Abstract
Purpose
Older patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) have often been excluded from myeloablative conditioning regimens containing busulfan because of non-disease related morbidity and mortality. We hypothesized that busulfan clearance (BuCL) in older patients (>60 years) would be reduced compared to that in younger patients, potentially explaining observed differences in busulfan tolerability.
Methods
AML patients in three CALGB hematopoietic cell transplantation studies were treated with a conditioning regimen using IV busulfan, dosed at 0.8 mg/kg. Plasma busulfan concentrations were determined by LC-MS, and analyzed by non-compartmental methods. BuCL was normalized to actual (ABW), ideal (IBW) or corrected (CBW) body weight (kg). Differences in BuCL between age groups were examined using the Wilcoxon rank sum test.
Results
185 patients were accrued; 174 provided useable pharmacokinetic data. Twenty-nine patients ≥ 60 years old (median 66; range 60–74) had a significantly higher BuCL vs those <60 years old (median 50; range 18–60): BuCL 236 vs 168 mL/min, p=0.0002; BuCL/ABW 3.0 vs 2.1 mL/min/kg, p=0.0001; BuCL/IBW 3.8 vs 2.6 mL/min/kg, p=0.0035; BuCL/CBW 3.4 vs 2.6 mL/min/kg, p=0.0005. Inter-patient variability in clearance (CV%) was up to 48% in both age groups. Phenytoin administration, a potential confounder, did not affect BuCL, regardless of weight normalization (p>0.34).
Conclusions
Contrary to our hypothesis, BuCL was significantly higher in older patients compared to younger patients in these studies and does not explain the previously reported increase in busulfan toxicity observed in older patients.
Keywords: busulfan, bone marrow transplant, elderly, pharmacokinetics
INTRODUCTION
While the incidence of cancer increases with age and the average age of the US population continues to rise, inclusion of older patients in clinical trials remains low [1]. The paucity of information about the pharmacokinetics and pharmacodynamics of anticancer drugs in older patients is an impediment to oncologists making optimal therapeutic decisions in this patient group, and may negatively affect treatment outcomes [2]. The number of people over 65 years of age in the US is projected to double from 35 million persons to 70 million by 2030, and thus account for an estimated 20% of Americans. Cancer rates for people aged 50–64 years are 7- to 16-fold higher than those for younger persons, and rates for persons aged 65–74 years are another 2- to 3-fold higher still [3,4].
Ageing is associated with several physiological changes which can affect drug pharmacokinetics and pharmacodynamics [5–13]. It is evident that there is a need for a better understanding of the interaction between age and anti-cancer drug toxicity and efficacy [14].
Busulfan is an alkylating agent commonly used in preparative regimens for patients undergoing hematopoietic cell transplantation for a variety of malignancies, including acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) [15]. Studies of myeloablative conditioning for AML and MDS have shown a poorer outcome in older patients due to causes other than relapse, such as transplant-related morbidity and mortality [16–19]. As a result, it has become routine practice to exclude older AML and MDS patients from treatment with myeloablative conditioning regimens [15] or alternatively, to lower the dose or number of doses of busulfan for older patients [20].
Both oral and intravenous busulfan are associated with wide interpatient variability in the busulfan area under the plasma concentration-time curve (AUC) achieved [4,21,22]. A high AUC (> 1,500 μmol/L • min) has been associated with a high risk of fatal veno-occlusive disease of the liver, especially in the context of allogeneic transplantation [23]. Other pharmacokinetic-pharmacodynamic correlations for busulfan include: Css and the risk for graft rejection and/or leukemic relapse with a matched sibling graft or HLA–partially mismatched related or HLA matched unrelated donor; Css / AUC and the risk of leukemia recurrence after hematopoietic cell transplantation for CML; Css / AUC and disease-free survival in older MDS patients undergoing allogeneic hematopoietic cell transplantation [24].
Because of the concern for treatment-related morbidity and mortality, older AML and MDS patients have generally been excluded from many myeloablative treatment regimens, and thus may receive less efficient cytoreduction [25]. Information on busulfan pharmacokinetics in older patients would allow better informed treatment decisions and potentially improve outcome. The goal of this study was to investigate whether older age is associated with lower busulfan clearance by pooling extensive pharmacokinetic data from three CALGB transplant studies using busulfan-containing conditioning regimens.
PATIENTS AND METHODS
Patients
Patients were enrolled on one of three studies at CALGB institutions, all of which were approved by the institutional review board of participating institutions. Each participant signed an IRB-approved, protocol-specific informed consent in accordance with federal and institutional guidelines. Two studies, CALGB 19808 (NCT00006363) and 10503 (NCT00416598) used autologous transplant as treatment for AML in first remission in patients under age 60, and CALGB 100103 (NCT00070135) used allogeneic transplant as treatment for AML in first remission for patients between age 60 and 74. All trials required patients to have adequate organ function [26,27]. The CALGB 19808 end date was March 2006. CALGB 10503 and 100103 were performed over the similar time span of 2004–2011.
Treatment
All study patients received 2 h infusions of busulfan at 0.8 mg/kg corrected bodyweight (CBW), every 6 h for a total of 16 doses (CALGB 10503 and 19808) or 8 doses (CALGB 100103). CBW was calculated from ideal bodyweight (IBW) and actual bodyweight (ABW) as follows: CBW = IBW + 0.25*(ABW - IBW). If ABW >150% IBW, 150% of IBW was used as the ABW (IBW would be 112.5% IBW). If ABW < IBW, ABW was used [28]. Supportive care included the use of anti-seizure medication (phenytoin) and anti-emetics, which was recorded. CALGB 10503 and CALGB 19808 involved remission induction with cytarabine, daunorubicin, and etoposide, PBSC mobilization with etoposide, high dose cytarabine and G-CSF, and transplant was preceded by sequential busulfan (days -7 through -4) and etoposide (day -3). In CALGB 100103, the transplant was preceded by 30 mg/m2/day fludarabine (days -7 through -3), busulfan (days -4 through -3), and thymoglobulin (days -4 through -2).
Pharmacokinetic sampling schedule and quantitation of busulfan concentrations
In CALGB 10503 and 19808, blood samples (5 mL collected in heparin anti-coagulated tubes) were obtained prior to, and at 125, 135 min, 4 h, and 6 h after the start of the first 2 h busulfan infusion. Samples were immediately placed on wet ice, refrigerated until processed, and processed within 1 h of collecting the last sample. Plasma was then separated by centrifugation at 1000 × g, for 10 min at 4 °C. Plasma aliquots were transferred to a cryovial and stored at −70 °C until analysis for busulfan concentrations. In CALGB 100103, samples were obtained prior to, and 115 min, 3, 4, 5, and 6 h after the start of the busulfan infusion. Peripheral blood sampling was preferred. If central line blood draws were performed, the central venous catheter was flushed with 5 mL of saline, and the initial 2 mL of blood drawn was discarded.
Plasma busulfan concentrations were quantitated with an LC-MS assay. Briefly, 0.2 mL aliquots of plasma sample, calibrator, or quality control were taken into preparation. Ten microliter of 1 μg/mL [D8]-busulfan in methanol:0.2% formic acid in 10 mM ammonium acetate (50:50, v/v) were added, followed by a double extraction with 1 mL of ethyl acetate. The pooled supernatants were evaporated to dryness at 40 °C under a gentle stream of nitrogen. Dried residues were reconstituted in 50 μL methanol, sonicated, and diluted with 50 μL 0.2% formic acid in 10 mM ammonium acetate. Reconstituted residues were then transferred to Spin-X centrifuge tubes and centrifuged for 2 minutes at 16,000 × g. Ultrafiltrates were transferred to autosampler vials, and 10 μL were injected. The LC system consisted of an Agilent (Palo Alto, CA, USA) 1100 autosampler and binary pump, a Phenomenex (Torrance, CA, USA) hydro-Synergi (4 μm, 100 mm × 2 mm) column at ambient temperature, and a gradient mobile phase of methanol (A) and 0.2% formic acid in 10 mM ammonium acetate buffer (B) pumped at 0.2 mL/min. Between 0 and 4 min, A was increased linearly from 25% to 50%, and kept at 50% for 1 min. Between 5 and 6 min, A was increased linearly from 50% to 99% and then maintained at 99% from 6–8 min at a flow rate of 0.4 mL/min, followed by re-equilibration for a total run-time of 15 min. Mass spectrometric detection was carried out using a ThermoFinnigan (San Jose, CA, USA) MSQ mass spectrometer with electrospray ionization in positive-ion mode. The settings of the mass spectrometer were as follows: capillary voltage 4.0 kV; cone voltage 10 V; probe temperature 400 °C. In single ion monitoring mode, the m/z values monitored were 263.9, and 271.9 for busulfan and [D8]-busulfan, respectively. The LC system and mass spectrometer were controlled by ThermoFinnigan Excalibur software (version 1.4), and data were collected with the same software. The analyte-to-internal standard ratio (response) was calculated for each standard by dividing the area of the analyte peak by the area of the internal standard peak. The assay was accurate (between 96.5 and 106.0 %) and precise (CV <9.3%) over the busulfan concentration range of 1 to 300 ng/mL. Any samples above the upper limit of quantitation (300 ng/mL) was diluted to within the concentration range and re-assayed. During the study period, the assay was externally assessed by participation in the North American Busulfan Proficiency Exchange.
Data Integrity and Statistical Analysis
Patient registration and data collection was managed by the Cancer and Leukemia Group B (CALGB) Statistical Center. Statistical analyses were performed by CALGB statisticians and were based on the study database that was locked on Dec 10, 2011.
The busulfan plasma concentration versus time data was analyzed non-compartmentally using the LaGrange function as implemented by the LAGRAN computer program [29]. Clearance was calculated from AUC0-inf. The percentage of the AUC that was extrapolated was on average 29%, with a median and SD of 27% and 15%, respectively.
The two-sided Fisher’s Exact test was utilized to test for a difference in BMI between age patients <60 years vs ≥60 years old. The Wilcoxon rank sum test [30] was used to test the effect of age <60 years vs ≥60 years old on busulfan clearance (BuCL) as calculated non-compartmentally (mL/min), normalized to actual (ABW), ideal (IBW), or corrected (CBW) body weight (in kg). The effect of body size category (BMI normal 18–26 kg/m2; obese 27–35 kg/m2; severely obese >35 kg/m2), was tested by Kruskal-Wallis rank sum test.
RESULTS
Patient characteristics
A total of 185 subjects were enrolled between August 2003 and July 2010, of which 11 were inevaluable for one or more of the following reasons: original blood sample drawn from busulfan infusion site, inadequate documentation of body weight or dose administered, and inadequate busulfan concentration–time data to perform the non-compartmental pharmacokinetic analysis. Detailed patient demographics are listed in Table 1.
Table 1.
Patient demographics of studies in subjects ≥60 years old (100103) and patients <60 years old (19808 and 10503).
| CALGB Study | 19808 | 10503 | 19808+10503 | 100103 |
|---|---|---|---|---|
|
| ||||
| Number of patients | 56 | 100 | 156 | 32 |
|
| ||||
| Useable pharmacokinetic data | 56 | 100 | 156 | 29 |
|
| ||||
| Median age (range) | 50.3 (19.3–59.6) | 49.6 (17.8–60.0) | 49.8 (17.8–60.0) | 66.3 (60.3–74.3) |
|
| ||||
| Sex | ||||
| Male (%) | 21 (37.5) | 50 (50) | 71 (45.5) | 20 (69) |
|
| ||||
| Race | ||||
| White n (%) | 46 (82) | 85 (85) | 131 (84) | 27 (93) |
| African n (%) | 6 (11) | 4 (4) | 10 (6) | 0 |
| Asian n (%) | 1 (2) | 1 (1) | 2 (1) | 0 |
|
| ||||
| ECOG Performance Status* | ||||
| 0 (%) | 26 (46) | 32 (32) | 58 (37) | 15 (52) |
| 1 (%) | 24 (43) | 62 (62) | 86 (55) | 10 (34) |
| 2 (%) | 5 (9) | 6 (6) | 11 (7) | 4 (14) |
| 3 (%) | 1 (2) | 0 | 1 (0.6) | 0 |
|
| ||||
| Phenytoin use (%) | 49 (88) | 55 (55) | 104 (67) | 13 (45) |
|
| ||||
| Weight (kg) | 76.1(49–172) | 82.3(46.6–171) | 79,8(46.6–172)** | 84(54.1–124)** |
|
| ||||
| BMI (kg/m2) | 27(18.9–57.9) | 29.4(17–54.4) | 28.4(17–57.9)*** | 27.1(19.8–35.4)*** |
Performance status was recorded at the time of diagnosis for patients on the 2 studies with younger patients, but only at the time of transplantation in the older patient study.
P-value by wilcox rank test: 0.7307
P-value by two-sided Fisher’s Exact test: 0.024
Busulfan pharmacokinetics and age
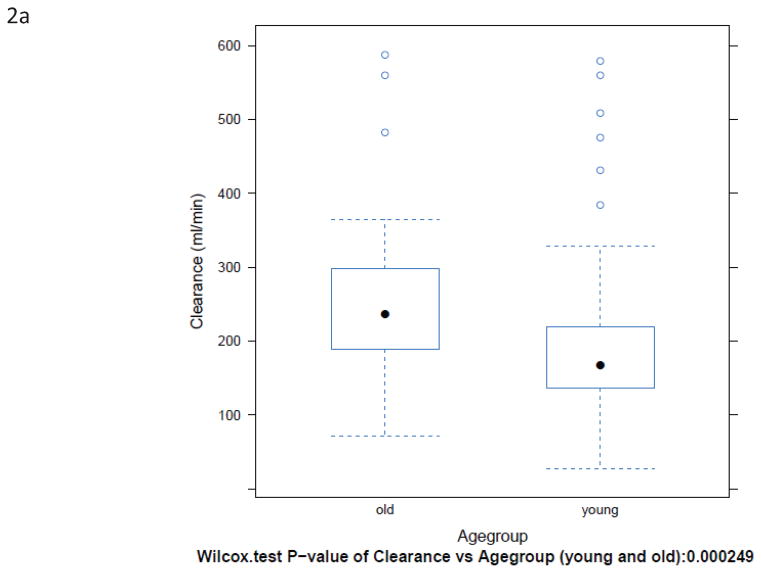
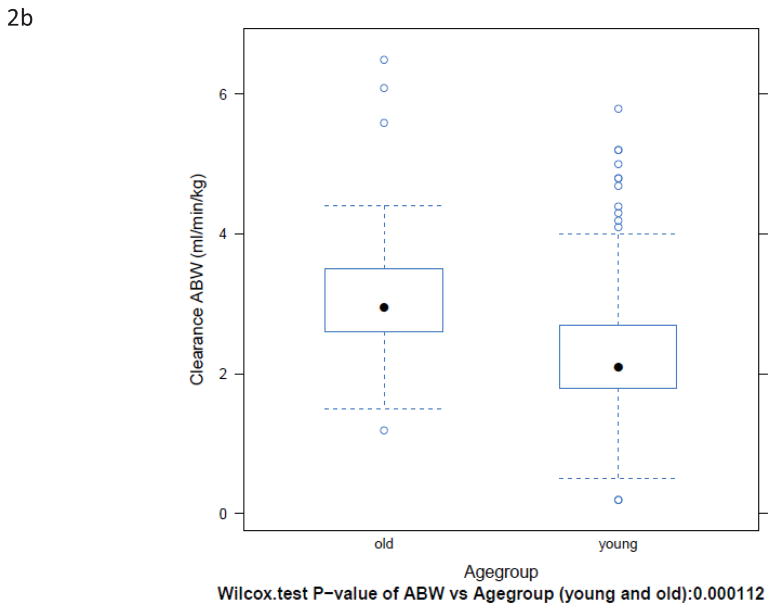
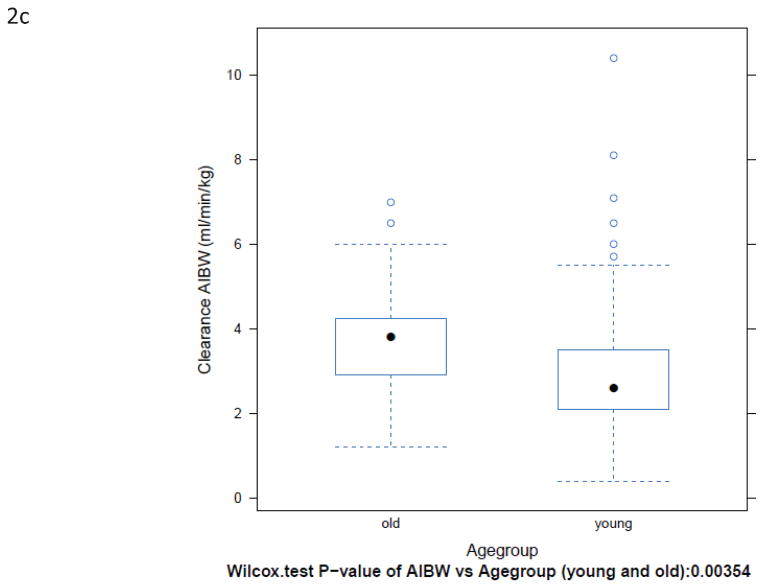
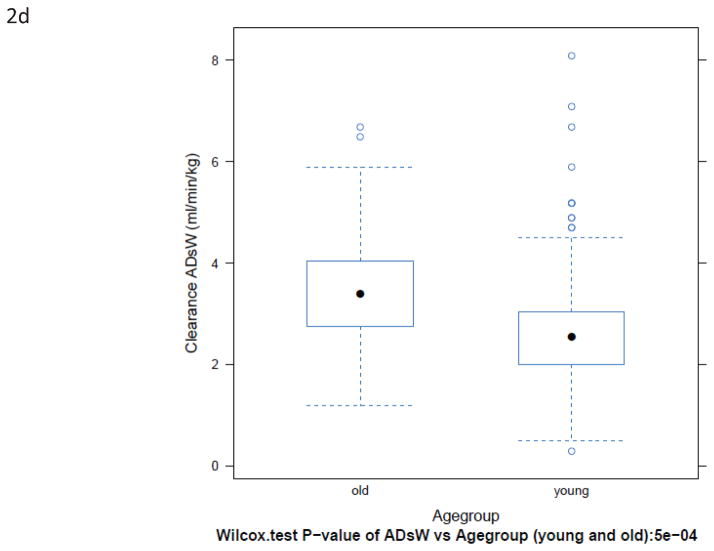
BuCL values of individual patients are shown in Fig. 1. Patients over the age of 60 had a significantly higher clearance than younger patients, regardless of how clearance was expressed (Fig. 2 and Table 2). Inter-patient variability in clearance (CV%) was up to 48% in those <60 years old and up to 47% in the patients ≥60 years old.
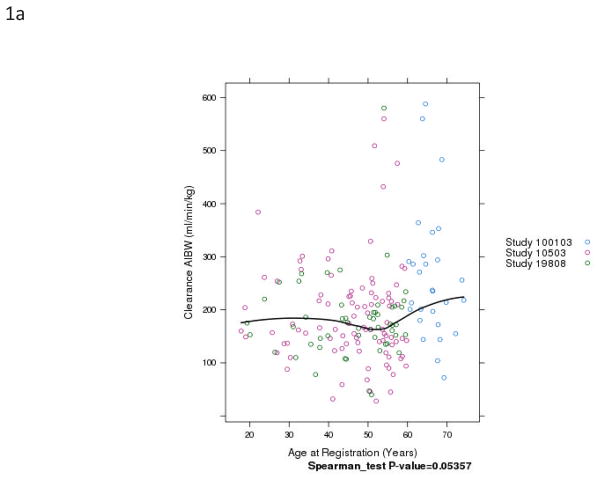
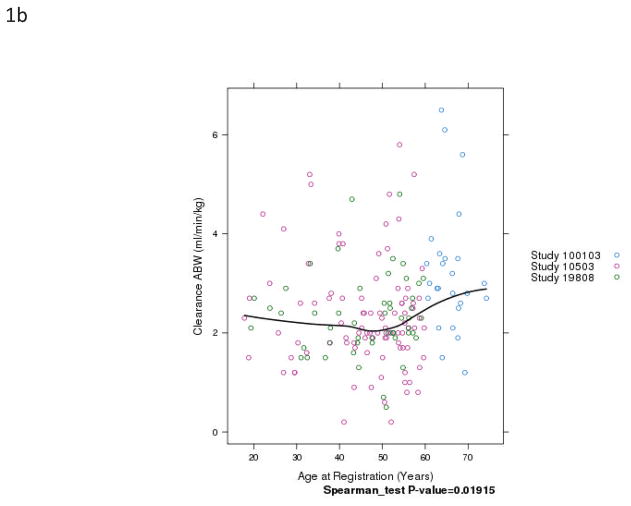
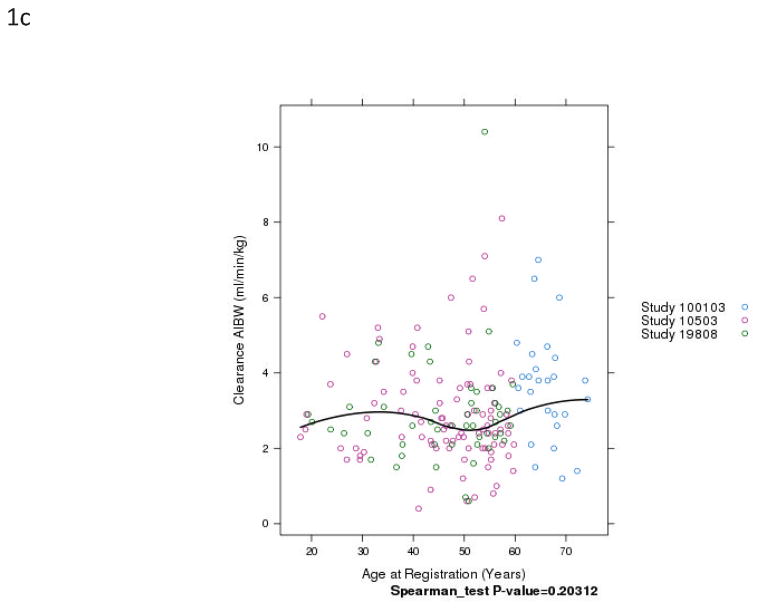
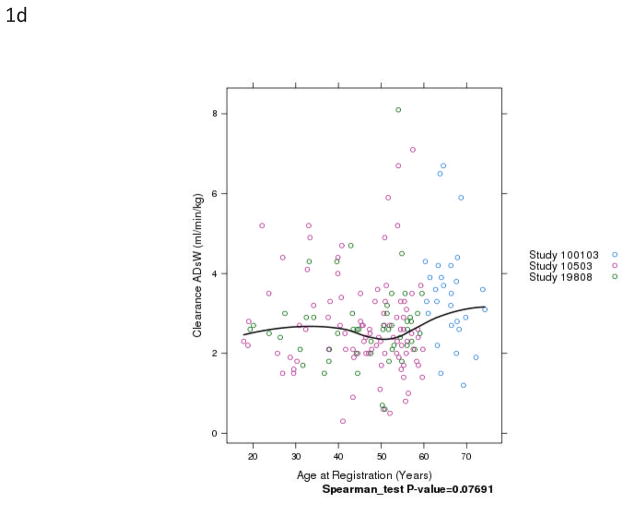
Fig. 1.
Relationship of patient age and busulfan clearance (BuCL). Points represent individual patient values of BuCL (a), BuCL/ABW (b), BuCL/IBW (c), and BuCL/CBW (d). A loess smoother spline was fitted to the data in each case and shows the trend of BuCL with age.
Fig. 2.
Box-plots of BuCL (a; p=0.0002), CL/ABW (b; p=0.0001), CL/IBW (c; p=0.0035), and CL/CBW (d, p=0.0005) in patients <60 years old and ≥60 years old.
Table 2.
Busulfan clearance in subjects ≥ 60 years old (100103) and patients <60 years old (19808 and 10503).
| CALGB Study | 19808 | 10503 | 19808+10503 | 100103 |
|---|---|---|---|---|
|
| ||||
| Median age (range) | 50.3(19.3–59.6) | 49.6(17.8–60.0) | 49.8 (17.8–60.0) | 66.3 (60.3–74.3) |
|
| ||||
| CL (mL/min) | ||||
| Mean | 180 | 190 | 186 | 263 |
| Median | 172 | 163 | 168 | 236 |
| Range | 40–580 | 28–560 | 28–580 | 72–588 |
| P-value* | 0.0002 | |||
|
| ||||
| CL/ABW (mL/min/kg) | ||||
| Mean | 2.34 | 2.34 | 2.34 | 3.22 |
| Median | 2.3 | 2.1 | 2.1 | 3.0 |
| Range | 0.5–4.8 | 0.2–5.8 | 0.2–5.8 | 1.2–6.5 |
| P-value* | 0.0001 | |||
|
| ||||
| CL/IBW (mL/min/kg) | ||||
| Mean | 2.91 | 2.90 | 2.91 | 3.63 |
| Median | 2.6 | 2.6 | 2.6 | 3.8 |
| Range | 0.6–10.4 | 0.4–8.1 | 0.4–10.4 | 1.2–7 |
| P-value* | 0.0035 | |||
|
| ||||
| CL/CBW (mL/min/kg) | ||||
| Mean | 2.7 | 2.7 | 2.7 | 3.5 |
| Median | 2.6 | 2.5 | 2.6 | 3.4 |
| Range | 0.6–8.1 | 0.3–8.1 | 1.2–6.7 | |
| P-value* | 0.0005 | |||
|
| ||||
| t½ (h) | ||||
| Mean | 3.88 | 3.93 | 3.91 | 3.70 |
| Median | 3.00 | 2.77 | 2.82 | 2.62 |
| Range | 1.05–29.4 | 0.28–33.0 | 0.28–33.0 | 1.24–12.4 |
| P-value* | 0.74 | |||
|
| ||||
| Vd (L) | ||||
| Mean | 49.4 | 50.9 | 50.4 | 77.6 |
| Median | 44.5 | 41.6 | 43.4 | 59.2 |
| Range | 20.5–125 | 7.3–302 | 7.3–302 | 15.5–228 |
| P-value* | 0.0023 | |||
patients <60 years old and ≥60 years old i.e CALGB100103 vs CALGB 19808 + CALGB 10503.
ABW, actual bodyweight; IBW, ideal bodyweight, CBW, corrected bodyweight (used to dose)
The elimination half-life was comparable between the studies in patients <60 years old and ≥60 years old. The volume of distribution was statistically higher in the older patients.
The use of phenytoin in our study population was more prevalent in the younger population, and did not affect busulfan clearance, regardless of normalization (p>0.34), as tested by the wilcoxon rank test.
Other co-medications documented in our study population included fluconazole, voriconazole, levofloxacin, flucloxacillin, co-trimoxazole, caspofungin, ondansetron, levetiracetam, lamotrigine, ibuprofen, and oxycodone, but these were not considered likely to be of relevance in modifying busulfan pharmacokinetics, as described previously [31].
Analysis of clearance across age and BMI categories showed that ABW normalized clearance is a function of BMI, while AIBW normalized clearance is not, see Table 3.
Table 3.
Busulfan clearance by different weight metrics and body mass index–based weight category, mean±SD (range).
| Clearance metric | Normal | Obese | Severely Obese | P-value** |
|---|---|---|---|---|
| All patients | ||||
| N (%) | 82 (44.3%)* | 66 (35.7%) | 37 (20.0%) | |
| CL (mL/min) | 183±86.8 (45–560) | 217±110 (40–588) | 200±102 (28–580) | 0.1411 |
| CL/ABW (mL/min/kg) | 2.64±1.1 (0.6–6.5) | 2.56±1.12 (0.5–6.1) | 1.95±0.94 (0.2–4.8) | 0.00097 |
| CL/IBW (mL/min/kg) | 2.82±1.17 (0.6–6.5) | 3.27±1.49 (0.6–8.1) | 3.09±1.78 (0.4–10.4) | 0.25 |
| CL/CBW (mL/min/kg) | 2.76±1.14 (0.6–6.5) | 3.04±1.34 (0.6–7.1) | 2.61±1.33 (0.3–8.1) | 0.2 |
| <60 years | ||||
| N (%) | 68 (43.6%) | 52 (33.3%) | 36 (23.1%) | |
| CL (mL/min) | 169±65.2 (45–384) | 200±103 (40–560) | 199±103 (28–580) | 0.22 |
| CL/ABW (mL/min/kg) | 2.52±0.98 (0.6–5.2) | 2.39±1.06 (0.5–5.8) | 1.92±0.93 (0.2–4.8) | 0.0022 |
| CL/IBW (mL/min/kg) | 2.71±1.06 (0.6–5.5) | 3.07±1.49 (0.6–8.1) | 3.09±1.81 (0.4–10.4) | 0.60 |
| CL/CBW (mL/min/kg) | 2.64±1.02 (0.6–5.2) | 2.86±1.34 (0.6–7.1) | 2.6±1.35 (0.3–8.1) | 0.60 |
| ≥60 years | ||||
| N (%) | 14 (48.3%) | 14 (48.3%) | 1 (3.4%) | |
| CL (mL/min) | 248±137 (72–560) | 283±113 (144–588) | 213 | 0.53 |
| CL/ABW (mL/min/kg) | 3.25±1.46 (1.2–6.5) | 3.22±1.16 (1.5–6.1) | 3 | 0.94 |
| CL/IBW (mL/min/kg) | 3.33±1.55(1.2–6.5) | 4.04±1.29 (1.5–7) | 3 | 0.23 |
| CL/CBW (mL/min/kg) | 3.31±1.48 (1.2–6.5) | 3.73±1.18 (1.5–6.7) | 3 | 0.38 |
One patient had marginal low BMI and was placed in the normal cohort to allow testing across 3 categories.
By Kruskal-Wallis rank sum test
ABW, actual bodyweight; IBW, ideal bodyweight, CBW, corrected bodyweight (used to dose)
DISCUSSION
The goal of this study was to investigate the relationship between busulfan pharmacokinetics and age. Age-dependent differences in the pharmacokinetics of busulfan have been reported previously with children having a higher distribution volume and clearance compared with adults after oral busulfan, resulting in both reduced toxicity and reduced efficacy [32]. When comparable exposures were achieved, toxicity and efficacy were more comparable in children and adults [33]. More recently, a potential age-related difference in pharmacodynamics susceptibility to busulfan was reported. Oral busulfan exposure determined graft rejection in children as had been documented in adults. However, severe regimen-related toxicity appeared unrelated to exposure in children, in contrast to reports in adults[34,23,35]. Our hypothesis was that older patients had a lower busulfan clearance than young patients.
Our study showed that patients older than 60 years did not have a lower busulfan clearance than the patients younger than 60 years. The pharmacokinetic disposition of busulfan in our study was concordant with previous published reports (mean busulfan clearance = 2.74 mL/min/kg ABW; mean t½ = 2.83 h) [24]. The slightly (but statistically significant) higher volume of distribution of busulfan in older patients might be explained by the older subjects having a body composition with more adipose tissue compared to younger study participants, in line with the higher proportion high BMI subjects in the older group. Previously, in both pediatrics and adults, the volume of distribution was shown to be a function of actual body weight [36–38]
Our hypothesis was based on a decreased metabolic capacity for busulfan in older subjects. Busulfan is predominantly conjugated to glutathione by glutathione-S-transferase 1A1 (GST1A1), and metabolized to a lesser extent by CYP3A4 [39]. The rate-limiting factor for glutathione conjugation via GST1A1 is the availability of the cofactor glutathione, the availability of which decreases with age, as reported in human lymphocytes, gastric mucosa, and erythrocytes, and rodent liver, kidney and heart [40–42]. This presumably explains shifts of human acetaminophen glutathionylation to oxidative metabolism with older age [43]. Therefore, we hypothesized that busulfan clearance would be lower in older patients, resulting in higher busulfan exposures, increasing non-disease related morbidity and mortality in older AML patients. The data from our studies suggested that busulfan clearance is increased (not lower) in patients ≥60 years old compared with patients <60 years. The effect of age on clearance appears opposite to our hypothesis, but the absolute difference in clearance of −68 mL/min (−127 to −27 mL/min 95% CI of the difference) equals 40% of the median clearance in patients <60 years. This effect is small relative to the observed inter-individual coefficient of variability in BuCL of 47%. The relatively large variability in clearance observed in our study relative to the 19–34% variability previously reported [44,4,45–49]is likely a reflection of the diverse population of patients participating in this multi-institutional cooperative group trial, as opposed to the previous, mostly single institution, studies.
Based on a recent report by Yeh et al.[50], we analyzed our data after categorizing patients based on BMI category and confirmed the previous report that ABW normalized clearance is a function of BMI, and that AIBW normalized clearance is not. Indeed, BMI was higher in the older group of patients (p=0.024). Although this could partially explain the observed difference in CL/ABW between patients age <60 years vs ≥60 years old, it does not explain the difference when expressed as CL/AIBW. The effect of body size on busulfan pharmacology deserves further characterization [50,44].
A number of medications may bias busulfan pharmacokinetics studies. Itraconazole, but not fluconazole, decreased busulfan clearance by 20% [51]. Metronidazole co-administration doubled busulfan trough concentrations, increasing toxicity, including multi-organ failure, veno-occlusive disease and hemorrhagic cystitis, likely through inhibition of CYP3A metabolism [52]. Risk factors for veno-occlusive disease of the liver included concomitant ketoconazole, a known pan-CYP450 inhibitor [53]. Phenytoin increased oral busulfan clearance by approximately 20% [54,55], likely due to induction of CYP3A4 [56], which may have predominated any reduction in glutathione conjugation [57–59]. Blood glutathione concentrations have been positively correlated with Bu clearance (adjusted R2 = 0.45; P = 0.009) [49]. The toxicological relevance of the shift of busulfan clearance from conjugation to oxidative metabolism is uncertain. Phenytoin use was documented in our study population, but could not explain the increased clearance in the older patients because its use was slightly more prevalent in the younger patients studied rather than the older patients. In addition, more recent data with intravenous busulfan has failed to confirm the interaction observed with oral busulfan [31,60,38,36]. Fluconazole and voriconazole were documented as administered concomitant medications, but neither ketoconazole nor itraconazole, the more potent inhibitors of CYP450 enzymes, were administered in our study populations [61]. No other co-medication was deemed able to explain the observed difference in busulfan clearance. Yeh et al. previously reported that concomitant fludarabine (used in the study of patients >60 years old) might decrease busulfan clearance from day 1 to day 4, though busulfan preceded by fludarabine did not result in any change in busulfan clearance [50]. Unfortunately data were only presented as relative change between days, not allowing comparison of absolute clearance values between these two scenarios. Furthermore, Bartelink et al. did not observe a changes in busulfan clearance between patients with or without fludarabine[31].
Previously, McCune et al. reported in a dataset of 1610 patients (92% children; 128 adults with a maximum age of 66 years old) that busulfan clearance normalized by body size essentially plateaued at age 2.5 years. However, their dataset did not have nearly as many elderly patients as the current study and utilized “size-standardized clearance”, which utilized “normal fat mass” as the size-metric, itself derived through a series of estimates and formulae. Visually, the cluster of patients age 32–66 years old might display higher clearances than the model estimated [44].
Busulfan exposure is correlated with both efficacy and toxicity in patients. The considerable differences in study design of the three trials from which the patients’ busulfan pharmacokinetic data were obtained, precluded a rigorous evaluation of the effect of age on non-disease related toxicities.
The increased clearance that we observed in the older patients should be interpreted with caution, and clearly needs to be confirmed in future studies. Potential limitations of our findings are that the pharmacokinetic studies were performed on the first day of busulfan dosing, which may not be reflective of the entire treatment period (usually 16 or 8 doses at 6-hourly intervals) with busulfan. Possibly, clearance may decrease as glutathione levels are depleted upon repeated doses, and it is not inconceivable that this effect would be more pronounced in older patients. The slightly different pharmacokinetic sampling in the studies is unlikely to have had an impact on calculated busulfan pharmacokinetic parameters. Potentially, there exists a selection bias in the older subjects for good performance status, with a better physiological state than less selected subjects of <60 years old. This notion is supported by the higher percentage of older patients with good performance status. An unplanned sub-analysis of the effect of age on BuCl within each of the three individual studies did not result in a significant effect (P-value>0.162).
In summary, we observed that there was increased BuCL in a study of patients ≥60 with AML undergoing transplantation compared to younger patients (<60 years) with AML in two other studies where busulfan was used as part of a myeloablative regimen. Future studies of this kind should recruit all age groups in a single protocol with a uniform study plan, and use measures to prevent selection bias. In addition, other possible covariates, such as functional age [62], and its relationship with drug tolerability, need to be studied in older patients to better optimize busulfan therapy for individuals ≥60 years old. Subsequently, potential differences in exposure-response relationships between these age-cohorts can be addressed. Population pharmacokinetic approaches and limited sampling strategies may then play a role in further optimizing therapy in this increasingly prevalent type of patient.
Acknowledgments
We thank the University of Pittsburgh Cancer Institute Hematology/Oncology Writing Group for constructive suggestions regarding the manuscript. This project used the UPCI Clinical Pharmacology Analytical Facility (CPAF) and was supported in part by award P30CA047904 (Jan H. Beumer). (Alliance) was supported, in part, by grants from the National Cancer Institute (CA31946) to the Alliance for Clinical Trials in Oncology (Monica M. Bertagnolli, M.D., Chair) and to the Alliance Statistics and Data Center (Daniel J. Sargent, Ph.D., CA33601). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
This manuscript is dedicated to Merrill J. Egorin for his many contributions to the CALGB and the field of cancer therapeutics.
The following institutions participated in this study: Christiana Care Health Services, Inc. CCOP, Wilmington, DE, Stephen Grubbs, M.D., supported by CA45418; Dana-Farber Cancer Institute, Boston, MA, Harold J. Burstein, M.D., Ph.D., supported by CA32291; Monter Cancer Center of North Shore - LIJ Health Systems, Lake Success, NY, Daniel Budman, MD, supported by CA35279; Massachusetts General Hospital, Boston, MA, Jeffrey W. Clark, M.D., supported by CA32291; Mount Sinai Medical Center, Miami, FL, Michael A. Schwartz, M.D., supported by CA45564; The Ohio State University Medical Center, Columbus, OH, Clara D. Bloomfield, M.D., supported by CA77658; Rhode Island Hospital, Providence, RI, William Sikov, M.D., supported by CA08025; Roswell Park Cancer Institute, Buffalo, NY, Ellis Levine, M.D., supported by CA59518; University of California at San Francisco, San Francisco, CA, Charles J. Ryan, M.D., supported by CA60138; University of Chicago, Chicago, IL, Hedy L. Kindler, M.D., supported by CA41287; University of Maryland Greenebaum Cancer Center, Baltimore, MD, Martin Edelman, M.D., supported by CA31983; University of Massachusetts Medical School, Worcester, MA, William V. Walsh, M.D., supported by CA37135; University of Minnesota, Minneapolis, MN, Bruce A. Peterson, M.D., supported by CA16450; University of Nebraska Medical Center, Omaha, NE, Apar Ganti, M.D., supported by CA77298; University of North Carolina at Chapel Hill, Chapel Hill, NC, Thomas C. Shea, M.D., supported by CA47559; University of Vermont, Burlington, VT, Steven M. Grunberg, M.D., supported by CA77406; Wake Forest University School of Medicine, Winston-Salem, NC, David D. Hurd, M.D., supported by CA03927; Washington University School of Medicine, St. Louis, MO, Nancy Bartlett, M.D., supported by CA77440; Weill Medical College of Cornell University, New York, NY, John Leonard, M.D., supported by CA07968; Western Pennsylvania Cancer Institute, Pittsburgh, PA, John Lister, M.D., supported by CA31946; Dartmouth Medical School-Norris Cotton Cancer Center, Lebanon, NH, Konstantin Dragnev, M.D., supported by CA04326; Walter Reed Army Medical Center, Washington, DC, David C. Van Echo, M.D., supported by CA26806.
Footnotes
Disclosures: None
Contributor Information
Jan H. Beumer, Email: beumerj@gmail.com.
Kouros Owzar, Email: kouros.owzar@duke.edu.
Lionel D. Lewis, Email: Lionel.D.Lewis@dartmouth.edu.
Chen Jiang, Email: chen.jiang@duke.edu.
Julianne L. Holleran, Email: HollJL@UPMC.EDU.
Susan M. Christner, Email: christners@upmc.edu.
William Blum, Email: William.Blum@osumc.edu.
Steven Devine, Email: Steven.Devine@osumc.edu.
Jonathan E. Kolitz, Email: kolitz@nshs.edu.
Charles Linker, Email: chas.linker@gmail.com.
Ravi Vij, Email: rvij@dom.wustl.edu.
Edwin P Alyea, Email: Edwin_Alyea@dfci.harvard.edu.
Richard A. Larson, Email: rlarson@medicine.bsd.uchicago.edu.
Mark J. Ratain, Email: mratain@medicine.bsd.uchicago.edu.
References
- 1.Chow SL, Lin Y, LoConte NK, Royds RB, Nekrassova TG, Ivy SP, Mauer J, Wilding G, Stoller R, Egorin MJ. Enrollment of and toxicity in patients 70 years and older on CTEP/NCI-sponsored, single-agent phase I studies from 1980 to 2005. Proceedings of the American Society of Clinical Oncology. 2010;28:15s. [Google Scholar]
- 2.Begg CB, Carbone PP. Clinical trials and drug toxicity in the elderly. The experience of the Eastern Cooperative Oncology Group. Cancer. 1983;52 (11):1986–1992. doi: 10.1002/1097-0142(19831201)52:11<1986::aid-cncr2820521103>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Edwards BK, Howe HL, Ries LAG, Thun MJ, Rosenberg HM, Yancik R, Wingo PA, Jemal A, Feigal EG. Annual Report to the Nation on the status of cancer, 1973–1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002;94 (10):2766–2792. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]
- 4.McCune JS, Holmberg LA. Busulfan in hematopoietic stem cell transplant setting. Expert opinion on drug metabolism & toxicology. 2009;5 (8):957–969. doi: 10.1517/17425250903107764. [DOI] [PubMed] [Google Scholar]
- 5.Adelman LS, Liebman J. Anatomy of body water and electrolytes. The American journal of medicine. 2011;27:256–277. doi: 10.1016/0002-9343(59)90346-8. [DOI] [PubMed] [Google Scholar]
- 6.Egorin MJ. Cancer pharmacology in the elderly. Semin Oncol. 1993;20 (1):43–49. [PubMed] [Google Scholar]
- 7.Forbes GB, Reina JC. Adult lean body mass declines with age: some longitudinal observations. Metabolism: clinical and experimental. 1970;19(9):653–663. doi: 10.1016/0026-0495(70)90062-4. 0026-0495(70)90062-4 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33 (4):278–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 9.Repetto L, Comandini D, Mammoliti S. Life expectancy, comorbidity and quality of life: the treatment equation in the older cancer patients. Crit RevOncolHematol. 2001;37(2):147–152. doi: 10.1016/s1040-8428(00)00104-9. S1040-8428(00)00104-9 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120 (2):104–110. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- 11.Shock NW, Watkin DM, Yiengst MJ, Norris AH, Gaffney GW, Gregerman RI, Falzone JA. Age differences in the water content of the body as related to basal oxygen consumption in males. J Gerontol. 1963;18:1–8. doi: 10.1093/geronj/18.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Sotaniemi EA, Arranto AJ, Pelkonen O, Pasanen M. Age and cytochrome P450-linked drug metabolism in humans: an analysis of 226 subjects with equal histopathologic conditions. Clinical pharmacology and therapeutics. 1997;61 (3):331–339. doi: 10.1016/S0009-9236(97)90166-1. [DOI] [PubMed] [Google Scholar]
- 13.Tumer N, Scarpace PJ, Lowenthal DT. Geriatric pharmacology: basic and clinical considerations. Annual review of pharmacology and toxicology. 1992;32:271–302. doi: 10.1146/annurev.pa.32.040192.001415. [DOI] [PubMed] [Google Scholar]
- 14.Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, Lichtman SM, Gajra A, Bhatia S, Katheria V, Klapper S, Hansen K, Ramani R, Lachs M, Wong FL, Tew WP. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457–3465. doi: 10.1200/JCO.2011.34.7625. JCO.2011.34.7625 [pii] [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alatrash G, de Lima M, Hamerschlak N, Pelosini M, Wang X, Xiao L, Kerbauy F, Chiattone A, Rondon G, Qazilbash MH, Giralt SA, de Padua Silva L, Hosing C, Kebriaei P, Zhang W, Nieto Y, Saliba RM, Champlin RE, Andersson BS. Myeloablative reduced-toxicity i.v. busulfan-fludarabine and allogeneic hematopoietic stem cell transplant for patients with acute myeloid leukemia or myelodysplastic syndrome in the sixth through eighth decades of life. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17 (10):1490–1496. doi: 10.1016/j.bbmt.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cahn JY, Labopin M, Schattenberg A, Reiffers J, Willemze R, Zittoun R, Bacigalupo A, Prentice G, Gluckman E, Herve P, Gratwohl A, Gorin NC. Allogeneic bone marrow transplantation for acute leukemia in patients over the age of 40 years. Acute Leukemia Working Party of the European Group for Bone Marrow Transplantation (EBMT) Leukemia. 1997;11 (3):416–419. doi: 10.1038/sj.leu.2400573. [DOI] [PubMed] [Google Scholar]
- 17.Ringden O, Labopin M, Ehninger G, Niederwieser D, Olsson R, Basara N, Finke J, Schwerdtfeger R, Eder M, Bunjes D, Gorin NC, Mohty M, Rocha V. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol. 2009;27(27):4570–4577. doi: 10.1200/JCO.2008.20.9692. JCO.2008.20.9692 [pii] [doi] [DOI] [PubMed] [Google Scholar]
- 18.Sutton L, Chastang C, Ribaud P, Jouet JP, Kuentz M, Attal M, Reiffers J, Tigaud JM, Rio B, Dauriac C, Legros M, Dreyfus F, Lioure B, Troussard X, Milpied N, Witz F, Oriol P, Cahn JY, Michallet M, Gluckman E, Ifrah N, Pico JL, Vilmer E, Leblond V. Factors influencing outcome in de novo myelodysplastic syndromes treated by allogeneic bone marrow transplantation: a long-term study of 71 patients Societe Francaise de Greffe de Moelle. Blood. 1996;88 (1):358–365. [PubMed] [Google Scholar]
- 19.Wallen H, Gooley TA, Deeg HJ, Pagel JM, Press OW, Appelbaum FR, Storb R, Gopal AK. Ablative allogeneic hematopoietic cell transplantation in adults 60 years of age and older. J Clin Oncol. 2005;23(15):3439–3446. doi: 10.1200/JCO.2005.05.694. JCO.2005.05.694 [pii] [doi] [DOI] [PubMed] [Google Scholar]
- 20.Yusuf RZ, Dey B, Yeap BY, McAfee S, Attar E, Sepe PS, Dube C, Spitzer TR, Ballen KK. Autologous SCT with a dose-reduced BU and CY regimen in older patients with non-Hodgkin’s lymphoma. Bone marrow transplantation. 2009;43 (1):37–42. doi: 10.1038/bmt.2008.298. [DOI] [PubMed] [Google Scholar]
- 21.Schuler US, Ehrsam M, Schneider A, Schmidt H, Deeg J, Ehninger G. Pharmacokinetics of intravenous busulfan and evaluation of the bioavailability of the oral formulation in conditioning for haematopoietic stem cell transplantation. Bone marrow transplantation. 1998;22 (3):241–244. doi: 10.1038/sj.bmt.1701322. [DOI] [PubMed] [Google Scholar]
- 22.Veal GJ, Nguyen L, Paci A, Riggi M, Amiel M, Valteau-Couanet D, Brock P, Ladenstein R, Vassal G. Busulfan pharmacokinetics following intravenous and oral dosing regimens in children receiving high-dose myeloablative chemotherapy for high-risk neuroblastoma as part of the HR-NBL-1/SIOPEN trial. Eur J Cancer. 2012;48(16):3063–3072. doi: 10.1016/j.ejca.2012.05.020. S0959-8049(12)00464-9 [pii] [doi] [DOI] [PubMed] [Google Scholar]
- 23.Grochow LB. Busulfan disposition: the role of therapeutic monitoring in bone marrow transplantation induction regimens. Seminars in oncology. 1993;20 (4 Suppl 4):18–25. quiz 26. [PubMed] [Google Scholar]
- 24.Andersson BS, Kashyap A, Gian V, Wingard JR, Fernandez H, Cagnoni PJ, Jones RB, Tarantolo S, Hu WW, Blume KG, Forman SJ, Champlin RE. Conditioning therapy with intravenous busulfan and cyclophosphamide (IV BuCy2) for hematologic malignancies prior to allogeneic stem cell transplantation: a phase II study. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2002;8 (3):145–154. doi: 10.1053/bbmt.2002.v8.pm11939604. [DOI] [PubMed] [Google Scholar]
- 25.Andersson BS, Thall PF, Madden T, Couriel D, Wang X, Tran HT, Anderlini P, de LM, Gajewski J, Champlin RE. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2002;8(9):477–485. doi: 10.1053/bbmt.2002.v8.pm12374452. S1083879102500082 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Devine SM, Ozwar K, Blum W, DeAngelo D, Stone RM, Hsu JW, Champlin RE, Chen YA, Vij R, Slack JL, Soiffer RJ, Larson RA, Shea TC, Hars V, Bennett E, Spangle S, Giralt SA, Carter SL, Horowitz MM, Linker C, Alyea EP. A Phase II Study of Allogeneic Transplantation for Older Patients with AML in First Complete Remission Using a Reduced Intensity Conditioning Regimen: Results from CALGB100103/BMT CTN 0502. Blood. 2012;120 (21):230. [Google Scholar]
- 27.Kolitz JE, George SL, Marcucci G, Vij R, Powell BL, Allen SL, DeAngelo DJ, Shea TC, Stock W, Baer MR, Hars V, Maharry K, Hoke E, Vardiman JW, Bloomfield CD, Larson RA Cancer, Leukemia Group B. P-glycoprotein inhibition using valspodar (PSC-833) does not improve outcomes for patients younger than age 60 years with newly diagnosed acute myeloid leukemia: Cancer and Leukemia Group B study 19808. Blood. 2010;116 (9):1413–1421. doi: 10.1182/blood-2009-07-229492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson JD, Lupkiewicz SM, Palenik L, Lopez LM, Ariet M. Determination of ideal body weight for drug dosage calculations. AmJHospPharm. 1983;40 (6):1016–1019. [PubMed] [Google Scholar]
- 29.Ediss C, Tam YK. An interactive computer program for determining areas bounded by drug concentration curves using lagrange interpolation. Journal of pharmacological and toxicological methods. 1995;34 (3):165–168. doi: 10.1016/1056-8719(95)00062-1. [DOI] [PubMed] [Google Scholar]
- 30.Altman DG. Practical Statistics for Medical Research. Chapman & Hall; London: 1991. [Google Scholar]
- 31.Bartelink IH, Boelens JJ, Bredius RG, Egberts AC, Wang C, Bierings MB, Shaw PJ, Nath CE, Hempel G, Zwaveling J, Danhof M, Knibbe CA. Body weight-dependent pharmacokinetics of busulfan in paediatric haematopoietic stem cell transplantation patients: towards individualized dosing. Clinical pharmacokinetics. 2012;51 (5):331–345. doi: 10.2165/11598180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 32.Grochow LB, Krivit W, Whitley CB, Blazar B. Busulfan disposition in children. Blood. 1990;75 (8):1723–1727. [PubMed] [Google Scholar]
- 33.Yeager AM, Wagner JE, Jr, Graham ML, Jones RJ, Santos GW, Grochow LB. Optimization of busulfan dosage in children undergoing bone marrow transplantation: a pharmacokinetic study of dose escalation. Blood. 1992;80 (9):2425–2428. [PubMed] [Google Scholar]
- 34.McCune JS, Gooley T, Gibbs JP, Sanders JE, Petersdorf EW, Appelbaum FR, Anasetti C, Risler L, Sultan D, Slattery JT. Busulfan concentration and graft rejection in pediatric patients undergoing hematopoietic stem cell transplantation. Bone marrow transplantation. 2002;30 (3):167–173. doi: 10.1038/sj.bmt.1703612. [DOI] [PubMed] [Google Scholar]
- 35.Dix SP, Wingard JR, Mullins RE, Jerkunica I, Davidson TG, Gilmore CE, York RC, Lin LS, Devine SM, Geller RB, Heffner LT, Hillyer CD, Holland HK, Winton EF, Saral R. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone marrow transplantation. 1996;17 (2):225–230. [PubMed] [Google Scholar]
- 36.Nguyen L, Fuller D, Lennon S, Leger F, Puozzo C. I.V. busulfan in pediatrics: a novel dosing to improve safety/efficacy for hematopoietic progenitor cell transplantation recipients. Bone marrow transplantation. 2004;33 (10):979–987. doi: 10.1038/sj.bmt.1704446. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen L, Leger F, Lennon S, Puozzo C. Intravenous busulfan in adults prior to haematopoietic stem cell transplantation: a population pharmacokinetic study. Cancer ChemotherPharmacol. 2006;57(2):191–198. doi: 10.1007/s00280-005-0029-0. [doi] [DOI] [PubMed] [Google Scholar]
- 38.Schechter T, Finkelstein Y, Doyle J, Verjee Z, Moretti M, Koren G, Dupuis LL. Pharmacokinetic disposition and clinical outcomes in infants and children receiving intravenous busulfan for allogeneic hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13 (3):307–314. doi: 10.1016/j.bbmt.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 39.Vassal G, Challine D, Koscielny S, Hartmann O, Deroussent A, Boland I, Valteau-Couanet D, Lemerle J, Levi F, Gouyette A. Chronopharmacology of high-dose busulfan in children. Cancer research. 1993;53 (7):1534–1537. [PubMed] [Google Scholar]
- 40.Hazelton GA, Lang CA. Glutathione contents of tissues in the aging mouse. The Biochemical journal. 1980;188 (1):25–30. doi: 10.1042/bj1880025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loguercio C, Taranto D, Vitale LM, Beneduce F, Del Vecchio Blanco C. Effect of liver cirrhosis and age on the glutathione concentration in the plasma, erythrocytes, and gastric mucosa of man. Free radical biology & medicine. 1996;20 (3):483–488. doi: 10.1016/0891-5849(96)02057-6. [DOI] [PubMed] [Google Scholar]
- 42.van Lieshout EM, Peters WH. Age and gender dependent levels of glutathione and glutathione S-transferases in human lymphocytes. Carcinogenesis. 1998;19 (10):1873–1875. doi: 10.1093/carcin/19.10.1873. [DOI] [PubMed] [Google Scholar]
- 43.Pickering G, Schneider E, Papet I, Pujos-Guillot E, Pereira B, Simen E, Dubray C, Schoeffler P. Acetaminophen metabolism after major surgery: a greater challenge with increasing age. Clinical pharmacology and therapeutics. 2011;90 (5):707–711. doi: 10.1038/clpt.2011.176. [DOI] [PubMed] [Google Scholar]
- 44.McCune JS, Bemer MJ, Barrett JS, Scott Baker K, Gamis AS, Holford NH. Busulfan in infant to adult hematopoietic cell transplant recipients: a population pharmacokinetic model for initial and Bayesian dose personalization. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20 (3):754–763. doi: 10.1158/1078-0432.CCR-13-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andersson BS, Madden T, Tran HT, Hu WW, Blume KG, Chow DS, Champlin RE, Vaughan WP. Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as pretransplantation conditioning therapy: a phase I study. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2000;6 (5A):548–554. doi: 10.1016/s1083-8791(00)70064-4. [DOI] [PubMed] [Google Scholar]
- 46.Madden T, de Lima M, Thapar N, Nguyen J, Roberson S, Couriel D, Pierre B, Shpall EJ, Jones RB, Champlin RE, Andersson BS. Pharmacokinetics of once-daily IV busulfan as part of pretransplantation preparative regimens: a comparison with an every 6-hour dosing schedule. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13 (1):56–64. doi: 10.1016/j.bbmt.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 47.Geddes M, Kangarloo SB, Naveed F, Quinlan D, Chaudhry MA, Stewart D, Savoie ML, Bahlis NJ, Brown C, Storek J, Andersson BS, Russell JA. High busulfan exposure is associated with worse outcomes in a daily i.v. busulfan and fludarabine allogeneic transplant regimen. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14 (2):220–228. doi: 10.1016/j.bbmt.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 48.Ryu SG, Lee JH, Choi SJ, Lee JH, Lee YS, Seol M, Hur EH, Lee SH, Bae KS, Noh GJ, Lee MS, Yun SC, Han SB, Lee KH. Randomized comparison of four-times-daily versus once-daily intravenous busulfan in conditioning therapy for hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13 (9):1095–1105. doi: 10.1016/j.bbmt.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Almog S, Kurnik D, Shimoni A, Loebstein R, Hassoun E, Gopher A, Halkin H, Nagler A. Linearity and stability of intravenous busulfan pharmacokinetics and the role of glutathione in busulfan elimination. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17 (1):117–123. doi: 10.1016/j.bbmt.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 50.Yeh RF, Pawlikowski MA, Blough DK, McDonald GB, O’Donnell PV, Rezvani A, Deeg HJ, McCune JS. Accurate targeting of daily intravenous busulfan with 8-hour blood sampling in hospitalized adult hematopoietic cell transplant recipients. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18 (2):265–272. doi: 10.1016/j.bbmt.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buggia I, Zecca M, Alessandrino EP, Locatelli F, Rosti G, Bosi A, Pession A, Rotoli B, Majolino I, Dallorso A, Regazzi MB. Itraconazole can increase systemic exposure to busulfan in patients given bone marrow transplantation. GITMO (Gruppo Italiano Trapianto di Midollo Osseo) Anticancer Res. 1996;16 (4A):2083–2088. [PubMed] [Google Scholar]
- 52.Nilsson C, Aschan J, Hentschke P, Ringden O, Ljungman P, Hassan M. The effect of metronidazole on busulfan pharmacokinetics in patients undergoing hematopoietic stem cell transplantation. Bone marrow transplantation. 2003;31 (6):429–435. doi: 10.1038/sj.bmt.1703896. [DOI] [PubMed] [Google Scholar]
- 53.Meresse V, Hartmann O, Vassal G, Benhamou E, Valteau-Couanet D, Brugieres L, Lemerle J. Risk factors for hepatic veno-occlusive disease after high-dose busulfan-containing regimens followed by autologous bone marrow transplantation: a study in 136 children. Bone Marrow Transplant. 1992;10 (2):135–141. [PubMed] [Google Scholar]
- 54.Hassan M, Oberg G, Bjorkholm M, Wallin I, Lindgren M. Influence of prophylactic anticonvulsant therapy on high-dose busulphan kinetics. Cancer chemotherapy and pharmacology. 1993;33 (3):181–186. doi: 10.1007/BF00686213. [DOI] [PubMed] [Google Scholar]
- 55.Sandstrom M, Karlsson MO, Ljungman P, Hassan Z, Jonsson EN, Nilsson C, Ringden O, Oberg G, Bekassy A, Hassan M. Population pharmacokinetic analysis resulting in a tool for dose individualization of busulphan in bone marrow transplantation recipients. Bone marrow transplantation. 2001;28 (7):657–664. doi: 10.1038/sj.bmt.1703229. [DOI] [PubMed] [Google Scholar]
- 56.Zhou SF. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. CurrDrug Metab. 2008;9 (4):310–322. doi: 10.2174/138920008784220664. [DOI] [PubMed] [Google Scholar]
- 57.Gallagher EP, Sheehy KM. Effects of phenytoin on glutathione status and oxidative stress biomarker gene mRNA levels in cultured precision human liver slices. Toxicological sciences : an official journal of the Society of Toxicology. 2001;59 (1):118–126. doi: 10.1093/toxsci/59.1.118. [DOI] [PubMed] [Google Scholar]
- 58.Kostrubsky SE, Sinclair JF, Strom SC, Wood S, Urda E, Stolz DB, Wen YH, Kulkarni S, Mutlib A. Phenobarbital and phenytoin increased acetaminophen hepatotoxicity due to inhibition of UDP-glucuronosyltransferases in cultured human hepatocytes. Toxicological sciences : an official journal of the Society of Toxicology. 2005;87 (1):146–155. doi: 10.1093/toxsci/kfi211. [DOI] [PubMed] [Google Scholar]
- 59.Ono H, Sakamoto A, Sakura N. Plasma total glutathione concentrations in epileptic patients taking anticonvulsants. Clin Chim Acta. 2000;298(1–2):135–143. doi: 10.1016/s0009-8981(00)00286-2. S0009898100002862 [pii] [DOI] [PubMed] [Google Scholar]
- 60.Trame MN, Bergstrand M, Karlsson MO, Boos J, Hempel G. Population pharmacokinetics of busulfan in children: increased evidence for body surface area and allometric body weight dosing of busulfan in children. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17 (21):6867–6877. doi: 10.1158/1078-0432.CCR-11-0074. [DOI] [PubMed] [Google Scholar]
- 61.Zhang S, Pillai VC, Mada SR, Strom S, Venkataramanan R. Effect of voriconazole and other azole antifungal agents on CYP3A activity and metabolism of tacrolimus in human liver microsomes. Xenobiotica; the fate of foreign compounds in biological systems. 2011 doi: 10.3109/00498254.2011.631224. [doi] [DOI] [PubMed] [Google Scholar]
- 62.Pallis AG, Hatse S, Brouwers B, Pawelec G, Falandry C, Wedding U, Lago LD, Repetto L, Ring A, Wildiers H. Evaluating the physiological reserves of older patients with cancer: the value of potential biomarkers of aging? Journal of geriatric oncology. 2014;5 (2):204–218. doi: 10.1016/j.jgo.2013.09.001. [DOI] [PubMed] [Google Scholar]