 3.Pinedo-Onofre JA, Robles-Perez A, Pena-Mirabal ES, et al. Giant solitary fibrous tumor of the pleura. Cir Cir. 2010;78(1):31–42. [PubMed] [Google Scholar]
- 4.Hezla M, Ashis KM. Natural history of multifocal solitary fibrous tumor of the pleura: a 25-year follow-up report. J Natl Med Assoc. 2004;96(5):659–64. [PMC free article] [PubMed] [Google Scholar]
- 5.Findik G, Ozturk F, Gunay E, et al. Surgical management of solitary fibrous tumors of the pleura – an analysis of 21 cases. Adv Clin Exp Med. 2011;20(3):363–69. [Google Scholar]
- 6.Agarwal VK, Plotkin BE, Dumani D, et al. Solitary fibrous tumor of pleura: a case report and review of clinical, radiographic and histologic findings. J Radiol Case Rep. 2009;3(5):16–20. doi: 10.3941/jrcr.v3i5.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chmielewski A, Trawiński J, Sroczyński P, et al. Przypadek uszypułowanego guza włóknistego (solitary fibrous tumor) opłucnej. Lekarz Wojskowy. 2009;2:100–3. [in Polish] [Google Scholar]
- 8.Abu Arab W. Solitary fibrous tumors of the pleura. Eur J Cardiothorac Surg. 2012;41(3):587–97. doi: 10.1093/ejcts/ezr009. [DOI] [PubMed] [Google Scholar]
- 9.Ali MJ, Honovar SG, Naik MN, et al. Orbital solitary fibrous tumor: a clinicopathologic correlation and review of literature. Oman J Ophthalmol. 2011;4(3):147–49. doi: 10.4103/0974-620X.91274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pływaczewski R, Hawryłkiewicz I, Langfort R, et al. Odosobniony guz włóknisty opłucnej u 75-letniej chorej. Pneumonol Alergol Pol. 2004;72(1–2):32–36. [in Polish] [PubMed] [Google Scholar]
- 11.Milchert M, Trzcińska-Butkiewicz B, Ostanek L, et al. Pierwotna osteoartropatia przerostowa jako rzadka przyczyna dolegliwości stawowych. Wiadomości Lekarskie. 2006;LIX(11–12):873–78. [in Polish] [PubMed] [Google Scholar]