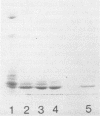
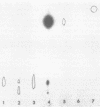
Abstract
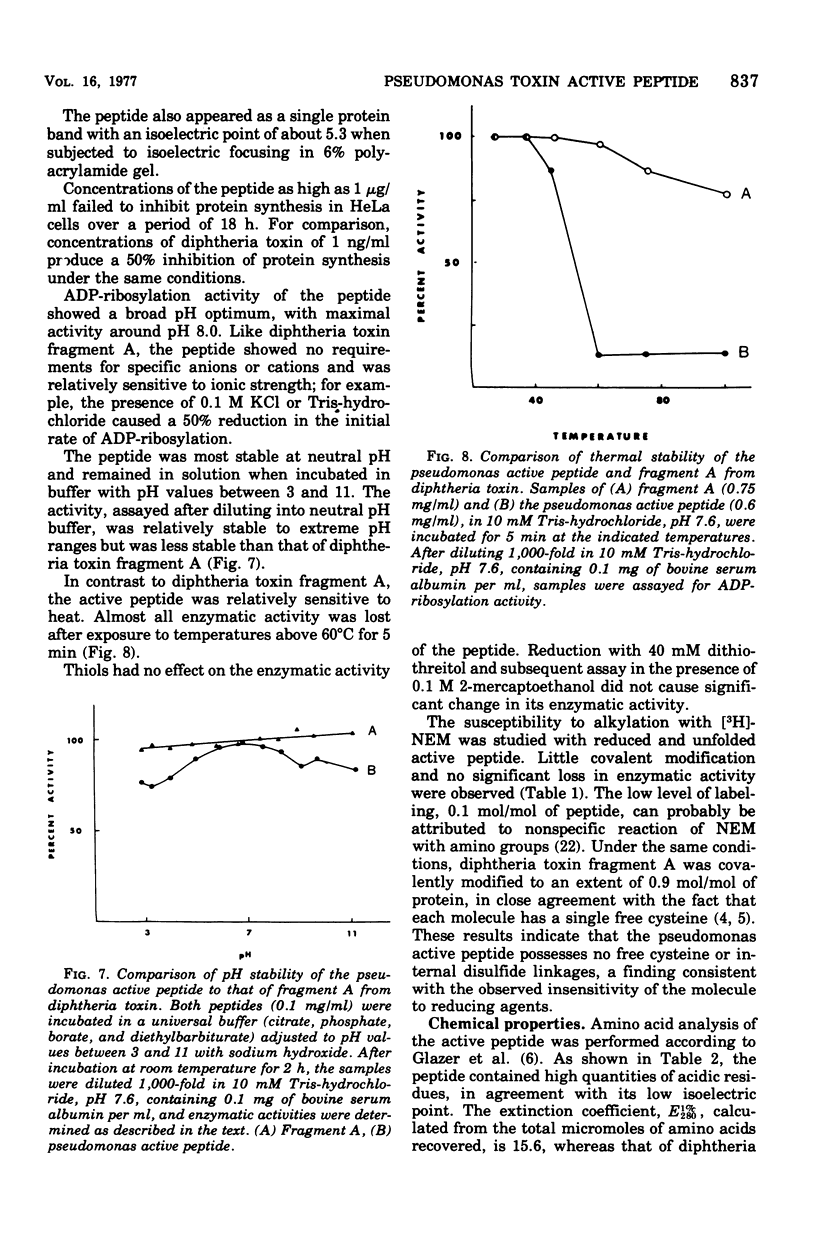
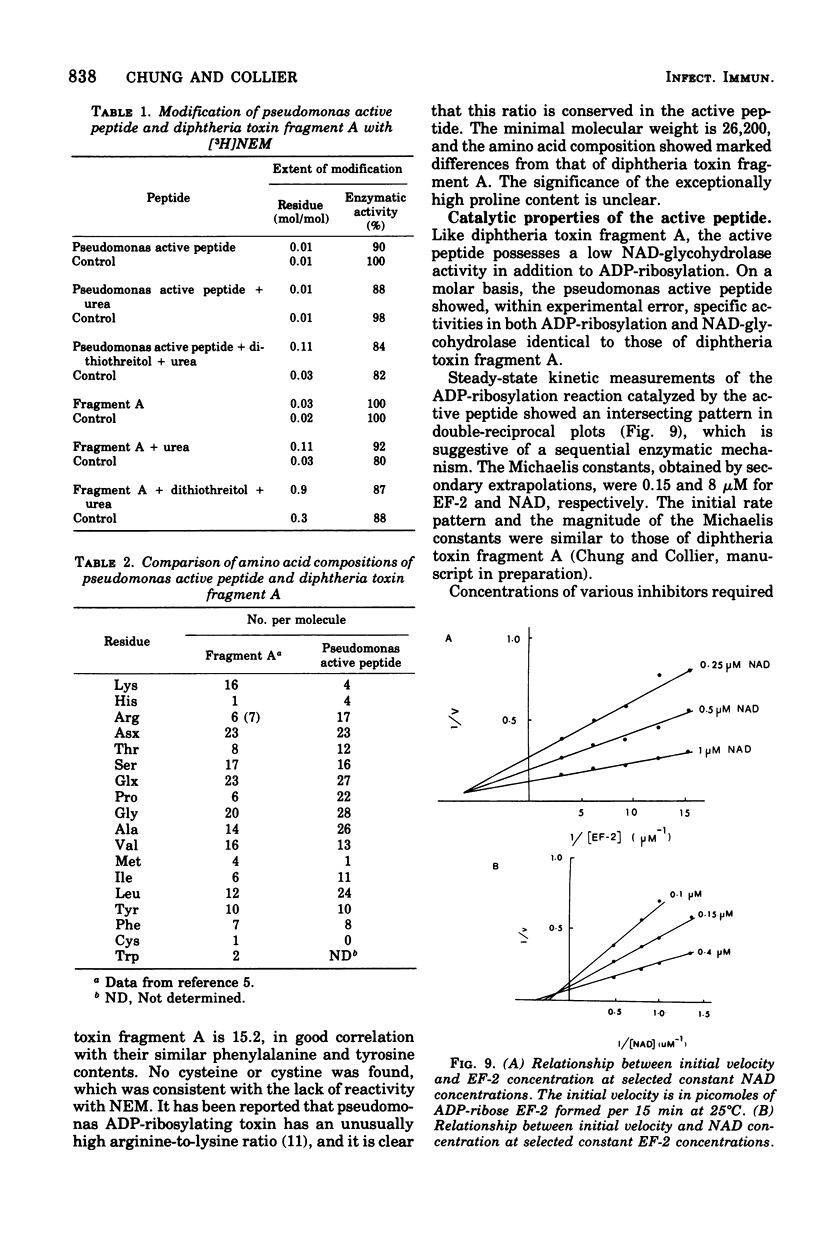
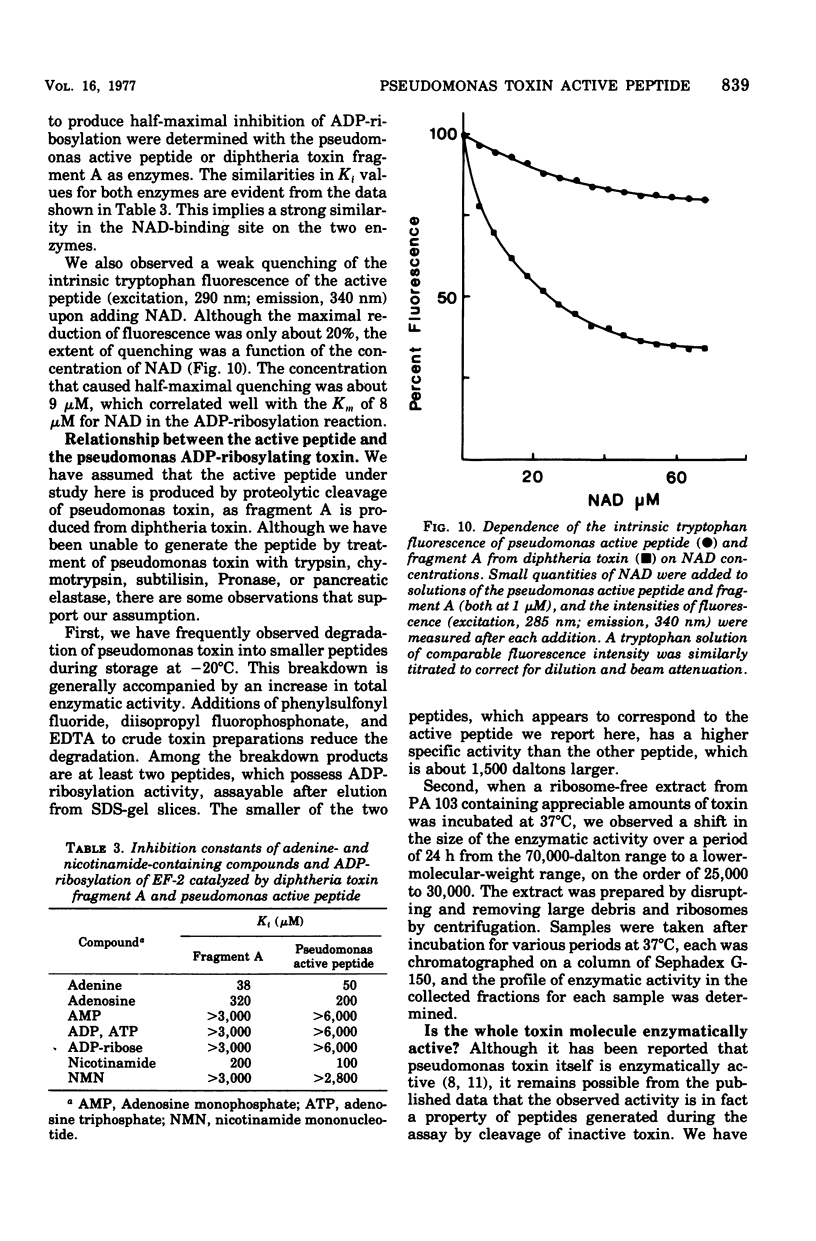
A nontoxic peptide (molecular weight, 26,000), which is active in catalyzing the adenosine diphosphate (ADP)-ribosylation of elongation factor 2, has been isolated from the culture supernatant of Pseudomonas aeruginosa strain 103 in stationary phase. Like fragment A from diphtheria toxin, the active peptide catalyzed the hydrolysis of nicotinamide adenine dinucleotide as well as the ADP-ribosylation of elongation factor 2 and showed similarities to fragment A in specific activity, kinetic constants, pH optimum, and ionic sensitivity. These results provide strong evidence for a high degree of homology in the structures of their active sites. That the peptide is not identical to fragment A is shown by the fact that it was not neutralized by fragment A-specific antiserum and was different in amino acid composition and pH and thermal labilities. Although definitive evidence is lacking, there are data suggesting that this peptide is a proteolytic fragment from the ADP-ribosylating toxin (exotoxin A; molecular weight, 66,000) produced by the same strain of P. aeruginosa.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C. Optimal conditions for counting of precipitated 3H-RNA on glass-fiber filters. Anal Biochem. 1970 Sep;37(1):178–182. doi: 10.1016/0003-2697(70)90275-7. [DOI] [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J., Kandel J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J Biol Chem. 1971 Mar 10;246(5):1496–1503. [PubMed] [Google Scholar]
- DeLange R. J., Drazin R. E., Collier R. J. Amino-acid sequence of fragment A, an enzymically active fragment from diphtheria toxin. Proc Natl Acad Sci U S A. 1976 Jan;73(1):69–72. doi: 10.1073/pnas.73.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legocki A. B., Marcus A. Polypeptide synthesis in extracts of wheat germ. Resolution and partial purification of the soluble transfer factors. J Biol Chem. 1970 Jun 10;245(11):2814–2818. [PubMed] [Google Scholar]
- Leppla S. H. Large-scale purification and characterization of the exotoxin of Pseudomonas aeruginosa. Infect Immun. 1976 Oct;14(4):1077–1086. doi: 10.1128/iai.14.4.1077-1086.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. V. Exotoxins of Pseudomonas aeruginosa. I. Factors that influence the production of exotoxin A. J Infect Dis. 1973 Oct;128(4):506–513. doi: 10.1093/infdis/128.4.506. [DOI] [PubMed] [Google Scholar]
- Liu P. V. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. 3. Identity of the lethal toxins produced in vitro and in vivo. J Infect Dis. 1966 Oct;116(4):481–489. doi: 10.1093/infdis/116.4.481. [DOI] [PubMed] [Google Scholar]
- Liu P. V., Yoshii S., Hsieh H. Exotoxins of Pseudomonas aeruginosa. II. Concentration, purification, and characterization of exotoxin A. J Infect Dis. 1973 Oct;128(4):514–519. doi: 10.1093/infdis/128.4.514. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Gill D. M. Diphtheria. Science. 1973 Oct 26;182(4110):353–358. doi: 10.1126/science.182.4110.353. [DOI] [PubMed] [Google Scholar]
- Pavlovskis O. R., Gordon F. B. Pseudomonas aeruginosa exotoxin: effect on cell cultures. J Infect Dis. 1972 Jun;125(6):631–636. doi: 10.1093/infdis/125.6.631. [DOI] [PubMed] [Google Scholar]
- Pavlovskis O. R., Shackelford A. H. Pseudomonas aeruginosa exotoxin in mice: localization and effect on protein synthesis. Infect Immun. 1974 Mar;9(3):540–546. doi: 10.1128/iai.9.3.540-546.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid M. S., Bieleski R. L. A simple apparatus for vertical flat-sheet polyacrylamide gel electrophoresis. Anal Biochem. 1968 Mar;22(3):374–381. doi: 10.1016/0003-2697(68)90278-9. [DOI] [PubMed] [Google Scholar]
- Seal S. N., Bewley J. D., Marcus A. Protein chain initiation in wheat embryo. Resolution and function of the soluble factors. J Biol Chem. 1972 Apr 25;247(8):2592–2597. [PubMed] [Google Scholar]