Biophysicists have had wonderful fun prodding and pulling on the molecular components of ion channels to uncover how the moving parts actually work. Such studies have provided an important platform for understanding the physiological relevance of channels in their native cells. More recently, reverse genetics and transgenic approaches have allowed ion channel researchers to create testable hypotheses in intact animals regarding why ion channels work the way they do. The BK potassium channel (a.k.a. Maxi-K) provides an excellent example of a complex ion channel protein for which biophysical and mouse genetic approaches are beginning to converge to yield an understanding of physiological function. Indeed, in the previous issue of the Journal of General Physiology, Martinez-Espinosa et al. (2014) have provided new insight into the roles of accessory subunits in shaping BK channel function. The context for these experiments is the neurosecretory adrenal chromaffin cells, where BK channels are key regulators of membrane excitability.
The BK potassium channel is unique in having both a voltage sensor and intrinsic calcium sensors that allosterically couple to the channel gate to promote opening (Rothberg and Magleby, 2000; Horrigan and Aldrich, 2002). BK channels have a highly selective potassium pore that nevertheless is of very large conductance (∼250 pS) compared with most other voltage-activated potassium channels (∼10–20 pS; Blatz and Magleby, 1984). It is perhaps not surprising that a channel that can conduct so much current would use a fast inactivation mechanism in some cell types to carefully limit the duration of channel opening. BK channel inactivation was first observed in adrenal chromaffin cells, where this channel transiently dominates the outward current before the inactivation process, with time constants of ∼25 to hundreds of milliseconds, occludes the pore (Fig. 1 A; Solaro and Lingle, 1992).
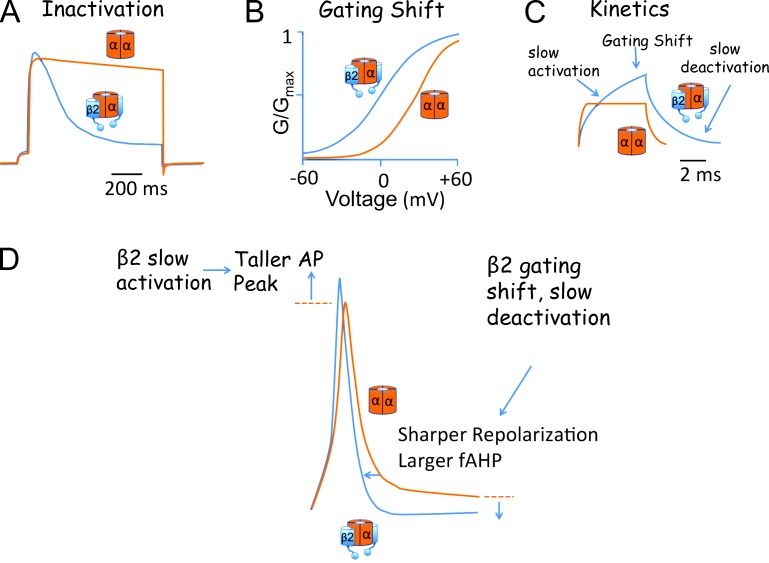
Figure 1.
Biophysical properties conferred by BK β2 subunits and their effect on the adrenal chromaffin AP. (A) Example of β2-mediated inactivation of BK currents in adrenal chromaffin cells (blue trace). Noninactivating BK currents (orange trace) are shown for comparison. (B) Chromaffin cells with inactivating BK currents (β2-expressing cells) have G-V relations that are shifted to negative membrane potentials (blue trace) relative to β2 KO chromaffin cells (orange trace). (C) Theoretical effect of β2 on BK currents (blue trace) during a short time period (before inactivation occurs) as compared with BK channels lacking β subunits (orange trace). β2 “slow activation” reduces early BK channel recruitment. However, the negative shift of the G-V relations (“gating shift”) promotes a larger current activation than BK channels lacking β2. In addition, the β2 “slow deactivation” sustains BK current after repolarization more than BK channels lacking β2. (D) Comparison of adrenal chromaffin APs from an inactivating wild-type (blue trace) and β2 KO (orange trace) cell evoked by short current injection (150 pA, 5 ms). Theoretically, β2 slow activation allows a larger AP peak, whereas the gating shift and slow deactivation promote a faster repolarization and larger AHP. Example data from A, B, and D are taken from Martinez-Espinosa et al. (2014). C is a schematic based on data from Brenner et al. (2000).
Cloning of the BK channel accessory β subunit family revealed that two of the four members conferred inactivation on BK channels. These include the β2 subunit (Wallner et al., 1999), which is expressed in adrenal chromaffin cells (Xia et al., 1999) and may also be expressed in various sensory and central neurons (McLarnon, 1995; Abdul-Ghani et al., 1996; Hicks and Marrion, 1998; Shao et al., 1999; Faber and Sah, 2003; Pyott et al., 2004; Li et al., 2007; Grimes et al., 2009). In addition, some splice isoforms of the β3 subunit, enriched in testis, cause a very fast, but incomplete inactivation that give the appearance of a rectifying current (Uebele et al., 2000; Xia et al., 2000). The accessory β subunits, as well as the more recently identify γ subunits (Yan and Aldrich, 2010, 2012), likely represent a mechanism to provide tissue-specific versatility to a channel pore-forming subunit (α subunit) encoded by only a single gene.
Although the inactivation mechanism draws the imagination because of its dramatic effect on BK currents, β2 also modifies BK channel gating properties. Most β subunit family members, β1, β2, and β4, cause a negative shift of the conductance-voltage relationship (G-V; Cox and Aldrich, 2000; Orio and Latorre, 2005; Jaffe et al., 2011). For β2, this gating shift can be as much as −75 mV, dramatically enhancing channel open probability (Orio and Latorre, 2005). The mechanism underlying the gating shift has been controversial, but several studies have converged on the conclusion that the β subunits shift the operational range of the α subunit voltage sensor to more negative membrane potentials (Bao and Cox, 2005; Orio and Latorre, 2005; Wang and Brenner, 2006; Wang et al., 2006; Contreras et al., 2012). β subunits also cause BK channels to open more slowly and to stay open longer (Brenner et al., 2000; Lippiat et al., 2003; Orio and Latorre, 2005). The effects of the accessory subunits on inactivation, open probability, and kinetics have been studied in great detail and beg the question of what are the physiological consequences of these adaptations to BK channels? Does β2 inactivation or slow gating reduce BK channel activation during action potentials (APs), or is the negative gating shift more important? Because β2 subunits are accessory proteins, pharmacological approaches cannot untangle their effects. In the recent publication, Martinez-Espinosa et al. (2014) report generation of a gene knockout (KO) of the β2 subunit, thereby providing initial glimpses into the physiological role of the β2 subunit in neurons.
To investigate BK β2 gene KO effects, the authors returned to adrenal chromaffin cells, the system in which inactivation of BK channels was first observed (Fig. 1 A; Solaro and Lingle, 1992). A key finding of studies of BK channel inactivation that had been suspected but perhaps not well appreciated is that β subunits likely assemble with BK channels at less than saturating stoichiometry. Historically, clear evidence of this was obtained from single-channel studies of inactivating BK channels. Slow trypsin digestion, which cleaves the inactivation domain, caused stepwise reductions in inactivation rates consistent with BK channel assembly with a maximum of four but usually two to three “inactivating particles” per channel in rat adrenal chromaffin cells (Ding et al., 1998). This “inactivation particle” was later provisionally identified as the accessory β2 subunit (Wallner et al., 1999; Xia et al., 1999). Like its effect on inactivation rate, the β subunit effect on gating is proportional to β:α stoichiometry (Wang et al., 2002). Martinez-Espinosa et al. (2014) conclusively showed that β2 KO eliminated fast inactivation, establishing that the β2 subunit is the inactivating particle in these cells. The authors used inactivation as a useful indicator of mean BK channel β2:α subunit stoichiometry in individual neurons and estimated that most BK channels in mouse chromaffin cells exist with only a mean of one to two β2 subunits per channel. Moreover, cells with a faster BK inactivation rate (indicative of an increased β2:α stoichiometry) correlated with a BK G-V relationship that is shifted to more negative membrane potentials. Thus, these experiments allow us to appreciate that BK channels may operate as a complex with few β subunits. β2 effect on voltage sensor and gate in most channels perhaps may be regarded as more of a nudge rather than a shove because a less than saturating stoichiometry of β2 subunits causes a more moderate shift on the G-V relationship than does a full complement. KO of β2 not only eliminated inactivation but also caused a shift of the G-V relationship to positive potentials (Fig. 1 B). Although the gating shift is more moderate than in vitro biophysical experiments using saturating β2 indicate (Martinez-Espinosa et al., 2014), it is certainly relevant to the cell because there were marked effects on AP shape and firing properties (see below). The concept that BK channels assemble with a subsaturating number of β subunits is likely relevant to the physiological function of other β subunit family members, such as β1 and β4, for which inactivation cannot be used as a reporter to indicate stoichiometry. Association of BK channels with other β subunits in their native cells is also not likely to be all or none. Variation in stoichiometry may serve as another means to generate diverse BK channels.
BK channels in neurons are arguably best recognized for shaping APs (Sah and Faber, 2002). Coincident depolarization and calcium influx generally activate BK channels during the AP repolarization phase and result in a sharpening of the width and a more negative afterhyperpolarization (AHP). However, in some neurons, BK channel activation is too slow to contribute to repolarization (Brenner et al., 2005; Alle et al., 2011). This is particularly relevant to BK channels assembled with β2 subunits, which confer a slower activation than BK channel lacking β subunits (Fig. 1 C). For example, in mossy fiber nerve endings, inactivating BK channels are outpaced by fast Kv3 channels, which mediate AP repolarization (Alle et al., 2011). However, inactivating BK channels in dorsal root ganglia neurons contribute to AP repolarization and the fast AHP (fAHP; Li et al., 2007). In adrenal chromaffin cells, inactivating BK channels are activated by spike voltage and calcium influx through L-type CaV1.3 voltage-dependent calcium channels to provide a sharper AP and a larger AHP (Marcantoni et al., 2007). The current study by Martinez-Espinosa et al. (2014) found that the β2 KO caused shorter and broader APs and a reduced AHP (Fig. 1 D). One can therefore infer that the complex effect of β2, the slow kinetics and the negative gating shift (Fig. 1 C), effectively shapes the timing and response of BK channels during the AP. The ability of β2 to slow BK channel recruitment during APs (Fig. 1 C) prevents BK channel activation during the rising phase so that APs are not truncated (Fig. 1 D). During the repolarization phase, however, the negative gating shift by β2 (Fig. 1 B), and perhaps the slow deactivation (Fig. 1 C), promotes BK channel activation, as indicated by the sharper APs and larger fAHP in wild-type but not in KO neurons (Fig. 1 D). These findings beautifully demonstrate how the complex effects observed under biophysical studies of the isolated channel become relevant in a physiological preparation.
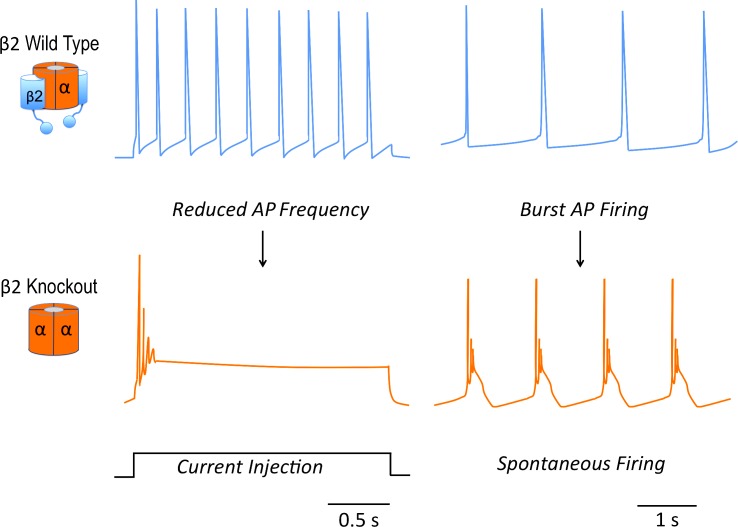
Adrenal chromaffin cells are catecholaminergic neurosecretory cells in which AP shape and frequency can affect cytosolic calcium and thereby influence quantity and frequency of hormone secretion (Marcantoni et al., 2007; De Diego et al., 2008). Early studies in adrenal chromaffin cells were among the first to ascribe a pro-excitatory role to BK channels (Lingle et al., 1996). Blocking BK channels in adrenal chromaffin cells reduces AP frequency during tonic firing. Computational modeling of adrenal chromaffin cells supports the concept that BK channel sharpening of APs and larger fAHP can theoretically promote a higher AP frequency by reducing sodium channel inactivation (Lovell and McCobb, 2001; Sun et al., 2009). Similar pro-excitatory effects have been observed in some central neurons (Gu et al., 2007; Shruti et al., 2008; Sheehan et al., 2009), and indeed it has been found that a BK channel gain-of-function mutation in humans causes spontaneous seizures (Du et al., 2005). The KO study by Martinez-Espinosa et al. (2014) finally provides a physiological examination of whether β2 effects and BK activation are pro-excitatory in chromaffin cells. Their results concurred with the computational models. Reduced activation of BK channels in the β2 KO reduced tonic firing during constant current injections (Fig. 2).
Figure 2.
β2 subunit modulation of BK channels has opposing effects on excitability during constant current injection and spontaneous firing. BK/β2 channels support greater tonic firing during constant current injection (top left), whereas the β2 KO cells fire less frequently (not depicted) or fail to maintain spiking (bottom left). Spontaneous firing chromaffin neurons (top right) undergo burst firing after KO of β2 (bottom right). Traces are modified from Martinez-Espinosa et al. (2014).
The study by Martinez-Espinosa et al. (2014) also provides new insights into β2 modulation of BK channels during spontaneous firing. Spontaneous firing in adrenal chromaffin cells is mediated by low-voltage activation of L-type, CaV1.3 calcium channels and inactivating BK channels that can act as pacemakers for the neuron. An unexpected finding was that β2 KO caused a large percentage of spontaneously firing neurons to undergo burst firing of APs (Fig. 2). Burst firing was not generally observed with KO of the pore-forming α subunit. Thus, burst firing is not a simple loss or gain-of-function of BK channels, but an emergent property of neurons expressing BK channels but lacking the β2 subunit. The mechanism of burst firing is not yet understood. In some manner, the burst firing is reminiscent of BK channel effect on pituitary somatotrophs (Van Goor et al., 2001). In those neurons, BK channels truncate the AP, similar to the adrenal chromaffin β2 KO (Fig. 1 D). The consequence of this is reduced recruitment of other voltage-sensitive potassium currents and a plateau potential that supports burst firing rather than a repolarizing trajectory (Van Goor et al., 2001).
Within the adrenal medulla, a subpopulation of chromaffin cells exist that lack apparent inactivating currents and likely have little expression of β2. The authors saw few of these neurons with burst firing. It is difficult to understand why those adrenal chromaffin neurons lacking significant inactivating currents rarely undergo burst firing, whereas genetic deletion of the β2 subunit causes frequent burst firing. Conceptually, the subpopulation of adrenal chromaffin cells containing noninactivating BK currents should be similar to the β2 subunit KO. One might speculate that those neurons that appear to have little inactivating currents may nevertheless have sufficient β2 subunits expressed to subtly adjust gating kinetics and voltage gating into ranges that prevent burst firing. Additionally, adrenal chromaffin cells are electrically coupled through gap junctions, and there is a growing appreciation that this may allow chromaffin cells to act in concert to control hormone release (Martin et al., 2001; Desarménien et al., 2013). It is therefore possible that neurons containing β2 subunits, through gap junctions, inhibit slow-wave bursting activity in the subpopulation of neighboring neurons lacking β2.
Having a KO animal in hand allows ion channel investigators to study effects from the perspective of the single channel to the whole organism. It will be interesting to see what the physiological consequences of the β2 KO mice are on catecholamine secretion. In pituitary somatotrophs, burst firing is much more efficient than tonic firing in raising cellular calcium and causing hormone secretion (Stojilkovic, 2006). One may speculate that β2 subunits maintain a low basal catecholamine secretion by preventing burst firing, but enhance evoked catecholamine secretion by increasing tonic firing rates when the cell is driven by depolarizing input. Future studies should allow a better appreciation of the effects of β2 subunits on sympathetic and neuroendocrine function in the animal.
Although it was the inactivation mechanism that first drew attention to the β2 subunit, the KO phenotype effectively refocuses our attention to the β2-mediated gating shift, and perhaps also the slow deactivation, as the key modulatory effect on BK channels that is relevant to adrenal chromaffin excitability. The question remains: What is the purpose of inactivation in BK channels? Although the study by Martinez-Espinosa et al. (2014) has broken new ground in our understanding of BK channel regulation, the β2 KO phenotype provides little insight into the effect of BK channel inactivation. Unlike voltage-dependent sodium channels, β2-mediated inactivation (τ >25 ms) is slow compared with the duration of most APs (1–2 ms) and therefore unlikely to substantially affect channel openings unless there are very large and sustained increases in calcium. It is feasible that, under conditions where preganglionic sympathetic nerves strongly drive the adrenal gland, calcium rises sufficiently to allow inactivation to come into play. In central neurons, inactivation of BK channels has been ascribed to a process of frequency-dependent AP broadening. Rather than inactivating during a single AP, cumulative inactivation of BK channels is proposed to ensue after multiple or high-frequency APs (Hicks and Marrion, 1998; Shao et al., 1999; Faber and Sah, 2003). Whether this is mediated by direct β2-mediated inactivation of BK channels or by inactivation of voltage-dependent calcium channel that serves as a calcium source for BK is still in question. Thus, whether inactivation is the evolutionary “appendix” of α/β2 BK channels in adrenal chromaffin neurons or a key component of β2 subunits in other neurons is yet to be determined. Certainly, this gene KO of the β2 subunit opens the field to many future studies addressing these and other questions.
Acknowledgments
The author acknowledges B. Wang for critical reading of the manuscript.
R. Brenner was supported by the Research Enhancement Program of the University of Texas Health Science Center at San Antonio and by National Institutes of Health grant 1R21AI113724.
The author declares no competing financial interests.
Elizabeth M. Adler served as editor.
References
- Abdul-Ghani M.A., Valiante T.A., Carlen P.L., and Pennefather P.S.. 1996. Tyrosine kinase inhibitors enhance a Ca2+-activated K+ current (IAHP) and reduce IAHP suppression by a metabotropic glutamate receptor agonist in rat dentate granule neurones. J. Physiol. 496:139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alle H., Kubota H., and Geiger J.R.. 2011. Sparse but highly efficient Kv3 outpace BKCa channels in action potential repolarization at hippocampal mossy fiber boutons. J. Neurosci. 31:8001–8012. 10.1523/JNEUROSCI.0972-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L., and Cox D.H.. 2005. Gating and ionic currents reveal how the BKCa channel’s Ca2+ sensitivity is enhanced by its β1 subunit. J. Gen. Physiol. 126:393–412. 10.1085/jgp.200509346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A.L., and Magleby K.L.. 1984. Ion conductance and selectivity of single calcium-activated potassium channels in cultured rat muscle. J. Gen. Physiol. 84:1–23. 10.1085/jgp.84.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R., Jegla T.J., Wickenden A., Liu Y., and Aldrich R.W.. 2000. Cloning and functional characterization of novel large conductance calcium-activated potassium channel β subunits, hKCNMB3 and hKCNMB4. J. Biol. Chem. 275:6453–6461. 10.1074/jbc.275.9.6453 [DOI] [PubMed] [Google Scholar]
- Brenner R., Chen Q.H., Vilaythong A., Toney G.M., Noebels J.L., and Aldrich R.W.. 2005. BK channel β4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat. Neurosci. 8:1752–1759. 10.1038/nn1573 [DOI] [PubMed] [Google Scholar]
- Contreras G.F., Neely A., Alvarez O., Gonzalez C., and Latorre R.. 2012. Modulation of BK channel voltage gating by different auxiliary β subunits. Proc. Natl. Acad. Sci. USA. 109:18991–18996 10.1073/pnas.1216953109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D.H., and Aldrich R.W.. 2000. Role of the β1 subunit in large-conductance Ca2+-activated K+ channel gating energetics. Mechanisms of enhanced Ca2+ sensitivity. J. Gen. Physiol. 116:411–432. 10.1085/jgp.116.3.411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Diego A.M., Gandía L., and García A.G.. 2008. A physiological view of the central and peripheral mechanisms that regulate the release of catecholamines at the adrenal medulla. Acta Physiol. (Oxf.). 192:287–301. 10.1111/j.1748-1716.2007.01807.x [DOI] [PubMed] [Google Scholar]
- Desarménien M.G., Jourdan C., Toutain B., Vessières E., Hormuzdi S.G., and Guérineau N.C.. 2013. Gap junction signalling is a stress-regulated component of adrenal neuroendocrine stimulus-secretion coupling in vivo. Nat. Commun. 4:2938 10.1038/ncomms3938 [DOI] [PubMed] [Google Scholar]
- Ding J.P., Li Z.W., and Lingle C.J.. 1998. Inactivating BK channels in rat chromaffin cells may arise from heteromultimeric assembly of distinct inactivation-competent and noninactivating subunits. Biophys. J. 74:268–289. 10.1016/S0006-3495(98)77785-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W., Bautista J.F., Yang H., Diez-Sampedro A., You S.A., Wang L., Kotagal P., Lüders H.O., Shi J., Cui J., et al. 2005. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat. Genet. 37:733–738. 10.1038/ng1585 [DOI] [PubMed] [Google Scholar]
- Faber E.S., and Sah P.. 2003. Ca2+-activated K+ (BK) channel inactivation contributes to spike broadening during repetitive firing in the rat lateral amygdala. J. Physiol. 552:483–497. 10.1113/jphysiol.2003.050120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes W.N., Li W., Chávez A.E., and Diamond J.S.. 2009. BK channels modulate pre- and postsynaptic signaling at reciprocal synapses in retina. Nat. Neurosci. 12:585–592. 10.1038/nn.2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N., Vervaeke K., and Storm J.F.. 2007. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J. Physiol. 580:859–882. 10.1113/jphysiol.2006.126367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks G.A., and Marrion N.V.. 1998. Ca2+-dependent inactivation of large conductance Ca2+-activated K+ (BK) channels in rat hippocampal neurones produced by pore block from an associated particle. J. Physiol. 508:721–734. 10.1111/j.1469-7793.1998.721bp.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan F.T., and Aldrich R.W.. 2002. Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J. Gen. Physiol. 120:267–305. 10.1085/jgp.20028605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe D.B., Wang B., and Brenner R.. 2011. Shaping of action potentials by type I and type II large-conductance Ca²+-activated K+ channels. Neuroscience. 192:205–218. 10.1016/j.neuroscience.2011.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Gao S.B., Lv C.X., Wu Y., Guo Z.H., Ding J.P., and Xu T.. 2007. Characterization of voltage-and Ca2+-activated K+ channels in rat dorsal root ganglion neurons. J. Cell. Physiol. 212:348–357. 10.1002/jcp.21007 [DOI] [PubMed] [Google Scholar]
- Lingle C.J., Solaro C.R., Prakriya M., and Ding J.P.. 1996. Calcium-activated potassium channels in adrenal chromaffin cells. Ion Channels. 4:261–301. 10.1007/978-1-4899-1775-1_7 [DOI] [PubMed] [Google Scholar]
- Lippiat J.D., Standen N.B., Harrow I.D., Phillips S.C., and Davies N.W.. 2003. Properties of BKCa channels formed by bicistronic expression of hSloα and β1–4 subunits in HEK293 cells. J. Membr. Biol. 192:141–148. 10.1007/s00232-002-1070-0 [DOI] [PubMed] [Google Scholar]
- Lovell P.V., and McCobb D.P.. 2001. Pituitary control of BK potassium channel function and intrinsic firing properties of adrenal chromaffin cells. J. Neurosci. 21:3429–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantoni A., Baldelli P., Hernandez-Guijo J.M., Comunanza V., Carabelli V., and Carbone E.. 2007. L-type calcium channels in adrenal chromaffin cells: role in pace-making and secretion. Cell Calcium. 42:397–408. 10.1016/j.ceca.2007.04.015 [DOI] [PubMed] [Google Scholar]
- Martin A.O., Mathieu M.N., Chevillard C., and Guérineau N.C.. 2001. Gap junctions mediate electrical signaling and ensuing cytosolic Ca2+ increases between chromaffin cells in adrenal slices: A role in catecholamine release. J. Neurosci. 21:5397–5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Espinosa P.L., Yang C., Gonzalez-Perez V., Xia X.-M., and Lingle C.J.. 2014. Knockout of the BK β2 subunit abolishes inactivation of BK currents in mouse adrenal chromaffin cells and results in slow-wave burst activity. J. Gen. Physiol. 144:275–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarnon J.G. 1995. Inactivation of a high conductance calcium dependent potassium current in rat hippocampal neurons. Neurosci. Lett. 193:5–8. 10.1016/0304-3940(95)11651-C [DOI] [PubMed] [Google Scholar]
- Orio P., and Latorre R.. 2005. Differential effects of β1 and β2 subunits on BK channel activity. J. Gen. Physiol. 125:395–411. 10.1085/jgp.200409236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyott S.J., Glowatzki E., Trimmer J.S., and Aldrich R.W.. 2004. Extrasynaptic localization of inactivating calcium-activated potassium channels in mouse inner hair cells. J. Neurosci. 24:9469–9474. 10.1523/JNEUROSCI.3162-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg B.S., and Magleby K.L.. 2000. Voltage and Ca2+ activation of single large-conductance Ca2+-activated K+ channels described by a two-tiered allosteric gating mechanism. J. Gen. Physiol. 116:75–100. 10.1085/jgp.116.1.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P., and Faber E.S.. 2002. Channels underlying neuronal calcium-activated potassium currents. Prog. Neurobiol. 66:345–353. 10.1016/S0301-0082(02)00004-7 [DOI] [PubMed] [Google Scholar]
- Shao L.R., Halvorsrud R., Borg-Graham L., and Storm J.F.. 1999. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J. Physiol. 521:135–146. 10.1111/j.1469-7793.1999.00135.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan J.J., Benedetti B.L., and Barth A.L.. 2009. Anticonvulsant effects of the BK-channel antagonist paxilline. Epilepsia. 50:711–720. 10.1111/j.1528-1167.2008.01888.x [DOI] [PubMed] [Google Scholar]
- Shruti S., Clem R.L., and Barth A.L.. 2008. A seizure-induced gain-of-function in BK channels is associated with elevated firing activity in neocortical pyramidal neurons. Neurobiol. Dis. 30:323–330. 10.1016/j.nbd.2008.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro C.R., and Lingle C.J.. 1992. Trypsin-sensitive, rapid inactivation of a calcium-activated potassium channel. Science. 257:1694–1698. 10.1126/science.1529355 [DOI] [PubMed] [Google Scholar]
- Stojilkovic S.S. 2006. Pituitary cell type-specific electrical activity, calcium signaling and secretion. Biol. Res. 39:403–423. 10.4067/S0716-97602006000300004 [DOI] [PubMed] [Google Scholar]
- Sun L., Xiong Y., Zeng X., Wu Y., Pan N., Lingle C.J., Qu A., and Ding J.. 2009. Differential regulation of action potentials by inactivating and noninactivating BK channels in rat adrenal chromaffin cells. Biophys. J. 97:1832–1842. 10.1016/j.bpj.2009.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebele V.N., Lagrutta A., Wade T., Figueroa D.J., Liu Y., McKenna E., Austin C.P., Bennett P.B., and Swanson R.. 2000. Cloning and functional expression of two families of β-subunits of the large conductance calcium-activated K+ channel. J. Biol. Chem. 275:23211–23218. 10.1074/jbc.M910187199 [DOI] [PubMed] [Google Scholar]
- Van Goor F., Li Y.X., and Stojilkovic S.S.. 2001. Paradoxical role of large-conductance calcium-activated K+ (BK) channels in controlling action potential-driven Ca2+ entry in anterior pituitary cells. J. Neurosci. 21:5902–5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M., Meera P., and Toro L.. 1999. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane β-subunit homolog. Proc. Natl. Acad. Sci. USA. 96:4137–4142. 10.1073/pnas.96.7.4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., and Brenner R.. 2006. An S6 mutation in BK channels reveals beta1 subunit effects on intrinsic and voltage-dependent gating. J. Gen. Physiol. 128:731–744. 10.1085/jgp.200609596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Rothberg B.S., and Brenner R.. 2006. Mechanism of β4 subunit modulation of BK channels. J. Gen. Physiol. 127:449–465. 10.1085/jgp.200509436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.W., Ding J.P., Xia X.M., and Lingle C.J.. 2002. Consequences of the stoichiometry of Slo1 α and auxiliary β subunits on functional properties of large-conductance Ca2+-activated K+ channels. J. Neurosci. 22:1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X.M., Ding J.P., and Lingle C.J.. 1999. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J. Neurosci. 19:5255–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X.M., Ding J.P., Zeng X.H., Duan K.L., and Lingle C.J.. 2000. Rectification and rapid activation at low Ca2+ of Ca2+-activated, voltage-dependent BK currents: consequences of rapid inactivation by a novel β subunit. J. Neurosci. 20:4890–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., and Aldrich R.W.. 2010. LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature. 466:513–516. 10.1038/nature09162 [DOI] [PubMed] [Google Scholar]
- Yan J., and Aldrich R.W.. 2012. BK potassium channel modulation by leucine-rich repeat-containing proteins. Proc. Natl. Acad. Sci. USA. 109:7917–7922. 10.1073/pnas.1205435109 [DOI] [PMC free article] [PubMed] [Google Scholar]