Abstract
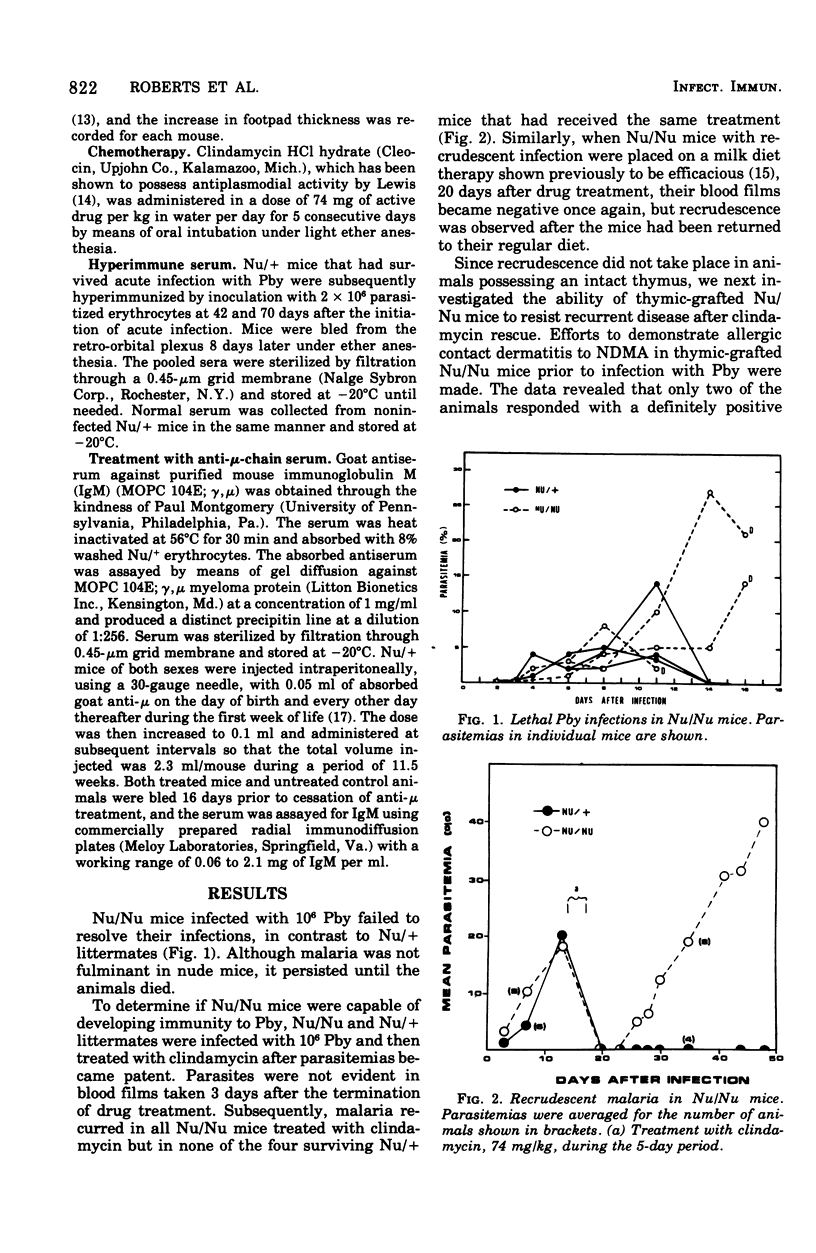
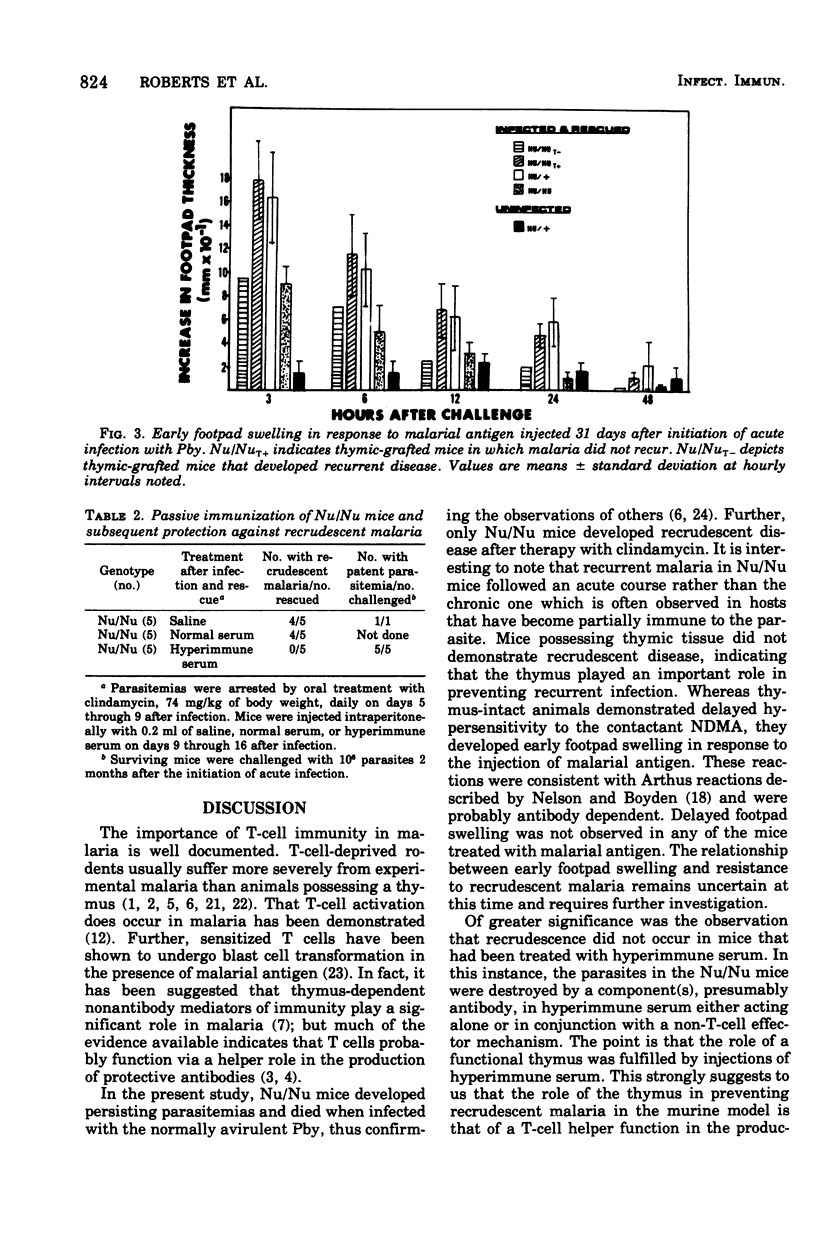
Nude mice died when infected with the normally avirulent malarial parasite Plasmodium berghei yoelii. Furthermore, malaria recrudesced in Nu/Nu mice after the termination of acute disease by treatment with clindamycin. Recrudescence was not observed in Nu/Nu mice that had been grafted with thymic tissue or treated with hyperimmune serum. Mice mad B cell deficient by treatment with anti-mu-chain serum also died when infected with P. berghei yoelii. The data suggest that a crucial role of the thymus in preventing recrudescent malaria in this model system is to provide a helper function in the production of protective antibody.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker L. R., Powers K. G. Impairment of antibody response and recovery in malarial rodents by antilymphocyte serum. Nature. 1971 Feb 5;229(5284):429–430. doi: 10.1038/229429a0. [DOI] [PubMed] [Google Scholar]
- Brown I. N., Allison A. C., Taylor R. B. Plasmodium berghei infections in thymectomized rats. Nature. 1968 Jul 20;219(5151):292–293. doi: 10.1038/219292a0. [DOI] [PubMed] [Google Scholar]
- Brown K. N., Jarra W., Hills L. A. T cells and protective immunity to Plasmodium berghei in rats. Infect Immun. 1976 Oct;14(4):858–871. doi: 10.1128/iai.14.4.858-871.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Chwatt L. J., Dorrell J., Topley E. Antilymphocyte serum and the course of rodent malaria in mice. Trans R Soc Trop Med Hyg. 1972;66(4):522–523. [PubMed] [Google Scholar]
- Clark I. A., Allison A. C. Babesia microti and Plasmodium berghei yoelii infections in nude mice. Nature. 1974 Nov 22;252(5481):328–329. doi: 10.1038/252328a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C., Cox F. E. Protection of mice against Babesia and Plasmodium with BCG. Nature. 1976 Jan 29;259(5541):309–311. doi: 10.1038/259309a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cox F. E., Allison A. C. Protection of mice against Babesia spp. and Plasmodium spp. with killed Corynebacterium parvum. Parasitology. 1977 Feb;74(1):9–18. doi: 10.1017/s003118200004748x. [DOI] [PubMed] [Google Scholar]
- Cohen S., Butcher G. A. The immunologic response to plasmodium. Am J Trop Med Hyg. 1972 Sep;21(5):713–721. doi: 10.4269/ajtmh.1972.21.713. [DOI] [PubMed] [Google Scholar]
- Finerty J. F., Krehl E. P. Cyclophosphamide pretreatment and protection against malaria. Infect Immun. 1976 Oct;14(4):1103–1105. doi: 10.1128/iai.14.4.1103-1105.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravely S. M., Kreier J. P. Adoptive transfer of immunity to Plasmodium berghei with immune T and B lymphocytes. Infect Immun. 1976 Jul;14(1):184–190. doi: 10.1128/iai.14.1.184-190.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardena A. N., Targett G. A., Leuchars E., Carter R. L., Doenhoff M. J., Davies A. J. T-cell activation in murine malaria. Nature. 1975 Nov 13;258(5531):149–151. doi: 10.1038/258149a0. [DOI] [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E. Potentiation of T-cell-mediated immunity by selective suppression of antibody formation with cyclophosphamide. J Exp Med. 1974 Jun 1;139(6):1529–1539. doi: 10.1084/jem.139.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAEGRAITH B. G., DEEGAN T., JONES E. S. Suppression of malaria (P. berghei) by milk. Br Med J. 1952 Dec 27;2(4799):1382–1384. doi: 10.1136/bmj.2.4799.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire H. C., Jr Specific acquired immune unresponsiveness to contact allergens with cyclophosphamide in the mouse. Int Arch Allergy Appl Immunol. 1976;50(6):651–658. doi: 10.1159/000231543. [DOI] [PubMed] [Google Scholar]
- Manning D. D., Jutila J. W. Immunosuppression in mice injected with heterologous anti-immunoglobulin antisera. J Immunol. 1972 Jan;108(1):282–285. [PubMed] [Google Scholar]
- NELSON D. S., BOYDEN S. V. THE CUTANEOUS REACTIVITY OF GUINEA PIGS TO PURE PROTEIN ANTIGENS. I. A CRITICAL EVALUATION OF METHODS FOR THE INDUCTION OF DELAYED-TYPE HYPERSENSITIVITY TO PURE PROTEINS. Int Arch Allergy Appl Immunol. 1964;25:279–303. doi: 10.1159/000229529. [DOI] [PubMed] [Google Scholar]
- Rank R. G., Weidanz W. P., Bondi A. Nonsterilizing immunity in avian malaria: an antibody-independent phenomenon. Proc Soc Exp Biol Med. 1976 Feb;151(2):257–259. doi: 10.3181/00379727-151-39186. [DOI] [PubMed] [Google Scholar]
- Spira D. T., Silverman P. H., Gaines C. Anti-thymocyte serum effects on Plasmodium berghei infection in rats. Immunology. 1970 Nov;19(5):759–766. [PMC free article] [PubMed] [Google Scholar]
- Stechschulte D. J. Plasmodium berghei infection in thymectomized rats. Proc Soc Exp Biol Med. 1969 Jul;131(3):748–752. doi: 10.3181/00379727-131-33968. [DOI] [PubMed] [Google Scholar]
- Weinbaum F. I., Evans C. B., Tigelaar R. E. An in vitro assay for T cell immunity to malaria in mice. J Immunol. 1976 May;116(5):1280–1283. [PubMed] [Google Scholar]
- Weinbaum F. I., Evans C. B., Tigelaar R. E. Immunity to Plasmodium Berghei yoelii in mice. I. The course of infection in T cell and B cell deficient mice. J Immunol. 1976 Nov;117(5 PT2):1999–2005. [PubMed] [Google Scholar]


