Abstract
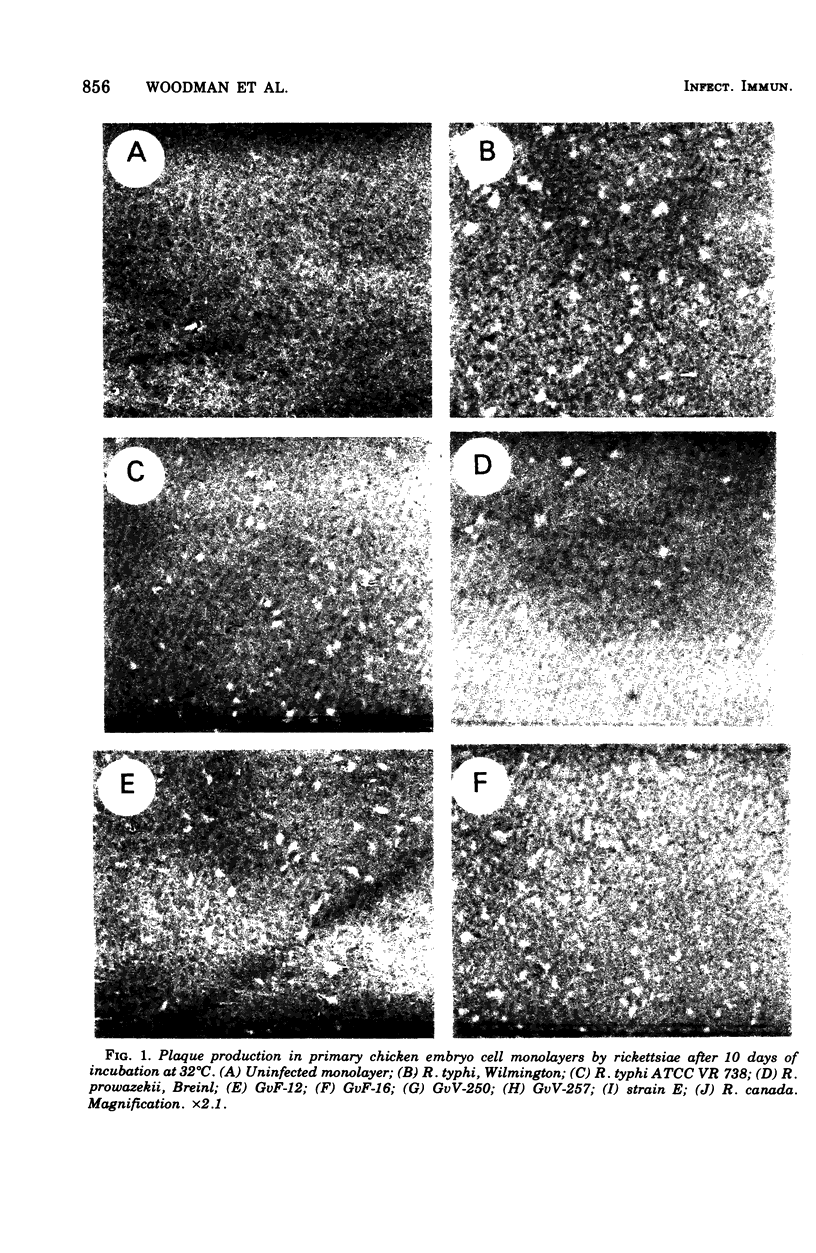
Four strains of Rickettsia prowazekii, isolated from flying squirrels (Glaucomys volans volans) from Florida and Virginia, were compared with other strains of the typhus biotype, two previously established strains each of R. prowazekii and R. typhi and one strain of R. canada, for similarities in a number of unrelated phenotypic characteristics. R. akari served as a spotted fever biotype control. All strains produced small plaques on chicken embryo cell monolayers that were clearly recognized only after 10 days of incubation at 32°C. All strains were highly susceptible to erythromycin. The Renografin density gradient centrifugation procedure of separating rickettsiae from the infected yolk sacs of surviving chicken embryos was equally satisfactory in all cases and resulted in moderate to large yields of purified rickettsiae. There was relatively small variation in specific hemolytic activity or specific CO2 formation from glutamate. None of the strains catabolized glucose. There was some strain variation in virulence for the chicken embryo, but none of the above tests separated the three species of the typhus biotype. On the other hand, R. akari was clearly distinguished by its more rapid plaque formation and by higher resistance to erythromycin. It is concluded that by the tests conducted thus far, the biological properties of the flying squirrel strains do not differ substantially from those of other strains of the typhus biotype.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bozeman F. M., Elisberg B. L., Humphries J. W., Runcik K., Palmer D. B., Jr Serologic evidence of Rickettsia canada infection of man. J Infect Dis. 1970 Apr;121(4):367–371. doi: 10.1093/infdis/121.4.367. [DOI] [PubMed] [Google Scholar]
- Bozeman F. M., Masiello S. A., Williams M. S., Elisberg B. L. Epidemic typhus rickettsiae isolated from flying squirrels. Nature. 1975 Jun 12;255(5509):545–547. doi: 10.1038/255545a0. [DOI] [PubMed] [Google Scholar]
- Coolbaugh J. C., Progar J. J., Weiss E. Enzymatic activities of cell-free extracts of Rickettsia typhi. Infect Immun. 1976 Jul;14(1):298–305. doi: 10.1128/iai.14.1.298-305.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasch G. A., Weiss E. Characterization of the Madrid E strain of Rickettsia prowazekii purified by renografin density gradient centrifugation. Infect Immun. 1977 Jan;15(1):280–286. doi: 10.1128/iai.15.1.280-286.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin C. S., Tyrrell D. A., Head B., Rees R. J. Inhibition of haemaggregation by lepromin and other mycobacterial substances. Nature. 1967 Dec 9;216(5119):1019–1020. doi: 10.1038/2161019a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McDade J. E., Stakebake J. R., Gerone P. J. Plaque assay system for several species of Rickettsia. J Bacteriol. 1969 Sep;99(3):910–912. doi: 10.1128/jb.99.3.910-912.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiel J. A., Bell E. J., Lackman D. B. Rickettsia canada: a new member of the typhus group of rickettsiae isolated from Haemaphysalis leporispalustris ticks in Canada. Can J Microbiol. 1967 May;13(5):503–510. doi: 10.1139/m67-065. [DOI] [PubMed] [Google Scholar]
- ORMSBEE R. A., PARKER H., PICKENS E. G. The comparative effectiveness of aureomycin, terramycin, chloramphenicol erythromycin, and thiocymetin in suppressing experimental rickettsial infections in chick embryos. J Infect Dis. 1955 Mar-Apr;96(2):162–167. doi: 10.1093/infdis/96.2.162. [DOI] [PubMed] [Google Scholar]
- Ormsbee R., Burgdorfer W., Peacock M., Hildebrandt P. Experimental infections of Rickettsia prowazeki among domestic livestock and ticks. Am J Trop Med Hyg. 1971 Jan;20(1):117–124. doi: 10.4269/ajtmh.1971.20.117. [DOI] [PubMed] [Google Scholar]
- REISS-GUTFREUND R. J. Un nouveau réservoir de virus pour Rickettsia prowazeki: les animaux domestiques et leurs tiques. Bull Soc Pathol Exot Filiales. 1956 Sep-Oct;49(5):946–1023. [PubMed] [Google Scholar]
- Rees H. B., Jr, Weiss E. Glutamate catabolism of Rickettsia rickettsi and factors affecting retention of metabolic activity. J Bacteriol. 1968 Feb;95(2):389–396. doi: 10.1128/jb.95.2.389-396.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS E., DRESSLER H. R., SUITOR E. C., Jr Selection of mutant strain of Rickettsia prowazeki resistant to p-aminobenzoic acid. J Bacteriol. 1957 Mar;73(3):421–430. doi: 10.1128/jb.73.3.421-430.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS E., DRESSLER H. R. Selection of an erythromycin-resistant strain of Rickettsia prowazekii. Am J Hyg. 1960 May;71:292–298. doi: 10.1093/oxfordjournals.aje.a120113. [DOI] [PubMed] [Google Scholar]
- WEISS E. Some aspects of variation in rickettsial virulence. Ann N Y Acad Sci. 1960 Nov 21;88:1287–1297. doi: 10.1111/j.1749-6632.1960.tb20120.x. [DOI] [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. Growth and physiology of rickettsiae. Bacteriol Rev. 1973 Sep;37(3):259–283. doi: 10.1128/br.37.3.259-283.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wike D. A., Ormsbee R. A., Tallent G., Peacock M. G. Effects of various suspending media on plaque formation by rickettsiae in tissue culture. Infect Immun. 1972 Oct;6(4):550–556. doi: 10.1128/iai.6.4.550-556.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wike D. A., Tallent G., Peacock M. G., Ormsbee R. A. Studies of the rickettsial plaque assay technique. Infect Immun. 1972 May;5(5):715–722. doi: 10.1128/iai.5.5.715-722.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Peterson J. C. Enzymatic activities leading to pyrimidine nucleotide biosynthesis from cell-free extracts of Rickettsia typhi. Infect Immun. 1976 Aug;14(2):439–448. doi: 10.1128/iai.14.2.439-448.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]




