Abstract
Rationale
Cyclic AMP (cAMP)-protein kinase A (PKA) signaling has been implicated in the regulation of ethanol consumption. Phosphodiesterase-4 (PDE4) specifically hydrolyzes cAMP and plays a critical role in controlling intracellular cAMP levels in the brain. However, the role of PDE4 in ethanol consumption remains unknown.
Objective
To examine whether PDE4 was involved in regulating ethanol intake.
Methods
The two-bottle choice paradigm was used to assess intake of ethanol, sucrose, and quinine in C57BL/6J mice treated with the selective PDE4 inhibitor rolipram or Ro 20-1724; locomotor activity was also monitored using the open-field test in mice treated with rolipram.
Results
Administration (i.p.) of either rolipram (0.25 and 0.5 mg/kg) or Ro 20-1724 (10 mg/kg) reduced ethanol intake and preference by 60-80%, but did not alter total fluid intake. In contrast, rolipram even at the higher dose of 0.5 mg/kg was not able to affect intake of sucrose or quinine, alcohol-induced sedation, or blood ethanol elimination. At 0.5 mg/kg, rolipram did decrease locomotor activity, but the effect only lasted for approximately 40 min, which did not likely affect behavior of ethanol drinking.
Conclusions
These results suggest that PDE4 is a novel target for drugs that reduce ethanol intake; PDE4 inhibitors may be used for treatment of alcohol dependence.
Keywords: Ethanol intake, phosphodiesterase-4 (PDE4), two-bottle choice, rolipram, locomotor activity, sucrose, quinine, cyclic AMP, mice
Introduction
It is established that cAMP-PKA signaling regulates ethanol intake in animal models (Misra and Pandey 2006; Pandey et al. 2004, 2005; Thiele et al. 2000). Stimulation of cAMP-PKA signaling decreases, whereas inhibition of this signaling pathway increases ethanol intake, although the actions appear to be brain region-specific (Misra and Pandey 2006; Pandey et al. 2005). Consistent with these, null mutation of the regulatory IIβ subunit of PKA decreases cAMP-stimulated PKA activity and increases ethanol intake in mice (Thiele et al. 2000). These results suggest that cAMP-PKA signaling plays an important role in the regulation of ethanol intake.
Phosphodiesterases (PDEs), a superfamily of enzymes that hydrolyze cAMP and/or cGMP, have 11 families, which are encoded by 21 different genes (Conti and Beavo 2007). PDE4 is the major PDE family in the control of intracellular cAMP levels (Houslay and Adams 2003). However, it is not known whether PDE4 is involved in the regulation of ethanol intake. There are four PDE4 subtypes (PDE4A, PDE4B, PDE4C, and PDE4D) in mammals, which are encoded by four distinct genes (Zhang 2009). While PDE4C is primarily distributed in peripheral tissues, PDE4A, PDE4B, and PDE4D are abundantly expressed in the brain (Cherry and Davis 1999; Perez-Torres et al. 2000). Specifically, PDE4B is predominantly expressed in the striatum and amygdala, whereas PDE4A and PDE4D are primarily expressed in the hippocampus and cerebral cortex. This distribution pattern appears to contribute to different roles of PDE4 subtypes in the central nervous system (CNS) functions. For instance, PDE4D is the major PDE4 subtype in the mediation of antidepressant activity (Zhang et al. 2002) and is important in the mediation of synaptic plasticity and memory (Rutten et al. 2008; Burgin et al. 2010); PDE4B is involved in the regulation of anxiety-like behavior (Zhang et al. 2008) and mediation of antipsychotic activity and striatal function (Siuciak et al. 2007, 2008).
Ethanol preference has been an important subject for studies in alcohol abuse and dependence (Green and Grahame 2008; Tabakoff et al. 2008). Oral ethanol consumption is widely used to investigate the genetic and neurobiological basis for high ethanol preference (Murphy et al. 2002; Tabakoff et al. 2008). The quantity of ethanol consumption is usually measured using the two-bottle choice test; animals are given a choice of either ethanol or water in their home cages and ethanol preference is determined based on the intake of ethanol and water (Green and Grahame 2008; Yoneyama et al. 2008). Although a great deal of effort has been made to investigate the neurobiology of ethanol preference, it remains a topic not well understood.
We hypothesized that PDE4, as a critical controller of intracellular cAMP signaling, should play a substantial role in regulating ethanol consumption. To test this hypothesis, we examined the effects of rolipram and Ro 20-1724, two selective PDE4 inhibitors, on ethanol intake and preference using the two-bottle choice paradigm in C57BL/6J mice, a strain of mice that drink a large amount of ethanol (Yoneyama et al. 2008). We found that both PDE4 inhibitors profoundly decreased ethanol intake without altering total fluid consumption.
Materials and methods
Animals
Adult male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME), 3-6 months old, were used for the experiments. Animals were housed in a temperature-controlled room with a 12-h light/dark cycle (lights on from 6:00 through 18:00) and had access to food and water ad libitum. Four batches of C57BL/6J mice were used for drug tests in order to minimize the potential interaction between drug treatments. The first batch of mice was used to test the effect of rolipram (0.5 mg/kg × 2 times/day; A.G. Scientific, San Diego, CA) on ethanol intake and preference; these mice were later used for the sucrose intake test. The second batch was used to test the effects of 0.1 and 0.25 mg/kg rolipram on ethanol intake and preference; the mice were later used for the quinine intake test and ethanol metabolism assay. The third batch of mice was used to examine the effect of Ro 20-1724 (Sigma-Aldrich, St. Louis, MO) on ethanol intake and preference. The fourth batch of mice was used to study the effects of rolipram on locomotor activity and ethanol-induced hypnosis. Animals were allowed 1-2 w for washout between experiments if they were used repeatedly for different tests.
All experiments were carried out according to the ‘NIH Guide for the Care and Use of Laboratory Animals’ (NIH Publications No. 80–23, revised 1996). The procedures were approved by the Animal Care and Use Committee of West Virginia University Health Sciences Center.
Ethanol two-bottle choice test
Ethanol consumption was measured by the two-bottle choice paradigm as described previously (Pandey et al. 2004). Mice were individually housed in standard clear plastic cages with wire lids holding the food and water/ethanol bottles (50 ml glass centrifuge tubes). The metal tubes attached to the stoppers for the glass tubes were long enough to allow animals to access to the liquid easily. Standard bedding (Bed-o-cobs) was used. Animals had ad libitum access to water in two bottles, from which animals were habituated to water drinking for up to 2 w. Bottle positions were switched daily to exclude position bias. Mice were provided with the ethanol solution (7%; v/v) in one bottle and water in the other bottle for 4 consecutive days; the ethanol concentration was then increased to 9% for 3 d, followed by 12% for another 3 d. Fresh ethanol solutions were provided every other day. During the test, mice were i.p. injected with vehicle [saline containing 5% dimethyl sulfoxide (DMSO)] or different doses (0.1, 0.25, and 0.5 mg/kg) of rolipram, which was dissolved in saline containing 5% DMSO, at 9 am and 6 pm each day. Consumption of ethanol and water was measured by weighing the bottles daily right before the 6 pm injections. The average daily intake of ethanol or water for each ethanol concentration was calculated from the drinking volumes starting from day 1. Leakage from the bottles was estimated by placing two of the same water bottles in empty cages without animals. The average volume depleted from these “control” bottles was subtracted from the daily drinking volume of ethanol or water.
In a separate experiment using naive animals, Ro 20-1724, another selective PDE4 inhibitor, was used to verify the effect of PDE4 inhibition on ethanol intake and preference. Similar to rolipram, Ro 20-1724 (10 mg/kg, dissolved in saline containing 1% Tween 80, which was used as vehicle) or vehicle was injected two times a day. In addition, we simply tested the median concentration (9%) of ethanol given the purpose of confirming the effect of rolipram on ethanol intake. Ethanol and water intake was measured using the two-bottle choice test as described above.
Sucrose or quinine intake
To determine whether the effect of rolipram on ethanol intake was related to taste preference, we measured consumption of sucrose or quinine in mice using the same two-bottle choice paradigm. Mice were provided with the sucrose solution (2%) in one bottle and water in the other bottle for 3 consecutive days before the sucrose concentration was increased to 4% for additional 3 d. Rolipram (0.5 mg/kg) or vehicle was injected twice a day following the procedures described above. Similarly, in the quinine intake test, 0.03 mM quinine was first provided for 3 d, followed by 0.1 mM quinine for another 3 d. Average daily intake of sucrose or quinine was calculated from all the three days of drinking.
Locomotor activity
This was measured by recording disruption of infrared photo beams (16 × 16) using the automated activity monitoring system PAS-Open Field (41 × 41 × 38 cm; San Diego Instruments, San Diego, CA). The experiment was performed between 6:00-10:00 pm the next day after 20-min habituation to the open-field chambers. Specifically, mice were injected with either rolipram (0.5 mg/kg) or its vehicle (saline containing 5% DMSO) and immediately placed into the chambers with the room lights off. The two treatment groups of animals were matched in the habituation and open-field test. Locomotor activity expressed as beam breaks was recorded every 10 min for 4 h.
Ethanol-induced sedation
This was carried out between 9:00 am - 12:00 pm as described previously (Radcliffe et al. 2005). Mice received rolipram (0.5 mg/kg) or its vehicle followed immediately by i.p. injections of 3.8 g/kg ethanol (20% w/v in 0.9% saline). The mice were then placed on a V-shaped trough once they became ataxic. Ethanol-induced sedation was calculated as the time interval between loss and regaining of righting reflex. Mice were considered to have regained their righting reflex when they were able to right themselves three times within 1 min.
Blood ethanol metabolism
To determine whether ethanol metabolism affected rolipram-induced reduction of ethanol intake, the blood ethanol concentration (BEC) was examined 2 w after the quinine intake test. The mice of the second batch were injected with vehicle or rolipram (0.5 mg/kg), followed 30 min later by ethanol. A lower dose (2 g/kg, i.p.) of ethanol was used given that the BEC is usually low in the two-bottle choice test (Belknap et al. 1977). Blood samples (25 μl) were collected from retro-orbital sinus at 15, 30, 60, and 120 min after the ethanol injection. Blood ethanol concentrations were determined with yeast alcohol dehydrogenase (ADH), which is a reliable enzyme assay for determination of ethanol contents (Lundquist, 1959). Yeast ADH catalyzes the oxidation of ethanol to acetyldehyde with the simultaneous reduction of nicotinamide adenine dinucleotide (NAD) to NADH. The consequent increase in absorbance at 340 nm measured by spectrophotometry is directly proportional to the ethanol concentration in the sample. Four mice in each group were used for this experiment.
Data analysis
Data were analyzed using two-way repeated measures ANOVA followed by post hoc Dunnett's tests. Statistical significance was considered when p < 0.05. Data shown are means ± SEM.
Results
Effects of PDE4 inhibitors on ethanol intake
To determine whether PDE4 was involved in the regulation of ethanol intake and preference, we examined the effects of PDE4 inhibitors rolipram and Ro 20-1724 on ethanol consumption in the two-bottle choice test in C57BL/6J mice, a strain that naturally drinks a large amount of alcohol (Yoneyama et al. 2008). The data were analyzed using two-way repeated measures ANOVA, followed by Dunnett's post hoc analysis. With regard to ethanol intake, rolipram treatment, ethanol concentration, and their interaction were all significant [treatment: F(3, 48) = 27.2, P < 0.001; ethanol concentration: F(2, 95) = 66.9, P < 0.001; interaction: F(6, 95) = 12.9, P < 0.001; Fig. 1]; with regard to alcohol preference, both treatment and the interaction of treatment × ethanol concentration were significant [F(3, 48) = 22.4, P < 0.001 and F(6, 95) = 2.68, P < 0.05, respectively], while ethanol concentration was not [F(2, 95) = 0.64, P > 0.05; Fig. 2]. Rolipram (0.1 - 0.5 mg/kg) decreased ethanol intake and ethanol preference in a dose-dependent manner. Specifically, although ineffective at 0.1 mg/kg, rolipram at higher doses (0.25 and 0.5 mg/kg) profoundly reduced ethanol intake compared to the vehicle control, regardless of the variation of ethanol concentrations, including 7%, 9%, and 12% (P < 0.05 or P < 0.001; Fig. 1). Ethanol preference was also decreased by rolipram treatment in a similar pattern (P < 0.01 or P < 0.001; Fig. 2). In addition, the higher ethanol concentration (e.g., 12%) was associated with more ethanol intake (F(2, 95) = 66.9, P < 0.001; Fig. 1), but not higher ethanol preference (F(2, 95) = 0.64, P > 0.05; Fig. 2). Rolipram did not change total fluid intake from the two bottles (P > 0.05; Table 1).
Fig. 1.
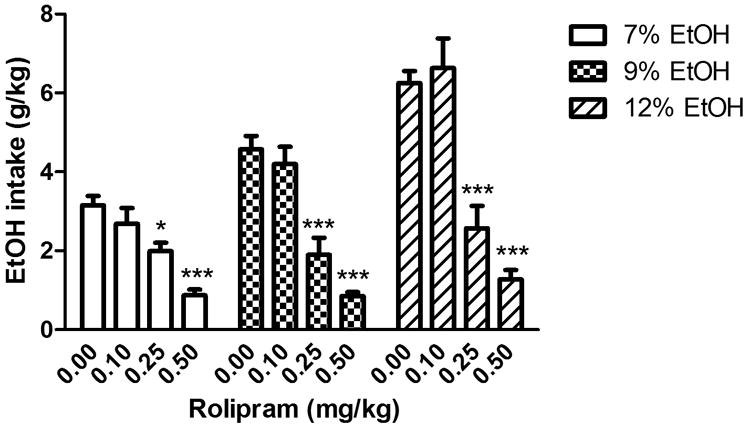
Rolipram reduced ethanol intake in the two-bottle choice paradigm in C57BL/6J mice. Animals were provided with one bottle containing the ethanol solution at the concentration of 7%, 9%, or 12%, each for 3-4 d and another bottle containing water. Rolipram (0.1, 0.25, or 0.5 mg/kg) or vehicle was injected twice daily. Ethanol intake (g) was adjusted by body weight (kg). Data were analyzed with two-way repeated measures ANOVA, followed by Dunnett's post hoc analysis. Rolipram at either 0.25 or 0.5 mg/kg profoundly reduced ethanol intake, while at 0.1 mg/kg it did not have a significant effect. Data shown are means ± SEM; n = 8 – 10 except for the vehicle control group (n = 26); * P < 0.05, *** P < 0.001 vs. corresponding vehicle.
Fig. 2.
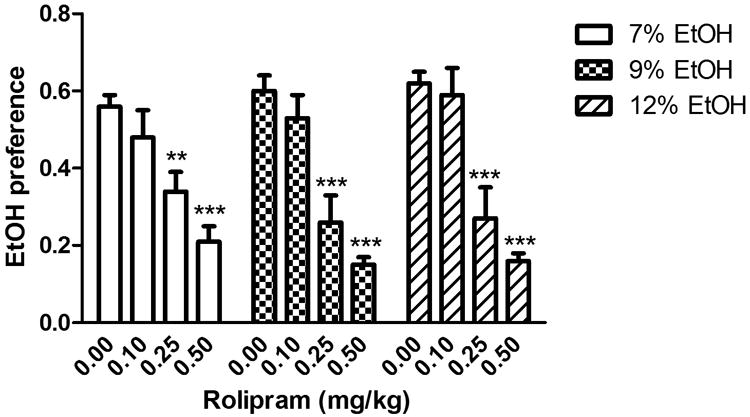
Rolipram reduced ethanol preference in the two-bottle choice paradigm in C57BL/6J mice. Animals were provided with one bottle containing the ethanol solution at the concentration of 7%, 9%, or 12%, each for 3-4 d, and another bottle containing water. Rolipram (0.1, 0.25, or 0.5 mg/kg) or vehicle was injected twice daily. Ethanol preference = ethanol intake/(ethanol intake + water intake). Data were analyzed with two-way repeated measures ANOVA, followed by Dunnett's post hoc analysis. Rolipram at either 0.25 or 0.5 mg/kg profoundly reduced ethanol preference, while at 0.1 mg/kg it did not have a significant effect. Data shown are means ± SEM; n = 8 - 10 except for the vehicle control group (n = 26); ** P < 0.01, *** P < 0.001 vs. corresponding vehicle.
Table 1.
Rolipram did not affect total fluid intake in the two-bottle choice test in mice.
| Treatment (mg/kg) | H2O + 7% EtOH (ml) | H2O + 9% EtOH (ml) | H2O + 12% EtOH (ml) |
|---|---|---|---|
| Vehicle | 2.83 ± 0.14 | 2.97 ± 0.14 | 2.98 ± 0.13 |
| Rolipram 0.1 | 2.75 ± 0.20 | 2.95 ± 0.13 | 3.09 ± 0.14 |
| Rolipram 0.25 | 2.95 ± 0.28 | 3.11 ± 0.38 | 2.96 ± 0.35 |
| Rolpram 0.5 | 2.80 ± 0.30 | 2.70 ± 0.26 | 2.65 ± 0.19 |
Animals were provided with one bottle containing the ethanol solution at the concentration of 7%, 9%, or 12%, each for 3-4 d, and another bottle containing water. Rolipram (0.1, 0.25, or 0.5 mg/kg) or vehicle was injected twice daily. Total fluid intake, which was calculated as the sum of intake from both bottles, was not changed by rolipram at any of the three doses (p > 0.05). Data shown are means ± SEM; n = 8 - 10.
The body weights of animals were also monitored during the tests. All the vehicle-treated mice (n = 26) weighed on average 27.9 ± 0.7 g and 27.5 ± 0.6 g before and after two-bottle choice, respectively, while rolipram-treated mice weighed 28.8 ± 0.9 g and 27.9 ± 0.7 g before and after the testing (all the mice treated with different doses of rolipram were combined since their body weights were not different between groups; n = 26). Overall, the body weights were not significantly changed, regardless of the presence or absence of rolipram (P > 0.05); no difference was observed between vehicle and rolipram treated animals (P > 0.05), suggesting changes in ethanol intake are independent of body weights or food consumption.
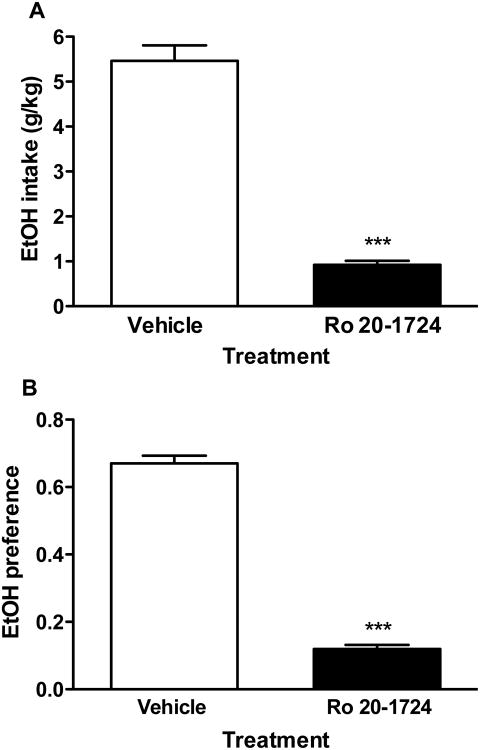
To confirm the role of PDE4 in regulating ethanol intake, we examined the effect of Ro 20-1724, another selective PDE4 inhibitor that is 15-30 times less potent compared to rolipram (Wachtel, 1983), on ethanol (9%) intake also in the two-bottle choice paradigm. Consistent with the results from rolipram treatment, mice treated with Ro 20-1724 at 10 mg/kg (i.p., twice a day), a dose equivalent to 0.33-0.67 mg/kg of rolipram, displayed profound decreases in ethanol intake (P < 0.001; Fig. 3A) and ethanol preference (P < 0.001; Fig. 3B). The extent of reduction in ethanol intake was similar to that from 0.5 mg/kg rolipram. In comparison with vehicle treatment, Ro 20-1724 did not change total fluid intake (3.71 ± 0.31 ml vs. 3.23 ± 0.48 ml; n = 7; P > 0.05).
Fig. 3.
The PDE4 inhibitor Ro 20-1724 reduced ethanol intake (A) and preference (B) in the two-bottle choice paradigm in C57BL/6J mice. Mice were provided with an ethanol (9%) bottle and a water bottle for 3 d. Ro 20-1724 (10 mg/kg) was administered (i.p.) twice a day. Data shown are means ± SEM; n = 7; *** P < 0.001 vs. corresponding vehicle.
Effect of rolipram on sucrose or quinine intake
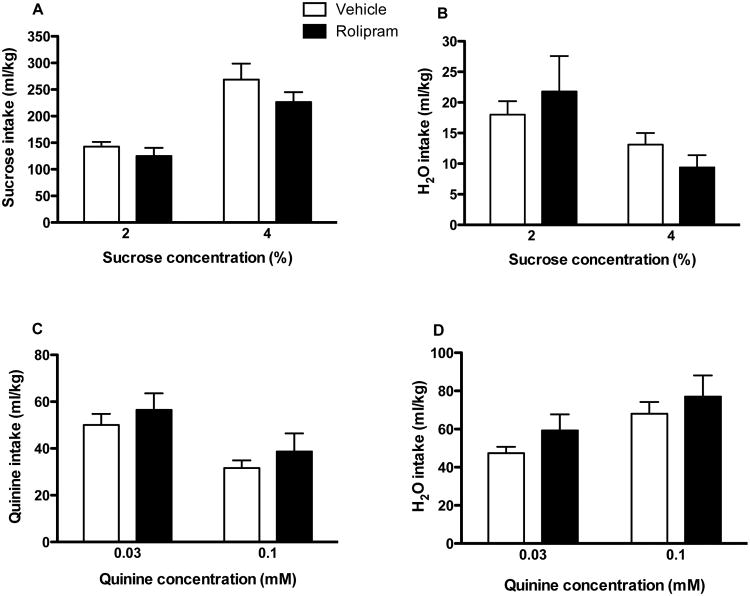
Taste preference may influence ethanol consumption (Carroll et al. 2008). To determine whether PDE4 inhibitor-induced decreases in ethanol intake were related to taste preference, we tested the effect of rolipram at the highest dose (0.5 mg/kg, twice a day) on sucrose (sweet) and quinine (bitter) intake in mice. As shown in Fig. 4, vehicle-treated mice drank daily on average 143 and 269 ml/kg of 2% and 4% sucrose solutions, respectively, and the preference ratios were 0.88 ± 0.02 and 0.94 ± 0.01, respectively; these were not changed by rolipram treatment (P > 0.05; Fig. 4A; preference ratios: 0.84 ± 0.05 for 2% and 0.96 ± 0.01 for 4% sucrose). Water intake was not altered either (P > 0.05; Fig. 4B). We also found that animals drank much less quinine solutions: vehicle-treated mice drank daily on average 50 and 32 ml/kg of 0.03 and 0.1 mM quinine solutions, respectively, and the preference ratios were 0.51 ± 0.03 and 0.32 ± 0.03, respectively; (Fig. 4C); rolipram did not change intake of either quinine at any of the concentrations (P > 0.05; preference ratios: 0.49 ± 0.07 for 0.03 mM and 0.35 ± 0.08 for 0.1 mM quinine); it did not alter water intake either (P > 0.05; Fig. 4D). These results suggest that rolipram does not affect taste preference at doses decreasing ethanol intake, although animals overall prefer the sweet taste and avoid the bitter taste.
Fig. 4.
Rolipram did not change intake of sucrose, quinine, or water. (A) Intake of sucrose at the concentrations of 2% and 4%; (B) water intake while the sucrose solution was provided; (C) intake of quinine at the concentrations of 0.03 or 0.1 mM; (D) water intake while the quinine solution was provided. The sweet (sucrose) or bitter (quinine) taste was tested for 3 d for each concentration also using the two-bottle choice paradigm in C57BL/6J mice. Rolipram (0.5 mg/kg) or vehicle was injected twice daily. Both vehicle- and rolipram-treated mice preferred 2% and 4% sucrose, while they avoided 0.1 mM quinine; however, there was no difference between the two treatment groups (P > 0.05). Data shown are means ± SEM; n = 8 - 10.
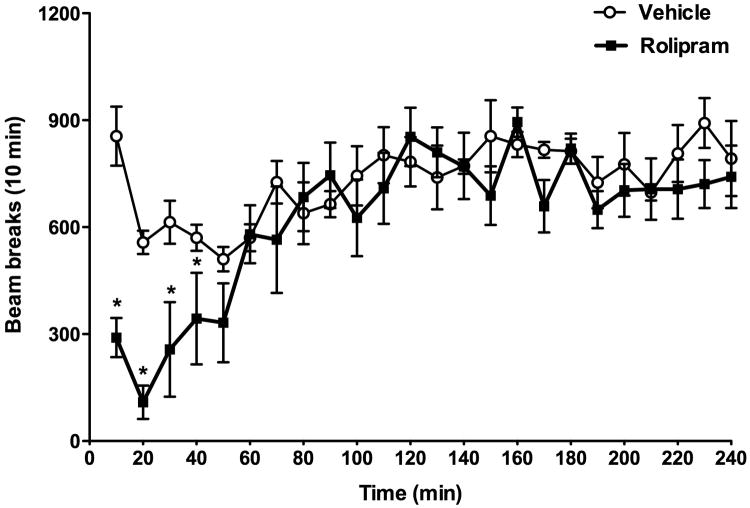
Effect of rolipram on locomotor activity
Rolipram has been reported to cause sedation when administered acutely in rodents (Wachtel 1983). To examine this effect of rolipram, mice were injected i.p. with 0.5 mg/kg rolipram or its vehicle, immediately after which locomotor activity expressed as beam breaks was recorded every 10 min for 4 h using the open-field test. Two-way repeated measures ANOVA showed that time was significant [F(23, 230) = 7.84, P < 0.001; Fig. 5], while treatment approached significance [F(1, 230) = 4.83, P = 0.05]. There was a significant interaction between treatment and time [F(23, 230) = 2.67, P < 0.001]. Post hoc Tukey analysis revealed that rolipram reduced locomotor activity only within 40 min of treatment (P < 0.05); it did not alter locomotor activity since 50 min after the injection (Fig. 5).
Fig. 5.
Effect of rolipram on locomotor activity in the open-field test in C57BL/6J mice. Animals were injected with vehicle or rolipram (0.5 mg/kg) immediately before the test; locomotor activity expressed as beam breaks was recorded every 10 min for 4 h using the PAS-Open Field (see text). Rolipram reduced locomotor activity only within 40 min of treatment. Data shown are means ± SEM; n = 6. * P < 0.05 vs. corresponding vehicle.
Effect of rolipram on ethanol-induced sedation
The cAMP/PKA signaling cascade has been shown to affect ethanol-induced sedation (Thiele et al. 2000). To determine whether PDE4 was also involved in ethanol-induced sedation, the sleep time was examined in mice treated with vehicle or rolipram (0.5 mg/kg) right before the injection of ethanol (20%, w/v) at the dose of 3.8 g/kg, which was chosen based on the significant sedation of ethanol at this dose in C57BL/6J mice (Fee et al. 2004). Ethanol-treated mice slept for 129 ± 9 min; this was not changed by administration of rolipram (129 ± 11 min; n = 8; P > 0.05), suggesting that rolipram does not alter ethanol-induced sedation.
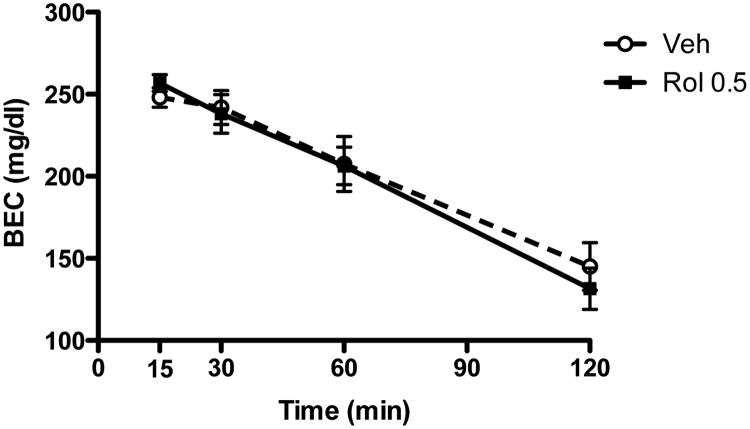
Effect of rolipram on ethanol metabolism
To determine whether rolipram affected ethanol metabolism, we examined blood ethanol concentrations in mice treated with rolipram (0.5 mg/kg) or vehicle at 15, 30, 60, or 120 min after the injection of 2 g/kg ethanol. During this period, the blood ethanol concentrations were gradually decreased over time, regardless of the treatment (Fig. 6). However, there was no difference between the rolipram treatment and vehicle control groups (P > 0.05), indicating that rolipram does not alter blood ethanol elimination.
Fig. 6.
Rolipram did not affect ethanol elimination. Rolipram (0.5 mg/kg) or vehicle was injected 30 min prior to the ethanol (2 g/kg) injection. Blood ethanol concentrations (mM) from the retro-orbital sinus were examined 15, 30, 60, and 120 min after the ethanol injection using an enzymatic assay (see the text). Data shown are means ± SEM; n = 4.
Discussion
In the present study, we demonstrated that inhibition of PDE4 by rolipram reduced ethanol consumption in C57BL/6J mice; this was independent of changes in the sweet or bitter taste. The effect of rolipram was verified by using Ro 20-1724, another selective PDE4 inhibitor, which decreased ethanol intake and preference to a similar extent as rolipram. Given that treatment with rolipram increases cAMP and phosphorylated cAMP response-element binding protein (pCREB) in the brain (Li et al. 2009 2011; Schneider 1984) and that cAMP signaling plays an important role in the regulation of ethanol preference (Misra and Pandey 2006; Pandey et al. 2004; Thiele et al. 2000), these results suggest that pharmacological inhibition of PDE4 and subsequent increases in cAMP signaling in the brain may be responsible for the reduction of ethanol preference. To our knowledge, this is the first study to investigate the role of PDEs in regulating ethanol consumption.
Since rolipram has been shown to cause sedation in rodents (Griebel et al. 1991; Smith 1990; Wachtel 1983), it is necessary to rule out the potential contribution of sedation to decreased ethanol intake after rolipram treatment. Our results did show that rolipram at 0.5 mg/kg reduced locomotor activity within 40 min after administration. This time course appears to be in agreement with our previous study, in which rolipram reverses scopolamine-induced memory deficit within 60 min after treatment (Zhang and O'Donnell 2000). The results are consistent with those in an earlier study (Smith, 1990), in which rolipram reduces locomotor activity as measured 30 min after administration for 10 min in C57BL/6J mice, although the dose (2 mg/kg) is much higher compared to that used in the present study. Regardless, rolipram-induced sedation appears not to contribute to decreased ethanol intake in rolipram-treated mice. This is because that the volume of ethanol drinking was measured before, instead of after, the rolipram injection. In addition, decreases in ethanol intake and preference produced by PDE4 inhibitors were accompanied by proportionate increases in water intake, leading to unaltered intake of total fluid, which indicates independence of sedation. Further, neither rolipram nor Ro 20-1724 affected sucrose intake. Finally, rolipram did not affect ethanol-induced hypnosis. Together, these results suggest that the sedative effect of rolipram is not involved in reduction of ethanol intake after rolipram treatment; other CNS effects rather than sedation may have caused the decrease in ethanol intake induced by PDE4 inhibitors. Nevertheless, we reduced the dose of rolipram to 0.5 mg/kg, twice a day, to minimize the potential sedative effect while sustaining the effect of rolipram on ethanol intake as long as possible. This treatment strategy was based on our and other studies showing that repeated administration of rolipram at 1.25 mg/kg once per day produces reliable behavioral and neurochemical effects in mice (Li et al. 2009 2011; Nakagawa et al. 2002), although the half-life of rolipram is only 1-3 h (Krause and Kuhne 1988). No tolerance was or has been observed with repeated rolipram treatment.
It was noted that C57BL/6J mice drank low volumes of total fluid in the present study (Table 1), leading to a relatively low baseline of ethanol intake even in the vehicle-treated animals, compared to the baseline published elsewhere using the same strain of mice (Yoneyama et al. 2008). Further reduction of ethanol intake by rolipram may cause fairly low blood ethanol concentrations (BECs), which would raise the concern of the interaction of rolipram and ethanol in the CNS. However, while the present study did not provide direct demonstrations to clarify this, it was most likely that rolipram reduced ethanol intake through the CNS. First, the mice displayed a high ethanol preference ratio (about 0.6), which falls to the ratio range of 0.4-0.8 (for 9-10% ethanol solutions) as published elsewhere focusing on the interaction between ethanol and cAMP signaling components in the brain (Pandey et al. 2004; Thiele et al. 2000; Yoneyama et al. 2008). Second, the volume of ethanol intake in the present study was comparable to that in some studies published earlier. For instance, Dole and Gentry have found that C57BL/6J mice voluntarily drink an average of 5 g/kg ethanol from a 10% (v/v) ethanol solution during a 24-hr period (Dole and Gentry 1984); 80% of the ethanol drinking occurs during the night. Consistent with this, the BECs rise to pharmacologically significant levels every night, although fluctuated and not for extended periods of time (Dole and Gentry 1984). Therefore, the levels of ethanol intake (e.g., about 5 g/kg from 9% ethanol) in the present study, although relatively low, should have provided BECs high enough during night for interaction with rolipram in the CNS. Third, rolipram did not affect ethanol elimination, suggesting that its effect on ethanol drinking is independent of ethanol metabolism. This is consistent with the finding that cAMP/PKA signaling is not likely involved in the regulation of ethanol metabolism (Fee et al. 2004; Pandey et al. 2004).
It has been shown that cAMP signaling in the brain is negatively correlated with ethanol intake; higher levels of cAMP-PKA signaling in the brain, especially in the nucleus accumbens (NAc) and amygdala, can cause lower levels of ethanol preference in animals (Misra and Pandey 2006; Pandey et al. 2004, 2005; Thiele et al. 2000). Rolipram, at doses (0.25 and 0.5 mg/kg) that reduced ethanol intake, increases cAMP levels in various brain regions, especially in the striatum (Schneider 1984), which has been shown important for ethanol drinking (Misra and Pandey 2006). In addition, studies have shown that rolipram may also reduce the use of other abused drugs such as cocaine, morphine, and methamphetamine (Iyo et al. 1996; Knapp et al. 1999; Mamiya et al. 2001; Thompson et al. 2004). For example, rolipram inhibits cocaine self-administration in animal models (Knapp et al. 1999) and cocaine- or morphine-induced conditioned place preference (Thompson et al. 2004), prevents methamphetamine-induced behavioral sensitization (Iyo et al. 1996), and blocks the development of morphine dependence (Mamiya et al. 2001). Therefore, it is possible that PDE4 inhibitors decrease ethanol intake and preference via increases in cAMP signaling in drug addiction-related brain regions such as the striatum and amygdala. Further studies are needed to examine the effects of PDE4 inhibitors on ethanol motivational (rewarding and aversive) properties.
Given the importance of cAMP-PKA in general drug abuse, for which the mesolimbic dopamine system is critical (Self 2004), reduction of ethanol intake in response to PDE4 inhibition may also be regulated by dopaminergic neurotransmissions. Rolipram, perfused into the jugular vein, decreases the firing of dopaminergic neurons in the ventral tegmental area (Scuvee-Moreau et al. 1987). It also enhances dopamine release and/or biosynthesis in the cultured mesencephalic neurons (Yamashita et al. 1997) and in the striatum in vivo (Nishi et al. 2008). At high doses such as 3 mg/kg, rolipram has been shown to reduce the binding of dopamine D1 and D2 receptors as well as muscarinic cholinergic receptors to their ligands in the brain, probably by changing cAMP-PKA signaling and protein phosphorylation (Hosoi et al. 2002, 2003). However, it is not known whether rolipram at much lower doses as used in the present study is able to produce similar effects on dopaminergic neurons.
While there is no evidence for the involvement of PDE4 subtypes in ethanol intake, PDE4B may be the PDE4 subtype of interest given that it is the predominant subtype expressed in the striatum, amygdala, and hypothalamus and the only PDE4 isoform expressed in the NAc, as evidenced by studies using in situ hybridization and immunohistochemistry (Cherry et al. 1999; Engels et al. 1995; Perez-Torres et al. 2000). These brain regions are involved in drug reinforcement (Koob and Volkow 2010) and stress-related processes (Shin and Liberzon 2010). In addition, mice deficient in PDE4B display more anxiety-like behavior and less exploration (Zhang et al. 2008). Novelty seeking or exploratory behaviors appear positively associated with ethanol intake or drug use (Davis et al. 2008; Nadal et al. 2002), although there are different observations (Bienkowski et al. 2001; Kliethermes et al. 2007). Furthermore, chronic ethanol exposure increases LPS-inducible expression of PDE4B in monocytes/macrophages (Gobejishvili et al. 2008); incubation of bovine bronchial epithelial cells with ethanol increases PDE4 activity (Forget et al. 2003). Further studies using mice deficient in PDE4B will help identify the role of PDE4B in the regulation of ethanol intake.
Overall, it has been established that cAMP signaling is importantly involved in alcohol dependence. We expanded this finding to PDE4, an enzyme that critically control intracellular cAMP levels, by providing promising demonstrations that pharmacological inhibition of PDE4 in the brain profoundly reduces ethanol intake and preference in mice. The results suggest that PDE4 may be a useful target for drugs to reduce ethanol consumption and that PDE4 inhibitors may be a novel class of drugs for treatment of alcohol dependence.
Acknowledgments
The work was supported by research funding from West Virginia University Research Funding Development Grants (RFDG). The authors thank Dr. Rae Matsumoto for her support in the open-field test and Mr. Chuang Wang for his assistance with manuscript preparation.
References
- Belknap JK, Belknap ND, Berg JH, Coleman R. Preabsorptive vs. postabsorptive control of ethanol intake in C57BL/6J and DBA/2J mice. Behav Genet. 1977;7:413–425. doi: 10.1007/BF01066776. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Koros E, Kostowski W. Novelty-seeking behaviour and operant oral ethanol self-administration in Wistar rats. Alcohol Alcohol. 2001;36:525–528. doi: 10.1093/alcalc/36.6.525. [DOI] [PubMed] [Google Scholar]
- Burgin AB, Magnusson OT, Singh J, Witte P, Staker BL, Bjornsson JM, Thorsteinsdottir M, Hrafnsdottir S, Hagen T, Kiselyov AS, Stewart LJ, Gurney ME. Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat Biotechnol. 2010;28:63–70. doi: 10.1038/nbt.1598. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Anker JJ, Perry JL, Dess NK. Selective breeding for differential saccharin intake as an animal model of drug abuse. Behav Pharmacol. 2008;19:435–460. doi: 10.1097/FBP.0b013e32830c3632. [DOI] [PubMed] [Google Scholar]
- Cherry JA, Davis RL. Cyclic AMP phosphodiesterases are localized in regions of the mouse brain associated with reinforcement, movement, and affect. J Comp Neurol. 1999;407:287–301. [PubMed] [Google Scholar]
- Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Ann Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- Davis BA, Clinton SM, Akil H, Becker JB. The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred High-Responder and Low-Responder rats. Pharmacol Biochem Behav. 2008;90:331–338. doi: 10.1016/j.pbb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole VP, Gentry RT. Toward an analogue of alcoholism in mice: scale factors in the model. Proc Natl Acad Sci USA. 1984;81:3543–3546. doi: 10.1073/pnas.81.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels P, Abdel'Al S, Hulley P, Lubbert H. Brain distribution of four rat homologues of the Drosophila dunce cAMP phosphodiesterase. J Neurosci Res. 1995;41:169–178. doi: 10.1002/jnr.490410204. [DOI] [PubMed] [Google Scholar]
- Fee JR, Sparta DR, Knapp DJ, Breese GR, Picker MJ, Thiele TE. Predictors of high ethanol consumption in RIIbeta knock-out mice: assessment of anxiety and ethanol-induced sedation. Alcohol Clin Exp Res. 2004;28:1459–1468. doi: 10.1097/01.ALC.0000141809.53115.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget MA, Sisson JH, Spurzem JR, Wyatt TA. Ethanol increases phosphodiesterase 4 activity in bovine bronchial epithelial cells. Alcohol. 2003;31:31–38. doi: 10.1016/j.alcohol.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Gobejishvili L, Barve S, Joshi-Barve S, McClain C. Enhanced PDE4B expression augments LPS-inducible TNF expression in ethanol-primed monocytes: relevance to alcoholic liver disease. Am J Physiol. 2008;295:G718–724. doi: 10.1152/ajpgi.90232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Misslin R, Vogel E, Bourguignon JJ. Behavioral effects of rolipram and structurally related compounds in mice: behavioral sedation of cAMP phosphodiesterase inhibitors. Pharmacol Biochem Behav. 1991;39:321–323. doi: 10.1016/0091-3057(91)90186-6. [DOI] [PubMed] [Google Scholar]
- Hebenstreit GF, Fellerer K, Fichte K, Fischer G, Geyer N, Meya U, Sastre-y-Hernandez M, Schony W, Schratzer M, Soukop W, et al. Rolipram in major depressive disorder: results of a double-blind comparative study with imipramine. Pharmacopsychiatry. 1989;22:156–160. doi: 10.1055/s-2007-1014599. [DOI] [PubMed] [Google Scholar]
- Hosoi R, Ishikawa M, Kobayashi K, Gee A, Yamaguchi M, Inoue O. Effects of rolipram on in vivo dopamine receptor binding. J Neural Transm. 2002;109:1139–1149. doi: 10.1007/s00702-001-0684-1. [DOI] [PubMed] [Google Scholar]
- Hosoi R, Ishikawa M, Kobayashi K, Gee A, Yamaguchi M, Inoue O. Effect of rolipram on muscarinic acetylcholine receptor binding in the intact mouse brain. J Neural Transm. 2003;110:363–372. doi: 10.1007/s00702-002-0797-1. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J. 2003;370:1–18. doi: 10.1042/BJ20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyo M, Bi Y, Hashimoto K, Inada T, Fukui S. Prevention of methamphetamine-induced behavioral sensitization in rats by a cyclic AMP phosphodiesterase inhibitor, rolipram. Eur J Pharmacol. 1996;312:163–170. doi: 10.1016/0014-2999(96)00479-7. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL, Kamens HM, Crabbe JC. Drug reward and intake in lines of mice selectively bred for divergent exploration of a hole board apparatus. Genes Brain Behav. 2007;6:608–618. doi: 10.1111/j.1601-183X.2006.00289.x. [DOI] [PubMed] [Google Scholar]
- Knapp CM, Foye MM, Ciraulo DA, Kornetsky C. The type IV phosphodiesterase inhibitors, Ro 20-1724 and rolipram, block the initiation of cocaine self-administration. Pharmacol Biochem Behav. 1999;62:151–158. doi: 10.1016/s0091-3057(98)00154-3. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause W, Kuhne G. Pharmacokinetics of rolipram in the rhesus and cynomolgus monkeys, the rat and the rabbit. Studies on species differences. Xenobiotica. 1988;18:561–571. doi: 10.3109/00498258809041693. [DOI] [PubMed] [Google Scholar]
- Li YF, Huang Y, Amsdell SL, Xiao L, O'Donnell JM, Zhang HT. Antidepressant- and anxiolytic-like effects of the phosphodiesterase-4 inhibitor rolipram on behavior depend on cyclic AMP response element binding protein-mediated neurogenesis in the hippocampus. Neuropsychopharmacology. 2009;34:2404–2419. doi: 10.1038/npp.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist F. The determination of ethyl alcohol in blood and tissue. Methods Biochem Anal. 1959;7:217–251. [Google Scholar]
- Mamiya T, Noda Y, Ren X, Hamdy M, Furukawa S, Kameyama T, Yamada K, Nabeshima T. Involvement of cyclic AMP systems in morphine physical dependence in mice: prevention of development of morphine dependence by rolipram, a phosphodiesterase 4 inhibitor. Br J Pharmacol. 2001;132:1111–1117. doi: 10.1038/sj.bjp.0703912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra K, Pandey SC. The decreased cyclic-AMP dependent-protein kinase A function in the nucleus accumbens: a role in alcohol drinking but not in anxiety-like behaviors in rats. Neuropsychopharmacology. 2006;31:1406–1419. doi: 10.1038/sj.npp.1300900. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Nadal R, Armario A, Janak PH. Positive relationship between activity in a novel environment and operant ethanol self-administration in rats. Psychopharmacology. 2002;162:333–338. doi: 10.1007/s00213-002-1091-5. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, Zhang YJ, Nestler EJ, Duman RS. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002;22:3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Kuroiwa M, Miller DB, O'Callaghan JP, Bateup HS, Shuto T, Sotogaku N, Fukuda T, Heintz N, Greengard P, Snyder GL. Distinct roles of PDE4 and PDE10A in the regulation of cAMP/PKA signaling in the striatum. J Neurosci. 2008;28:10460–10471. doi: 10.1523/JNEUROSCI.2518-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H, Xu T. Partial deletion of the cAMP response element-binding protein gene promotes alcohol-drinking behaviors. J Neurosci. 2004;24:5022–5030. doi: 10.1523/JNEUROSCI.5557-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Xu T. Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J Clin Invest. 2005;115:2762–2773. doi: 10.1172/JCI24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Torres S, Miro X, Palacios JM, Cortes R, Puigdomenech P, Mengod G. Phosphodiesterase type 4 isozymes expression in human brain examined by in situ hybridization histochemistry and[3H]rolipram binding autoradiography. Comparison with monkey and rat brain. J Chem Neuroanat. 2000;20:349–374. doi: 10.1016/s0891-0618(00)00097-1. [DOI] [PubMed] [Google Scholar]
- Radcliffe RA, Floyd KL, Drahnak JA, Deitrich RA. Genetic dissociation between ethanol sensitivity and rapid tolerance in mouse and rat strains selectively bred for differential ethanol sensitivity. Alcohol Clin Exp Res. 2005;29:1580–1589. doi: 10.1097/01.alc.0000179208.05882.1f. [DOI] [PubMed] [Google Scholar]
- Rutten K, Misner DL, Works M, Blokland A, Novak TJ, Santarelli L, Wallace TL. Enhanced long-term potentiation and impaired learning in phosphodiesterase 4D-knockout (PDE4D) mice. Eur J Neurosci. 2008;28:625–632. doi: 10.1111/j.1460-9568.2008.06349.x. [DOI] [PubMed] [Google Scholar]
- Schneider HH. Brain cAMP response to phosphodiesterase inhibitors in rats killed by microwave irradiation or decapitation. Biochem Pharmacol. 1984;33:1690–1693. doi: 10.1016/0006-2952(84)90295-8. [DOI] [PubMed] [Google Scholar]
- Scuvee-Moreau J, Giesbers I, Dresse A. Effect of rolipram, a phosphodiesterase inhibitor and potential antidepressant, on the firing rate of central monoaminergic neurons in the rat. Arch Int Pharmacodyn Ther. 1987;288:43–49. [PubMed] [Google Scholar]
- Self DW. Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology. 2004;47:242–255. doi: 10.1016/j.neuropharm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, Chapin DS, McCarthy SA, Martin AN. Antipsychotic profile of rolipram: efficacy in rats and reduced sensitivity in mice deficient in the phosphodiesterase-4B (PDE4B) enzyme. Psychopharmacology (Berl) 2007;192:415–424. doi: 10.1007/s00213-007-0727-x. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, McCarthy SA, Chapin DS, Martin AN. Behavioral and neurochemical characterization of mice deficient in the phosphodiesterase-4B (PDE4B) enzyme. Psychopharmacology (Berl) 2008;197:115–126. doi: 10.1007/s00213-007-1014-6. [DOI] [PubMed] [Google Scholar]
- Smith DF. Effects of lithium and rolipram enantiomers on locomotor activity in inbred mice. Pharmacol Toxicol. 1990;66:142–145. doi: 10.1111/j.1600-0773.1990.tb00721.x. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Saba L, Kechris K, Hu W, Bhave SV, Finn DA, Grahame NJ, Hoffman PL. The genomic determinants of alcohol preference in mice. Mamm Genome. 2008;19:352–365. doi: 10.1007/s00335-008-9115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Willis B, Stadler J, Reynolds JG, Bernstein IL, McKnight GS. High ethanol consumption and low sensitivity to ethanol-induced sedation in protein kinase A-mutant mice. J Neurosci. 2000;20:RC75. doi: 10.1523/JNEUROSCI.20-10-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BE, Sachs BD, Kantak KM, Cherry JA. The Type IV phosphodiesterase inhibitor rolipram interferes with drug-induced conditioned place preference but not immediate early gene induction in mice. Eur J Neurosci. 2004;19:2561–2568. doi: 10.1111/j.0953-816X.2004.03357.x. [DOI] [PubMed] [Google Scholar]
- Wachtel H. Species differences in behavioural effects of rolipram and other adenosine cyclic 3H, 5H-monophosphate phosphodiesterase inhibitors. J Neural Transm. 1983;56:139–152. doi: 10.1007/BF01243273. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Miyashiro M, Baba J, Sawa A. Rolipram, a selective inhibitor of phosphodiesterase type 4, pronouncedly enhanced the forskolin-induced promotion of dopamine biosynthesis in primary cultured rat mesencephalic neurons. Jpn J Pharmacol. 1997;75:91–95. doi: 10.1254/jjp.75.91. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HT. Cyclic AMP-specific phosphodiesterase-4 as a target for the development of antidepressant drugs. Curr Pharm Des. 2009;15:1688–1698. doi: 10.2174/138161209788168092. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Jin SL, Frith SA, Suvarna N, Conti M, O'Donnell JM. Antidepressant-like profile and reduced sensitivity to rolipram in mice deficient in the PDE4D phosphodiesterase enzyme. Neuropsychopharmacology. 2002;27:587–595. doi: 10.1016/S0893-133X(02)00344-5. [DOI] [PubMed] [Google Scholar]
- Zhang HT, O'Donnell JM. Effects of rolipram on scopolamine-induced impairment of working and reference memory in the radial-arm maze tests in rats. Psychopharmacology (Berl) 2000;150:311–316. doi: 10.1007/s002130000414. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Masood A, Stolinski LR, Li Y, Zhang L, Dlaboga D, Jin SL, Conti M, O'Donnell JM. Anxiogenic-like behavioral phenotype of mice deficient in phosphodiesterase 4B (PDE4B) Neuropsychopharmacology. 2008;33:1611–1623. doi: 10.1038/sj.npp.1301537. [DOI] [PMC free article] [PubMed] [Google Scholar]