Abstract
Background
An accurate and rapid serologic method to differentiate HIV-2 from HIV-1 infection is required since the confirmatory HIV-1 Western Blot (WB) may demonstrate cross-reactivity with HIV-2 antibodies.
Objectives
To evaluate the performance of the Bio-Rad Multispot HIV-1/HIV-2 rapid assay as a supplemental test to correctly identify HIV-2 infection and identify HIV-1 WB cross-reactivity with HIV-2 in clinical samples tested at an academic medical center.
Study design
Between August 2008 and July 2012, clinical samples were screened for HIV using either 3rd-or 4th-generation HIV-1/2 antibody or combination antibody and HIV-1 p24 antigen assays, respectively. All repeatedly reactive samples were reflexed for Multispot rapid testing. Multispot HIV-2 and HIV-1 and HIV-2-reactive samples were further tested using an HIV-2 immunoblot assay and HIV-1 or HIV-2 RNA assays when possible. The HIV-1 WB was performed routinely for additional confirmation and to assess for HIV-2 antibody cross-reactivity.
Results
Of 46,061 samples screened, 890 (89.6%) of 993 repeatedly reactive samples were also Multispot-reactive: 882 for HIV-1; three for only HIV-2; and five for both HIV-1 and HIV-2. All three HIV-2-only Multispot-positives along with a single dually reactive HIV-1/2 Multispot-positive were also HIV-2 immunoblot-positive; the latter was HIV-1 RNA negative and HIV-2 RNA positive.
Conclusions
The Multispot rapid test performed well as a supplemental test for HIV-1/2 diagnostic testing. Four new HIV-2 infections (0.45%) were identified from among 890 Multispot-reactive tests. The use of HIV-1 WB alone to confirm HIV-1/2 screening assays may underestimate the true prevalence of HIV-2 infection in the United States.
Keywords: Multispot, HIV-2, HIV testing algorithms, Nucleic acid amplification tests, Orthogonal supplemental testing
1. Background
Cases of infection with Human Immunodeficiency Virus type 2 (HIV-2) are predominantly found in West Africa [1–3]. However, an increasing number of HIV-2 cases have been recognized worldwide and in the United States (US). Between 1987 and 2009, the Centers for Disease Control and Prevention (CDC) confirmed 166 HIV-2 cases in the US, representing only 0.01% of new HIV infections diagnosed over this time period [4]. An update reported at the 2012 HIV Diagnostic Conference confirmed 33 more HIV-2 cases between 2010 and 2011. However, given the large number of immigrants from HIV-2 endemic areas living in the US it is likely that the current number of cases is underestimated [5]. Among the 166 confirmed HIV-2 cases, 97 (58.4%) were HIV-1 Western Blot (WB) positive [4], explaining why HIV-2 infection is often diagnosed only after immunologic deterioration occurs in patients with undetectable HIV-1 viral loads [6].
Currently available HIV-1/2 diagnostic 3rd-generation enzyme immunoassays (EIAs) or 4th-generation EIAs and chemiluminescence microparticle immunoassays (CMIAs) do not distinguish between HIV-1 and HIV-2 antibodies and the confirmatory HIV-1 Western Blot (WB) assay may misclassify HIV-2 infection as HIV-1 due to antibody cross-reactivity [7]. Thus, an efficient and rapid supplemental orthogonal serologic method is required to differentiate HIV-2 from HIV-1 infection [8]. The only FDA-approved supplemental test with the capacity to differentiate HIV-1 from HIV-2 is the Multispot HIV-1/HIV-2 rapid test (Bio-Rad Laboratories, Redmond, WA). As a single-use qualitative immunoconcentration assay, the Multispot rapid test is designed to detect and differentiate circulating HIV IgG antibodies via two HIV-1 (gp41 recombinant and gp41 peptide) and one HIV-2 (gp36 peptide) antigen-containing spots. A reactive result for HIV-2 antibody should be confirmed with an immunoblot assay or HIV-2 specific nucleic-acid amplification test or both [3,6].
2. Objectives
To correctly distinguish HIV-2 from HIV-1 infection, our study evaluated the clinical laboratory performance of the Multispot HIV-1/HIV-2 rapid test as an orthogonal supplemental test following 3rd- and 4th-generation HIV-1/2 assay screening in an academic center clinical testing laboratory. We also assessed the level of HIV-antibody cross-reactivity between HIV-1 and HIV-2 infection with the HIV-1 WB.
3. Study design
A retrospective analysis of clinical HIV-1/2 test results obtained between August 2008 and July 2012 was performed. All specimens were submitted for routine HIV diagnostic testing at Harborview and University of Washington Medical Centers, Seattle, WA. Almost 99.6% of the specimens were EDTA plasma. Between August 2008 and April 2011, all clinical samples were screened using the 3rd-generation Genetic Systems HIV-1/2 plus O EIA assay (Bio-Rad Laboratories, Redmond, WA); between May 2011 and July 2012, all clinical samples were screened using the 4th-generation Abbott Architect HIV Ag/Ab Combo assay (Abbott Laboratories, Abbot Park, IL). The following algorithm was used: All repeatedly-reactive samples using ether 3rd- or 4th-generation assays were reflexed to the Bio-Rad Multispot HIV-1/2 rapid test to facilitate same-day reporting of “presumptive” results. All Multispot HIV-1-reactive samples were tested using the Genetic Systems HIV-1 WB assay (Bio-Rad Laboratories, Redmond, WA). All Multispot HIV-2-reactive samples were forwarded for HIV-2 immunoblot (IB) testing (Focus Diagnostic). All Multispot HIV-1 and HIV-2 dually-reactive samples were also tested with the Abbott m2000 HIV-1 RNA assay and an HIV-2 RNA real time in-house assay whenever possible [9]. The last two groups of Multispot-reactive specimens were also tested using the HIV-1 WB assay to evaluate for HIV-2 antibody cross-reactivity. All 4th-generation reactive and Multispot-negative specimens were reflexed to HIV-1 RNA testing to identify acute HIV-1 infection [10].
According to the Multispot rapid test insert, the appearance of any color in any of the spots, regardless of intensity, was considered to be reactive. In addition, all samples that were Multispot-reactive for both HIV-1 and HIV-2 were retested after being diluted 1:10 and then 1:100 to dilute out any cross-reactivity. If the 1:100 dilution of the sample still showed reactivity for both HIV-1 and HIV-2, the sample was reported as “HIV undifferentiated”.
4. Results
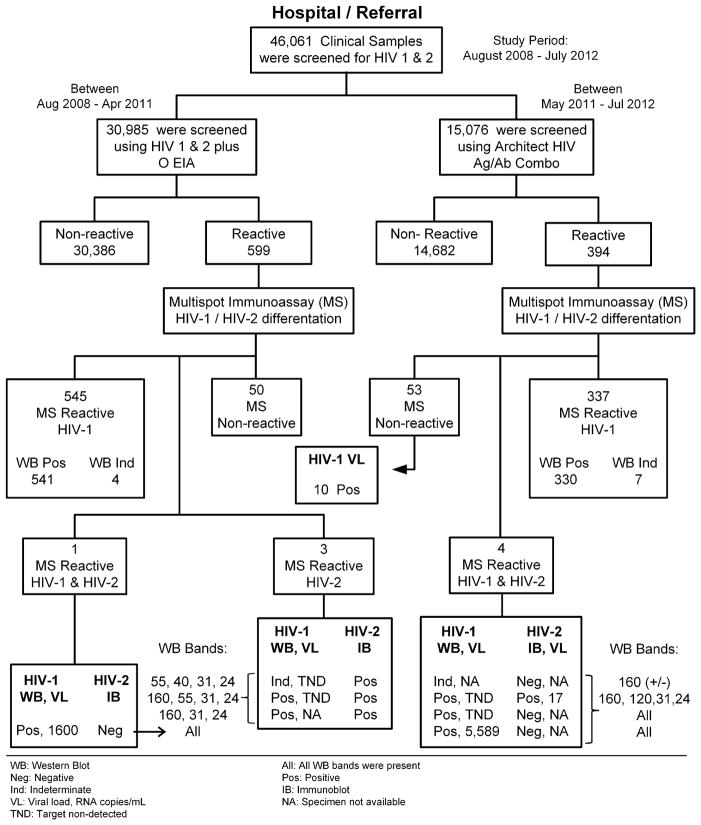
A total of 46,061 clinical samples were screened for HIV: 30,885 samples were screened using the 3rd-generation assay of which 599 (1.94%) were repeatedly-reactive and 15,076 samples were screened using the 4th-generation assay of which 394 (2.6%) were repeatedly-reactive. Thus, 993 (2.15%) repeatedly-reactive samples were tested using the Multispot rapid test, yielding 882 samples reactive for HIV-1 only, three samples reactive for HIV-2 only, five samples reactive for both HIV-1 and HIV-2 (HIV undifferentiated) and 103 non-reactive samples (50 screened by the 3rd-generation assay and 53 screened by the 4th-generation assay of which 10/53 (18.9%) were HIV-1 RNA positive for acute infection as shown in Fig. 1.
Fig. 1.
Schematic diagram of the HIV testing algorithms that used the Multispot rapid assay. HIV-1/2 RNA and Western Blot bands results for the Multispot dual-reactive HIV-1 and HIV-2 and HIV-2-reactive only samples are also shown. The 15,076 specimens screened using the Architect HIV-1/2 Ag/Ab Combo assay comprised a subset of the 21,317 specimen test results reported elsewhere [10].
Of the 882 samples Multispot-reactive for HIV-1, 871 were HIV-1 WB-positive and 11 were HIV-1 WB-indeterminate. Of the indeterminate samples, six patients were eventually traced and found to have serologically confirmed HIV-1 infection. Of the five samples that were Multispot-reactive for both HIV-1 and HIV-2 (HIV undifferentiated), three had strong HIV-1 spots and weak HIV-2 spots, one had strong spots for both HIV-1 and HIV-2, and one had weak spots for both HIV-1 and HIV-2 (Table 1). All three HIV-2 only positives and the sample with strong reactive spots for both HIV-1 and HIV-2 were both IB-positive for HIV-2. This last sample also had 17 HIV-2 RNA copies/mL and no detectable HIV-1 RNA. These four samples were reported as positive for HIV-2 infection. To evaluate HIV-2 antibody cross-reactivity, the HIV-1 WB test was also performed on these four samples. All had three or four HIV-1 bands: three samples (75%) were classified as HIV-1 WB-positive and one sample (25%) as HIV-1 WB indeterminate (Table 1). The most commonly observed HIV-1 WB bands were gag p24 and pol p31 (100%), followed by env gp160 (75%), gag p55 (50%), and env p120 and gag p40 (25%).
Table 1.
Spot intensity and HIV-1 Western Blot bands for Multispot HIV-2 and HIV-1 and HIV-2 reactive specimens. The spot intensity was defined as follows: no spot visible (−); light purple spot color (+/−); and clearly defined purple spot color (+).
| Samples number | Screening assay | Supplemental confirmatory test
|
WB bands | Infection status | |||
|---|---|---|---|---|---|---|---|
| Multispot
|
Immunoblot | Western Blot | |||||
| HIV-1 spots | HIV-2 spot | HIV-2 | HIV-1 | ||||
| 1 | 3rd Gen | − | + | Pos | Ind | 55, 40, 31, 24 | HIV-2 |
| 2 | 3rd Gen | − | + | Pos | Pos | 160, 55, 31, 24 | HIV-2 |
| 3 | 3rd Gen | − | + | Pos | Pos | 160, 31, 24 | HIV-2 |
| 4 | 3rd Gen | + | +/− | Neg | Pos | All | HIV-1 |
| 5 | 4th Gen | + | + | Pos | Pos | 160, 120, 31, 24 | HIV-2 |
| 6 | 4th Gen | + | +/− | Neg | Pos | All | HIV-1 |
| 7 | 4th Gen | + | +/− | Neg | Pos | All | HIV-1 |
| 8 | 4th Gen | +/− | +/− | Neg | Ind | 160 (+/−) | HIV-1 NC |
HIV-1 NC: inadequate specimen for HIV RNA confirmation; 3rd Gen: 3rd generation assay; 4th Gen: 4th generation assay; Ind: indeterminate; Pos: positive; Neg: negative.
The three samples with strong HIV-1 spots and weak HIV-2 spots were HIV-2 IB-negative and were positive for all HIV-1 WB bands; these samples were reported as positive for HIV-1 infection. Upon dilution, the sample with weak spots for HIV-1 and HIV-2 was reactive in both the HIV-2 spot and the HIV-1 peptide spot and nonreactive in the HIV-1 recombinant spot, HIV-2 IB-negative and HIV-1 WB indeterminate with only one weak band at gp160. This sample lacked sufficient volume for HIV-1 nucleic acid testing and was reported as “indeterminate, HIV-1 infection not confirmed”.
5. Discussion
The results of this study show that the Multispot rapid test performed well as an orthogonal supplemental antibody test to correctly classify HIV-2 from HIV-1 infection in diagnostic testing algorithms that used either 3rd- or 4th-generation HIV-1/2 assays. In order to assess the performance of the Multispot rapid test for detecting HIV-2 infection, it is important to first discuss the performance of this test for classifying HIV-1 infection. Of the Multispot HIV-1-reactive samples, 877/882 (99.4%) were confirmed by HIV-1 WB and reported as HIV-1 infection (including the six initially WB-indeterminate patients who were later documented to have HIV-1 infection). The Multispot rapid test demonstrated slightly more sensitivity and faster turnaround time than the WB, which is in agreement with previous studies [7,11,12]. More Multispot rapid test negative results (0.35% vs. 0.16%) were found with the 4th-generation compared to 3rd-generation assays, which would be expected since only the 4th generation assay can detect HIV-1 p24 antigen; thus, all discordant results should go to a viral load assay according to the algorithm proposed by the CDC to determine probable acute infection [8]. Finally, the Multispot rapid test correctly identified four HIV-2 infections: three samples were Multispot rapid test reactive for HIV-2 only and were confirmed with HIV-2 immunoblot (http://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-hiv-2-infection; last accessed on July 23, 2013), while one sample demonstrated cross-reactivity with HIV-1 (HIV undifferentiated) and was confirmed as HIV-2 infection with an HIV-2 RNA of 17 copies/mL.
The availability of a reliable HIV-2 viral nucleic acid assay is necessary for supplemental diagnostic testing and monitoring of known HIV-2 infections. The main Multispot rapid test reactivity characteristic of this group of samples was the strong spot for HIV-2 antibody. The purple color developed is proportional with the amount of HIV-2 antibody circulating in plasma (package insert), which is associated with the longer asymptomatic phase and slower progression of HIV-2 infection; thus, many of these patients were chronically infected at the time of the HIV-2 diagnosis [6]. Three of these HIV-2 infected samples had positive HIV-1 WB profiles (including the undifferentiated sample), which would have resulted in an incorrect diagnosis of HIV-1 infection had only the HIV-1 WB been used for the confirmatory test. One sample was HIV-1 WB indeterminate and cross-reactive with four HIV-1 bands (Table 1). Samples reactive for all WB bands reflected HIV-1 infection and not dual infection as determined by subsequent HIV nucleic acid testing [7,13]. Four samples were classified by the Multispot rapid test as “HIV undifferentiated”. Upon dilution, three of these “undifferentiated” samples had both weakly-reactive HIV-2 spots and strongly-reactive HIV-1 spots, which likely reflected cross-reactivity with HIV-1 antibody; two of these three samples had detectable HIV-1 viral RNA and both were HIV-1 WB-positive with all bands present and negative confirmatory HIV-2 immunoblots. The remaining sample with only a weakly reactive HIV-1 Multispot test and a concomitant HIV-2 spot had insufficient volume for further HIV NAAT confirmation and was reported as “HIV-infection not confirmed.”
The confirmation of chronic HIV-2 infection using HIV-2 RNA is problematic given that 25–35% of HIV-2-infected persons will have very low (as was the case here) or undetectable HIV-2 RNA [9]. As such, the optimal confirmatory test for an HIV-2 reactive Multispot is either the HIV-2 specific immunoblot assay or a total blood HIV-2 NAAT that includes both viral RNA and cell-associated HIV-2 DNA, or both.
6. Conclusion
The Multispot performed well as a supplemental test for screening algorithms that used either 3rd or 4th-generation HIV-1/2 assays. Importantly, there were four (0.46%) HIV-2 infections detected by the Multispot test and confirmed with HIV-2 IB and HIV-2 viral load assay from among 879 reactive and rapid tests; one HIV-2 sample was HIV-1 WB-indeterminate and three HIV-2 samples were also HIV-1 WB-positive. These samples would have been reported as either HIV-1 infection or probable HIV-1 infection based on the HIV-1 WB result alone without further information from the supplemental Multispot rapid test. As such, current HIV-1 WB confirmation of HIV-1/2 screening assays may underestimate the true prevalence of HIV-2 infection in the United States by up to 46-fold as shown by our current HIV screening algorithm.
Acknowledgments
Funding
This study was supported by the follow grants: ACTG Virology Specialty Laboratory (AI-38858), HVTN HIV Diagnostic Laboratory (AI-68618) and UW CFAR Clinical Retrovirology Core (AI-27757).
The authors would like to thank the clinical Retrovirology Laboratory staff for their contribution to the processing and diagnostic testing of specimens for HIV.
Footnotes
Authorship
All authors have made substantial contributions to each of the following: (1) the concept and design of the study (EMR, SH, JD, RWC), or acquisition of data (EMR, SH, JD), or analysis and interpretation of data (EMR, SH, JD, RWC); (2) drafting of the article or revising it critically for important intellectual content (EMR, JD, RWC); (3) final approval of the version to be submitted (EMR, SH, JD, RWC).
This study was presented at the 2012 HIV Diagnostics Conference, Atlanta, GA; December 12–14, 2012.
Competing interests
The authors declare no financial or other conflict of interest.
Ethical approval
University of Washington Human Subjects application #29860, titled “Laboratory Medicine Quality Assurance Project Research.”
References
- 1.Arien KK, Abraha A, Quinones-Mateu ME, Kestens L, Vanham G, Arts EJ. The replicative fitness of primary human immunodeficiency virus type 1 (HIV-1) group M, HIV-1 group O, and HIV-2 isolates. J Virol. 2005;79:8979–90. doi: 10.1128/JVI.79.14.8979-8990.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Silva TI, Cotten M, Rowland-Jones SL. HIV-2: the forgotten AIDS virus. Trends Microbiol. 2008;16:588–95. doi: 10.1016/j.tim.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Campbell-Yesufu OT, Gandhi RT. Update on human immunodeficiency virus (HIV)-2 infection. Clin Infect Dis. 2011;52:780–7. doi: 10.1093/cid/ciq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) HIV-2 infection surveillance –United States, 1987–2009. MMWR. 2011;60:985–8. [PubMed] [Google Scholar]
- 5.U.S. Census Bureau. The foreign-born population in the US 2010 (ACS-19) Report. 2012 May; Available at: http://www.census.gov/prod/2012pubs/acs-19.pdf.
- 6.Torian LV, Eavey JJ, Punsalang AP, Pirillo RE, Forgione LA, Kent SA, et al. HIV type 2 in New York City, 2000–2008. Clin Infect Dis. 2010;51:1334–42. doi: 10.1086/657117. [DOI] [PubMed] [Google Scholar]
- 7.Nasrullah M, Ethridge SF, Delaney KP, Wesolowski LG, Granade TC, Schwen-demann J, et al. Comparison of alternative interpretive criteria for the HIV-1 Western blot and results of the Multispot HIV-1/HIV-2 Rapid Test for classifying HIV-1 and HIV-2 infections. J Clin Virol. 2011;52(Suppl 1):S23–7. doi: 10.1016/j.jcv.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm – United States, 2011–2013. MMWR. 2013;62:489–94. [PMC free article] [PubMed] [Google Scholar]
- 9.Chang M, Gottlieb GS, Dragavon JA, Cherne SL, Kenney DL, Hawes SE, et al. Validation for clinical use of a novel HIV-2 plasma RNA viral load assay using the Abbott m2000 platform. J Clin Virol. 2012;55:128–33. doi: 10.1016/j.jcv.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramos EM, Harb S, Dragavon J, Swenson P, Stekler J, Coombs RW. Performance of an alternative HIV diagnostic algorithm using the ARCHITECT HIV Ag/Ab Combo assay and potential utility of sample-to-cutoff ratio to discriminate primary from established infection. J Clin Virol. 2013;58(Suppl 1):e38–43. doi: 10.1016/j.jcv.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Styer LM, Sullivan TJ, Parker MM. Evaluation of an alternative supplemental testing strategy for HIV diagnosis by retrospective analysis of clinical HIV testing data. J Clin Virol. 2011;52(Suppl 1):S35–40. doi: 10.1016/j.jcv.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Torian LV, Forgione LA, Punsalang AE, Pirillo RE, Oleszko WR. Comparison of Multispot EIA with Western blot for confirmatory serodiagnosis of HIV. J Clin Virol. 2011;52(Suppl 1):S41–4. doi: 10.1016/j.jcv.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Styer L, Sullivan T, Parker M. Detection of a rare HIV-1/HIV-2 co-infection using the new supplemental testing strategy. 2012 HIV diagnostic conference. 2012 Abstract. [Google Scholar]